Abstract
Rats raised in an isolated condition (IC) are impulsive and hyperactive compared to rats raised in an enriched condition (EC), suggesting that isolation rearing may be a preclinical model of attention-deficit/hyperactivity disorder (ADHD). The current study determined if administration of methylphenidate (MPH), a dopamine transporter (DAT) blocker used in the treatment of ADHD, reduces the hyperactivity observed in IC rats toward levels observed in EC rats. Another goal was to determine if chronic MPH treatment differentially alters DAT function in EC and IC rats in medial prefrontal cortex (mPFC) or orbital frontal cortex (OFC). IC and EC rats were treated with either MPH (1.5 mg/kg, p.o.) or vehicle from postnatal days (PND) 28–51. On PND 28 and 51, rats were evaluated for MPH-induced locomotor activity. On PND 55–63, in vitro [3H]DA uptake assays were performed in mPFC and OFC. At both PND 28 and 51, IC rats were hyperactive compared to EC rats. At PND 28, MPH increased activity in EC rats only. At PND 51, MPH did not alter locomotor activity in IC or EC rats. Beginning at PND 55, basal uptake of [3H]dopamine in IC rats was higher in mPFC and lower in OFC compared to EC rats. The basal differences in DAT function were normalized by MPH treatment in mPFC, but not in OFC. These findings suggest that isolation rearing may not represent a valid predictive model for screening effective medications in the treatment of hyperactivity associated with ADHD.
Keywords: Isolation rearing, Locomotor activity, Methylphenidate, Dopamine transporter, Attention-deficit/hyperactivity disorder, Rat
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by inattention, impulsivity, and hyperactivity [1]. ADHD is often diagnosed in children, affecting 8–12% of children worldwide [2]. Twin studies and adoption studies suggest that ADHD is a heritable disorder [3,4]. Several gene variants have been correlated with ADHD, such as the exon III 7-repeat allele of the dopamine (DA) D4 receptor gene and the 148-bp allele of the DA D5 receptor gene [2,5]. Preclinical research using inbred or knockout rodents are used to model genetic-based ADHD-like behaviors and associated neural changes [6,7].
Although evidence suggests that ADHD is influenced by genetic factors, environmental factors are also implicated. One environmental factor associated with ADHD is psychosocial adversity, including low social class and poverty [2,8]. Preclinical research suggests that one method that may be useful for studying the effects of environment on ADHD-like behaviors is to raise laboratory animals in different housing conditions. Rats raised in an isolated condition (IC) show greater impulsivity than rats raised in an enriched condition (EC) across various tasks. IC rats make more premature nose pokes in a sucrose-reinforced nose-poke task [9], have lower efficiency scores in a differential reinforcement of low rate of responding (DRL) task [10], and choose a small, immediate reward in a delay discounting task [11, but see 12]. IC rats treated with methylphenidate (MPH) increase their preference for a large, delayed reward (i.e., become less impulsive), whereas EC rats do not [11]. Because MPH is efficacious in treating ADHD [2], these results indicate that IC rats may be a preclinical model of environment-based ADHD.
IC rats also show neuroanatomical and neurochemical differences compared to EC rats [13,14]. IC rats have decreased brain weight and size [15] and thinner cortices, particularly the occipital cortex [16,17]. IC rats have an increase in functioning of the dopamine (DA) transporter (DAT) in medial prefrontal cortex (mPFC), as indicated by an increase in the maximum velocity of [3H]DA uptake [18]. Furthermore, IC rats have more DAT protein at the cell surface compared to EC rats [19]. These results are important because the mPFC is implicated in hyperactivity. For example, direct administration of the DA D1/D2 receptor antagonist cis-flupenthixol into mPFC reduces locomotor activity [20]. The mPFC also is implicated in impulsivity, as mPFC lesions increase impulsivity in a five-choice serial reaction time task (5CSRTT) [21] and DA depletion in mPFC increases impulsivity in a DRL task [22]. Thus, the IC model may be useful for determining the role of mPFC in environment-based ADHD-like behaviors.
The main goal of the present experiment was to determine if oral MPH ameliorates the hyperactivity in adolescent IC rats relative to EC rats. An oral dose of MPH was used (1.5 mg/kg, p.o.) to model the clinical route and blood levels obtained in humans [23]. Since the primary mechanism of action for MPH is to increase extracellular DA via a blockade of DAT [24,25], another goal was to determine how long-term oral MPH treatment differentially alters DAT function in mPFC in IC and EC rats. In addition to mPFC, the orbitofrontal cortex (OFC) was examined because electrolytic OFC lesions increase locomotor activity [26].
2. Materials and Methods
2.1. Materials
[3H]DA (3,4-ethyl-2 [N-3H]dihydroxyphenylethylamine (specific activity, 31 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). Dopamine HCl, desipramine HCl, nomifensine maleate, pargyline HCl, catechol, and L-ascorbic acid were purchased from Sigma–Aldrich (St. Louis, MO). D-Glucose was purchased from Aldrich Chemical Co. (Milwaukee, WI). Paroxetine HCl was generously provided by Beecham Pharmaceuticals (Surrey, UK). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Animals
Thirty-six male Sprague Dawley rats were obtained from Harlan Laboratories (Indianapolis IN) at postnatal day (PND) 21 and were housed in an IC or EC environment. IC rats were single-housed in stainless steel hanging cages (17 × 24 × 20 cm) and were handled as briefly as possible during experimentation. EC rats were housed in large steel wire cages (122 × 61 × 45.5 cm), with solid steel floors and pine bedding changed weekly. EC rats were housed 9 per cage and were handled daily. EC rats were given an assortment of plastic objects (14 per cage) that were replaced and rearranged daily to maximize novelty. The objects varied in color, shape, and size. The objects included plastic tubes, various children’s toys, and large plastic balls. Rats remained in these conditions for the duration of the experiment. All rats were housed in a colony room held at constant temperature. Light and dark phases were on a 12:12-h cycle (lights on at 6:00; lights off at 18:00), and all experimentation occurred during the light phase. Rats had unlimited access to food and water in their home cage. Rats were cared for in accordance with the “Guide for the Care and Use of Laboratory Animals” [27] and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.3. Behavioral Apparatus
Locomotor activity was measured in 12 clear acrylic chambers (42 × 42 × 30 cm), each with a 16 × 16 grid of photobeam sensors. Activity was recorded with a monitoring system (AccuScan Instruments Inc., Columbus, OH) and was expressed as the total horizontal distance traveled.
2.4. Procedure
2.4.1. Locomotor activity
Beginning at PND 21, IC and EC rats were divided into two groups (MPH vs. vehicle). From PND 21–27, all rats were removed from their home cage once daily and given apple juice (vehicle; p.o.; 1 ml/kg) to drink through a silastic tube connected to a syringe. During treatment, each rat was gently restrained by hand; the experimenter administered the fluid slowly (over approximately 5 s) and observed the rat to ensure that no spillover occurred. Immediately following consumption, rats were placed back into their home cage. From PND 28–51, one group of rats continued to receive vehicle only and the other group received a solution of vehicle + MPH (1.5 mg/kg). This dose was selected to ensure that peak plasma drug levels were therapeutically relevant [23,28]. Thus, there were 4 groups in this study: (1) IC vehicle; (2) IC MPH; (3) EC vehicle; and (4) EC MPH (n = 9 per group).
On PND 28 and 51, locomotor activity was assessed. On these two test days, rats were placed individually in the locomotor chamber for 30 min to habituate them to the chamber. Immediately following habituation, rats were allowed to drink vehicle alone or vehicle + MPH in the same manner as during habituation; however, rats were placed back into the locomotor chamber for 1 h instead of being placed into their home cage.
2.4.2. Synaptosomal preparation and [3H]DA uptake
At PND 55–63, the kinetic parameters (Vmax and Km) of [3H]DA uptake were determined using a previously published method [18]. mPFC and OFC from individual rats were homogenized separately in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 2000g for 10 min at 4°C, and resulting supernatants were centrifuged at 20,000g for 15 min at 4°C. Resulting pellets were resuspended in 2.2 ml of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4) to obtain synaptosomal suspensions. Nonspecific [3H]DA uptake was determined in the presence of 10 μM nomifensine. Since DA is transported by the norepinephrine transporter (NET) and the serotonin transporter (SERT) in prefrontal cortex [29,30], kinetic analysis of [3H]DA uptake by DAT in mPFC and OFC was assessed in the presence of desipramine (5 nM) and paroxetine (5 nM) to prevent [3H]DA uptake into norepinephrine- and serotonin-containing nerve terminals, respectively, thereby isolating uptake of DA via DAT [18].
mPFC and OFC synaptosomes containing approximately 40 and 50 μg protein/100 μl, respectively, were incubated in a metabolic shaker for 5 min at 34°C and then incubated for 5 min at 34°C after adding 1 of 7 [3H]DA concentrations (0.01–1 μM) in 250 μl total volume. Incubations were terminated by addition of 3 ml of ice-cold assay buffer, followed by immediate filtration through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 h). Filters were washed three times with 3 ml of ice-cold buffer containing pyrocatechol using a Brandel cell harvester (model MP-43RS; Brandel Inc., Gaithersburg, MD). Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences). Protein concentrations were determined, with bovine serum albumin as the standard [31]. Vmax and Km were determined using the commercially available Graph- Pad Prism 4.0 program (GraphPad Software Inc., San Diego, CA).
2.5. Drug
Methylphenidate HCl (Mallinckrodt, St. Louis, MO) was prepared in apple juice (from concentrate; Kroger Value, Kroger Co., Cincinnati, OH), with dose based on the salt weight.
2.6. Statistical Analysis
Body weight was analyzed using a mixed-factor analysis of variance (ANOVA) with Rearing Condition (IC vs. EC) and Drug (Vehicle vs. MPH) as between-subjects factors and Age (PND 28 vs. 51) as a within-subjects factor. Locomotor activity during the 30 min of the baseline session was analyzed with a mixed-factor ANOVA with Rearing Condition and Drug as between-subjects factors and Age as a within-subjects factor; rats did not receive vehicle or MPH treatment during the baseline session. Locomotor activity for the first 30 min of the test session was analyzed with a mixed-factor ANOVA with Rearing Condition and Drug as between-subjects factors and Age as a within-subjects factor. Only the first 30 min were analyzed because rats primarily engaged in non-ambulatory behavior, such as grooming and sleeping, during the last 30 min of the session. Because it was hypothesized that MPH would decrease the hyperactivity observed in IC rats relative to EC rats, planned comparisons (unpaired Student’s t tests) were conducted across treatment groups. For [3H]DA uptake, nonlinear curve fits of data used the Michaelis-Menten equation to obtain Vmax and Km values. Vmax and Km values for [3H]DA uptake were analyzed separately using a two-way ANOVA with Rearing Condition and Drug as between-subjects factors. Log transformed Km values were used for statistical analyses. Some samples were excluded from neurochemical analyses due to experimental error (1 mPFC sample from IC MPH and EC vehicle groups; 3 mPFC samples from EC MPH group; 1 OFC sample from IC and EC vehicle groups; 4 OFC samples from IC MPH group; 2 OFC samples from EC MPH group). Significant main effects were probed with Bonferroni post hoc tests, and interactions were probed using unpaired or paired Student’s t tests. In all cases, significance was declared at p < 0.05.
3. Results
3.1. Body Weight
Fig 1 shows body weight collapsed across drug treatment because MPH did not alter body weight in either IC or EC rats. Body weights were higher in IC rats than EC rats across development. Three-way ANOVA revealed main effects of Rearing Condition (F(1, 32) = 17.39, p < .01) and Age (F(23, 736) = 2172.64, p < .01), as well as a significant Rearing Condition × Age interaction (F(23, 736) = 1.78, p < .05). Post hoc tests revealed that IC rats weighed significantly more than EC rats from PND 28–44, PND 46–48, and PND 50–51 (t’s > 2.13). Fig 1 shows body weight collapsed across drug treatment because MPH did not alter body weight in either IC or EC rats.
Fig 1.
Mean (±S.E.M.) body weight, collapsed across drug treatment, for IC and EC rats from PND 28–51. *p < .05 compared to EC rats; **p < .01 compared to EC rats.
3.2. Locomotor activity
Table 1 shows baseline activity (before vehicle or MPH treatment) for each group on PND 28 and 51. Three-way ANOVA revealed main effects of Rearing Condition (F(1, 32) = 29.11, p < .01) and Age (F(1, 32) = 23.62, p < .01). IC rats were more active relative to EC rats at PND 28 and 51. IC and EC rats were more active at PND 51 than PND 28.
Table 1.
Mean (± S.E.M.) total distance traveled (in cm) for IC and EC rats during the 30 min baseline session at PND 28 and 51. Note that the MPH groups did not receive MPH during the baseline session.
| PND 28 | PND 51a | |||
|---|---|---|---|---|
|
| ||||
| Group | Vehicle | MPH | Vehicle | MPH |
| ICb | 3296.33 (± 587.56) | 3527.89 (± 541.13) | 5358.00 (± 924.60) | 5355.00 (± 1053.96) |
| EC | 1050.33 (± 117.41) | 1288.22 (± 194.24) | 2206.11 (± 174.20) | 2370.67 (± 217.00) |
p < .01, compared to PND 28.
p < .01, compared to EC rats.
Fig 2 shows activity for each group on PND 28 and 51. Three-way ANOVA revealed main effects of Rearing Condition (F(1, 32) = 41.16, p < .01) and Age (F(1, 32) = 13.94, p < .01), as well as a significant Rearing Condition × Age interaction (F(1, 32) = 16.04, p < .01). There was a trend for a significant Rearing Condition × Age × Drug interaction (F(1, 32) = 4.05, p =.053). Regardless of drug treatment, Bonferroni post hoc tests revealed that IC rats displayed more activity relative to EC rats at both ages; there was also a significant overall increase in activity from PND 28 to 51 in IC rats, but not in EC rats. Planned comparisons (unpaired Student’s t tests) were conducted to determine the effects of MPH in EC and IC rats on PND 28 and 51. On PND 28, EC rats treated with MPH had significantly increased activity compared to EC rats treated with vehicle (t(16) = 2.58, p < .05). No other significant effects of MPH were found.
Fig 2.
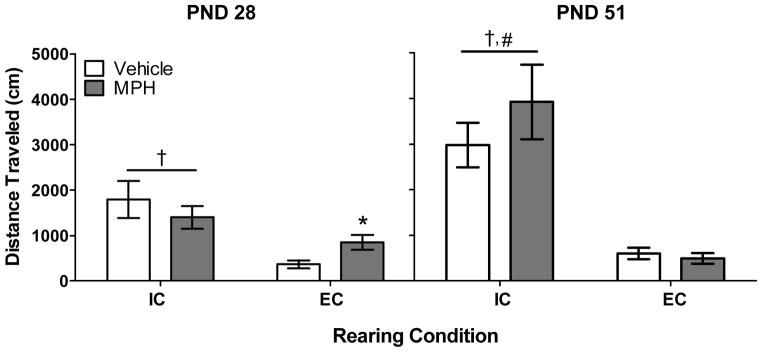
Mean (±S.E.M.) total distance traveled (in cm) for IC and EC rats treated with vehicle or MPH (n = 9 per group). *p < .05 compared to EC rats treated with vehicle; #p < .01 compared to IC rats on PND 28, collapsed across drug treatment; †p < .01 compared to EC rats, collapsed across drug treatment.
3.3. [3H]DA uptake in mPFC and OFC
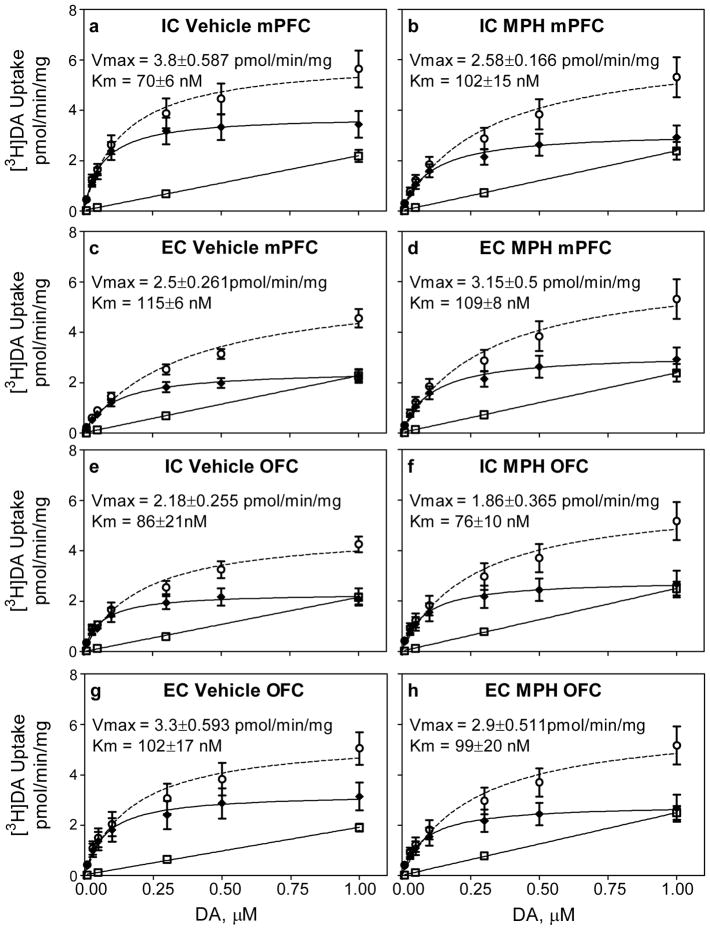
Fig 3 shows the concentration-dependent uptake of [3H]DA used to determine kinetic parameters (Vmax and Km; mPFC, Fig 3a-d and OFC, Fig 3e-h) for each group. DAT-mediated [3H]DA uptake was determined by subtracting nonspecific uptake at each concentration of [3H]DA from total uptake. In all groups, across the range of concentrations used, saturation was obtained in specific [3H]DA uptake, but not with nonspecific [3H]DA uptake.
Fig 3.
Mean (±S.E.M.) uptake of [3H]DA across varying concentrations used to define kinetic parameters (Vmax and Km) in mPFC and OFC across treatment groups. Panels a–d: Uptake in mPFC of IC rats treated with vehicle (panel a; n = 9) or MPH (panel b; n = 8) and EC rats treated with vehicle (panel c; n = 8) or MPH (panel d; n =6). Panel e-h: Uptake in OFC of IC rats treated with vehicle (panel e; n = 8) or MPH (panel f; n = 5) and EC rats treated with vehicle (panel g; n = 8) or MPH (panel h; n = 7). DAT-mediated [3H]DA uptake (—◆—) was determined by subtracting nonspecific uptake (---□---) at each concentration of DA from total uptake (—○—). Nonlinear curve fits of data for uptake used the Michaelis-Menten equation to obtain Vmax and Km values.
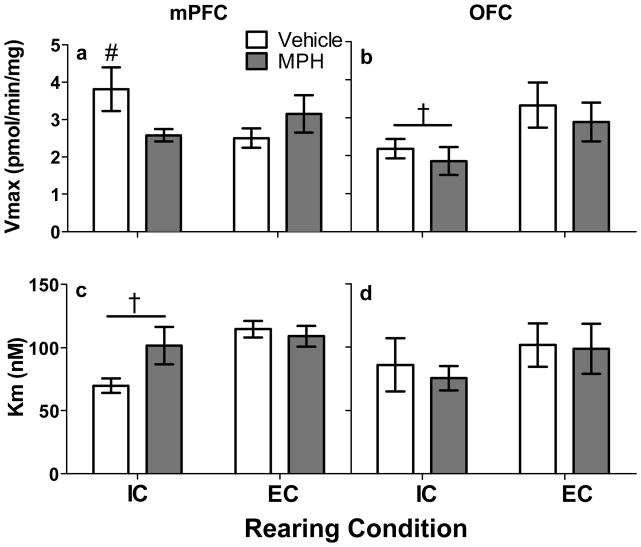
Fig 4 summarizes the kinetic parameters of [3H]DA uptake (Vmax and Km in mPFC, Fig 4a and 4c and in OFC, Fig 4b and 4d) for each group. In mPFC, two-way ANOVA revealed a Rearing Condition × Drug interaction (F(1, 27) = 4.82, p < .05) for Vmax. To explore this interaction, unpaired Student’s t tests were conducted to compare IC and EC rats. IC rats treated with vehicle had higher Vmax for DA uptake compared to EC rats treated with vehicle (t(15) = 1.95, p < .05); however, there was no difference between IC and EC rats treated with MPH. Two-way ANOVA on Km values in mPFC revealed only a significant main effect of rearing condition, with IC rats having a lower Km compared to EC rats, regardless of treatment (F(1, 27) = 7.34, p < .01; Fig. 4c). There was a nonsignificant trend for a Rearing Condition × Drug interaction (F(1,27) = 3.76, p = .06). In OFC, two-way ANOVA revealed a main effect of Rearing Condition (F(1, 24) = 5.19, p < .05). Regardless of drug treatment, IC rats had lower Vmax values in OFC compared to EC rats (Fig. 4b). There were no significant differences among groups in Km in OFC (Fig. 4d).
Fig 4.
Mean (±S.E.M.) Vmax and Km values obtained from mPFC and OFC across treatment groups. Panel a: Vmax of [3H]DA uptake in mPFC from IC rats treated with vehicle (n = 9) or MPH (n = 8) and EC rats treated with vehicle (n = 8) or MPH (n = 6). #p < .05 compared to EC rats treated with vehicle. Panel b: Vmax of [3H]DA uptake in OFC from IC rats treated with vehicle (n = 8) or MPH (n = 5) and EC rats treated with vehicle (n = 8) or MPH (n = 7). †p < .05 compared to EC rats, regardless of drug treatment. Panel c: Km values of [3H]DA uptake in mPFC. †p < .05 compared to EC rats, regardless of drug treatment. Panel d: Km values of [3H] DA uptake in OFC.
4. Discussion
The present findings demonstrate that rats reared in an isolated condition are hyperactive relative to rats reared in an enriched condition, and this difference increases over time. Although EC rats were more sensitive to the acute stimulant effects of MPH at PND 28, MPH did not alter locomotor activity in IC rats at either PND 28 or 51. Furthermore, IC rats had increased transporter function in mPFC, but decreased transporter function in OFC. MPH treatment decreased transporter function in mPFC to levels similar to EC rats. Despite the MPH-induced change in DAT function in mPFC, these results suggest that isolation rearing models the hyperactivity component of ADHD, but not the ability of MPH to ameliorate hyperactivity. Thus, isolation rearing does not model all aspects of the hyperactivity component of ADHD.
IC rats weighed more relative than EC rats during the course of the experiment, which is consistent with previous findings [32–34, but see 35]. Previous research has suggested that this difference is associated with physical enrichment because rats reared in an impoverished environment weigh less than rats reared in an enriched environment after controlling for the number of conspecifics in each condition [34]. The difference in weights also can be explained by alterations in caloric metabolism in IC and EC rats [32,33]. Importantly, oral MPH did not alter body weight in IC or EC rats, which is consistent with previous clinical studies [36,37]. This suggests that MPH did not alter locomotor activity in EC rats at PND 28 through nonspecific alterations in metabolism.
IC rats had higher baseline levels of locomotor activity compared to EC rats, which is consistent with previous research [18,34,38–45]. The current results extend this work by showing that the magnitude of difference between IC and EC rats increased with length of exposure to rearing conditions across development. In contrast to the baseline difference in activity, oral MPH increased activity acutely in EC rats (PND 28), whereas MPH did not alter activity either acutely or repeatedly in IC rats (PND 28 or 51). These findings are similar to previous research showing that IC rats are less sensitive to the acute stimulant effects of amphetamine on locomotor activity relative to EC rats [38–40]. However, repeated administration of MPH did not produce hyperactivity in either IC or EC rats. This latter finding contrasts with previous reports showing that IC rats show locomotor sensitization that is either similar to or greater than that observed in EC rats [38,46,47]. Direct comparison of results across these studies is tenuous, however, as the current study used the oral route of administration, whereas previous reports used systemic injections. Systemic administration of MPH produces a larger increase in extracellular release of DA in nucleus accumbens compared to oral administration [48], and DA within the mesolimbic system modulates locomotor activity [49]. According to Kuczenski and Segal [23], high doses of MPH predominately promote DA activity, whereas low doses promote norepinephrine activity. Regardless of the dose-dependent mechanisms, the current results show that a clinically relevant oral dose of MPH does not produce locomotor sensitization in either IC or EC rats during periadolescent development.
Sagvolden et al. [50] proposed that a valid animal model of ADHD must show that hyperactivity develops gradually over time. Consistent with this criterion, hyperactivity was present during the first locomotor session in IC rats relative to EC rats. Moreover, activity increased from PND 28 to 51 in IC rats, but not in EC rats. While this finding suggests that IC rats may serve as an animal model of ADHD with hyperactivity, the failure of oral MPH to decrease the hyperactivity is problematic. Strong clinical evidence demonstrates that MPH is effective in reducing the major symptoms of ADHD, including hyperactivity and impulsivity, in adolescent and young adult humans [51–54]. Although MPH reduces hyperactivity in clinical populations, previous preclinical research using genetic models has shown that MPH (2.5 and 10.0 mg/kg, i.p.) increases activity in the spontaneously hypertensive rat (SHR), a genetic animal model of ADHD [55–59, but see 60]. At a lower oral dose similar to that used in the current report, MPH (1.5 mg/kg, p.o.) has no effect on hyperactivity in SHR rats [61], which is consistent with the current finding that a low oral dose of MPH also does not alter hyperactivity in IC rats. Thus, to date, neither genetic- nor environment-based preclinical approaches model completely the MPH-sensitive hyperactive component of ADHD.
One limitation to the current study is that only one dose of MPH was tested. Although the aim of the present study was to assess the effects of a clinically relevant dose of MPH on the hyperactivity facet of ADHD, we cannot rule out the possibility that a reduction in hyperactivity may be observed in IC rats across varying doses of MPH. For example, MPH dose-dependently reduces impulsive choice in IC rats [11]. Also, dose-dependent effects have been observed in locomotor activity in IC and EC rats following repeated MPH treatment. IC rats show increased locomotor activity following repeated systemic administration of MPH (3 and 10 mg/kg), whereas EC rats only show increased activity following treatment with 10 mg/kg [47]. Generating dose-effect functions for the locomotor effects of orally delivered MPH will better determine if isolation rearing models the hyperactivity component of ADHD.
In addition to the isolated-induced hyperactivity, DAT function in frontal cortex differed between IC and EC rats. In IC rats, basal DAT function (no MPH) as measured by Vmax was increased in mPFC, but decreased in OFC. IC rats also had increased affinity for DAT in mPFC as measured by Km. These findings are consistent with previous reports showing that IC rats have increased DAT function in mPFC [18] and that DA activity in mPFC is implicated in hyperactivity [20,62]. The current results extend that previous work by showing that DAT function in OFC is decreased in IC rats. While a paucity of research has examined the possible role of OFC in hyperactivity, OFC has been implicated in impulsivity. OFC damage with excitotoxic or DA-depleting agents increases impulsivity measured by delay discounting [63–65, but see 66]. Furthermore, OFC damage impairs performance in a stop signal reaction time task [67] and reversal-learning tasks [68–71]. Thus, the decrease in DAT function in OFC may be related more to inhibitory deficits (impulsivity) in IC rats [11], whereas the increase in DAT function in mPFC may be related more to the hyperactivity in IC rats.
Repeated MPH treatment had differential effects on DAT function in mPFC and OFC in IC and EC rats. While MPH produced no changes in either Vmax or Km in OFC, MPH decreased Vmax in IC rats only. There was also a trend for MPH to increase Km in IC rats, although this effect did not reach statistical significance. More important, following MPH treatment, there were no longer any differences between IC and EC rats in Vmax, suggesting that MPH normalized the isolation-induced increase in DAT function in mPFC. This effect of MPH may reflect a down-regulation of functional DAT protein [28,47,72]. However, since the hyperactivity in IC rats was not normalized by MPH, it is unlikely that DAT activity in mPFC mediates directly the behavioral effects observed. Decreased DAT function may be a possible mechanism by which impulsive choice is reduced in IC rats following MPH treatment [11].
In conclusion, IC rats became hyperactive over time, which is one criterion for animal models of ADHD [50]. A clinically relevant dose of oral MPH normalized DAT function in mPFC in IC rats, whereas repeated MPH had no effect on DAT function in EC rats. However, MPH did not reduce hyperactivity in IC rats, suggesting that isolation rearing models some, but not all, of the features of ADHD. Although isolation rearing may not model the MPH-sensitive hyperactivity component of ADHD, future work is needed to determine if oral doses of MPH delivered during development affects the impulsivity and inattentive components of ADHD in IC rats.
Acknowledgments
This research was funded by NIH grants P50 DA05312, R01 DA12964 and R01 DA11716.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington: American Psychiatric Association; 1994. DSM-IV. [Google Scholar]
- 2.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 3.Sprich S, Biederman J, Crawford MH, Mundy E, Faraone SV. Adoptive and biological families of children and adolescents with ADHD. J Am Child Adolesc Psychiatry. 2000;39:1432–37. doi: 10.1097/00004583-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Thapar A, Holmes J, Poulton K, Harrington R. Genetic basis of attention deficit and hyperactivity. Br J Psychiatry. 1999;174:105–11. doi: 10.1192/bjp.174.2.105. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:718–26. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- 7.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 8.Thapar A, Cooper M, Jefferies R, Stergiakouli E. What causes attention deficit hyperactivity disorder? Arch Dis Child. 2012;97:260–5. doi: 10.1136/archdischild-2011-300482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Phys Behav. 2006;88:132–7. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Ough BR, Beatty WW, Khalili J. Effects of isolated and enriched rearing on response inhibition. Psychon Sci. 1972;27:293–4. [Google Scholar]
- 11.Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellemans KGC, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: Relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–20. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats—Behavioral and neurochemical aspects. Behav Brain Res. 2011;222:246–64. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochm Behav. 2009;92:377–82. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett EL, Rosenzweig MR, Diamond MC. Rat brain: Effects of environmental enrichment on wet and dry weights. Science. 1969;163:825–26. doi: 10.1126/science.163.3869.825. [DOI] [PubMed] [Google Scholar]
- 16.Diamond MC, Krech D, Rosenzweig MR. The effects of an enriched environment on the histology of the rat cerebral cortex. J Comp Neurol. 1964;123:111–20. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–17. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–43. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- 20.Bast T, Pezze MA, Feldon J. Dopamine receptor blockade in the rat medial prefrontal cortex reduces spontaneous and amphetamine-induced activity and does not affect prepulse inhibition. Behav Pharmacol. 2002;13:669–73. doi: 10.1097/00008877-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology. 2009;202:307–13. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- 22.Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994;131:23–33. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- 23.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: Preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–71. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: The actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–74. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–31. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 26.de Bruin JP, van Oyen HG, Van de Poll N. Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behav Brain Res. 1983;10:209–32. doi: 10.1016/0166-4328(83)90032-3. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council. Guide for the care and use of laboratory animals. 8. Washington: National Academy Press; 2010. [Google Scholar]
- 28.Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with attention deficit/hyperactivity disorder phenotype: Cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–47. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–95. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JM, Steketee JD. Characterization of dopamine transport in crude synaptosomes prepared from rat medial prefrontal cortex. J Neurosci Methods. 2004;137:161–65. doi: 10.1016/j.jneumeth.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Hellemans KGC, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Dev Brain Res. 2004;150:103–15. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Peña Y, Prunell M, Rotllant D, Armario A, Escorihuela RM. Enduring effects of environmental enrichment from weaning to adulthood on pituitary-adrenal function, pre-pulse inhibition and learning in male and female rats. Psychoneuroendocrinology. 2009;34:1390–1404. doi: 10.1016/j.psyneuen.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Zaias J, Queeney TJ, Kelley JB, Zakharova ES, Izenwasser S. Social and physical environmental enrichment differentially affect growth and activity of periadolescent and adolescent male rats. J Am Assoc Lab Anim Sci. 2008;47:30–4. [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann LC, Schütte SRM, Koch M, Schwabe K. Effect of “enriched environment” during development on adult rat behavior and response to the dopamine receptor agonist apomorphine. Neuroscience. 2009;158:1589–98. doi: 10.1016/j.neuroscience.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Biederman J, Spencer TJ, Monuteaux MC, Faraone SV. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: Sex and treatment effects. J Pediatr. 2010;157:635–40. doi: 10.1016/j.jpeds.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Du M, Zhuang S. Impact of long-term treatment of methylphenidate on height and weight of school age children with ADHD. Neuropediatrics. 2010;41:55–9. doi: 10.1055/s-0030-1261893. [DOI] [PubMed] [Google Scholar]
- 38.Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharamcol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- 39.Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–64. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 40.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropsychopharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 41.Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197:125–37. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Brenes JC, Rodríguez O, Fornaguera J. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav. 2008;89:85–93. doi: 10.1016/j.pbb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Dev Brain Res. 2004;153:213–23. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Schrijver NCA, Bahr NI, Weiss IC, Würbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–24. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- 45.Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: Effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–73. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- 46.Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology. 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- 47.Wooters TE, Bardo MT, Dwoskin LP, Middle NM, Gomez AM, Mactutus CF, et al. Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res. 2011;219:98–107. doi: 10.1016/j.bbr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: A mircrodialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–7. [PubMed] [Google Scholar]
- 49.Galey D, Simon H, Le Moal M. Behavioral effects of lesions in the A10 dopaminergic area of the rat. Brain Res. 1977;124:83–97. doi: 10.1016/0006-8993(77)90865-4. [DOI] [PubMed] [Google Scholar]
- 50.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbah M. Rodent models of attention- deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–47. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, et al. Assessing medication effects in the MTA study using neuropsychological outcomes. J Child Psychol Psychiatry. 2006;47:446–56. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- 52.McGough JJ, Biederman J, Wigal SB, Lopez FA, McCracken JT, Spencer T, et al. Long-term tolerability and effectiveness of once-daily mixed amphetamine salts (Adderall XR) in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44:530–38. doi: 10.1097/01.chi.0000157550.94702.a2. [DOI] [PubMed] [Google Scholar]
- 53.Prince JB. Pharmacotherapy of attention-deficit hyperactivity disorder in children and adolescents: Update on new stimulant preparations, atomoxetine, and novel treatments. Child Adolesc Psychiatr Clin N Am. 2006;15:13–50. doi: 10.1016/j.chc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Steinhoff KW. Attention-deficit/hyperactivity disorder: Medication treatment-dosing and duration of action. Am J Manag Care. 2004;10:S99–106. [PubMed] [Google Scholar]
- 55.Amini B, Yang PB, Swann AC, Dafny N. Differential locomotor responses in male rats from three strains to acute methylphenidate. Int J Neurosci. 2004;114:1063–84. doi: 10.1080/00207450490475526. [DOI] [PubMed] [Google Scholar]
- 56.Barron E, Yang PB, Swann AC, Dafny N. Adolescent and adult male spontaneous hyperactive rats (SHR) respond differently to acute and chronic methylphenidate (Ritalin) Int J Neurosci. 2009;119:40–58. doi: 10.1080/00207450802330546. [DOI] [PubMed] [Google Scholar]
- 57.Wultz B, Sagvolden T, Moser EI, Moser MB. The spontaneously hypertensive rat as an animal model of attention-deficit hyperactive disorder: Effects of methylphenidate on exploratory behavior. Behav Neural Biol. 1990;53:88–102. doi: 10.1016/0163-1047(90)90848-z. [DOI] [PubMed] [Google Scholar]
- 58.Yang PB, Cuellar DO, 3rd, Swann AC, Dafny N. Age and genetic strain differences in response to chronic methylphenidate administration. Behav Brain Res. 2011;218:206–17. doi: 10.1016/j.bbr.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 59.Yang PB, Swann AC, Dafny N. Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Res Bull. 2006;71:301–10. doi: 10.1016/j.brainresbull.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong Q, Zhang M, Pan XQ, Guo M, Li F, Tong ML, et al. Prefrontal cortex Homer expression in an animal model of attention-deficit/hyperactivity disorder. J Neurol Sci. 2009;287:205–11. doi: 10.1016/j.jns.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 61.Warton FL, Howells FM, Russell VA. Increased glutamate-stimulated release of dopamine in substantia nigra of a rat model for attention-deficit/hyperactivity disorder—Lack of effect of methylphenidate. Metab Brain Dis. 2009;24:599–613. doi: 10.1007/s11011-009-9166-1. [DOI] [PubMed] [Google Scholar]
- 62.Lacroix L, Broersen LM, Weiner I, Feldon J. The effects of excitotoxic lesion of the medial prefrontal cortex on latent inhibition, prepulse inhibition, food hoarding, elevated plus maze, active avoidance and locomotor activity in the rat. Neuroscience. 1998;84:431–42. doi: 10.1016/s0306-4522(97)00521-6. [DOI] [PubMed] [Google Scholar]
- 63.Kheramin S, Body S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, et al. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: A quantitative analysis. Psychopharmacology. 2004;175:206–14. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- 64.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;152:390–97. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- 65.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–68. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 66.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: Interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–85. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 67.Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5 choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: Differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–19. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–80. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–40. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, et al. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–91. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]