Abstract
The ability to recognize kin is an important social skill for primates. Humans are adept at using facial similarity to recognize likely kin, and there is evidence that nonhuman primates are also able to use facial similarity to make judgments about kinship. However, if and how nonhuman primate faces actually contain kinship information remains unclear. To test whether there is objectively measurable facial similarity in related nonhuman primates, we compared facial measurements from related (paternal half-sisters) and unrelated adult female rhesus macaques (Macaca mulatta). Facial measurements were first summarized into 5 factors using a principal component analysis. Differences in these factors between the faces of related macaques were compared with differences between the faces of random unrelated macaques and of age-matched unrelated macaques. The difference in facial measurements between related macaques was significantly smaller than the difference in facial measurements of either group of unrelated macaques, constituting an objective measure of facial similarity in macaque kin. These results indicate that kinship information is contained in the rhesus macaque face and suggest that nonhuman primates may rely in part on facial similarity to distinguish kin.
Keywords: kin recognition, facial similarity, rhesus macaque, principal component analysis
Recognizing kin or those likely to be kin is an evolutionarily important task that is especially relevant for social species. Humans commonly treat facial similarity as indicative of kinship and can accurately detect kin relationships based on facial similarity, even in unfamiliar faces (Alvergne et al., 2009; Kaminski, Dridi, Graff, & Gentaz, 2009). Parr and de Waal (1999) demonstrated a similar ability in chimpanzees (Pan troglodytes). Subjects were able to match facial photographs of chimp mothers and their male (but not female) offspring. Despite these findings, it is not clear what role (if any) facial information plays with regard to kin recognition for nonhuman primates. If facial similarity is a valid indicator of kinship for nonhuman primates as it is for humans, it is likely that primate species other than humans make use of facial similarity to recognize kin.
Much of the evidence for kin recognition in nonhuman primates suggests that familiarity is a common source of kin information (Rendall, 2004; Widdig, 2007). Widdig, Nürnberg, Krawczak, Streich, and Bercovitch (2001) studied levels of affiliation among adult females in a group of rhesus macaques, Macaca mulatta. The highest levels of affiliation were found between maternal half-sisters, while the lowest levels were found between unrelated females. Paternal half-sisters affiliated significantly more than unrelated females, but less than maternal half-sisters. In addition, there was a clear effect for birth cohort regardless of kinship, suggesting a strong role for simple familiarity. Similar paternal kin bias was confirmed in other studies of rhesus macaques (Widdig, Nürnberg, Krawczak, Streich, & Bercovitch, 2002; Widdig et al., 2006) as well as in baboons (Smith, Alberts, & Altmann, 2003; Silk, Altmann, & Alberts, 2006) and mandrills (Mandrillus sphinx; Charpentier, Peignot, Hossaert-Mckey, & Wickings, 2007). Other studies found maternal kin bias, but no evidence for paternal kin bias in chimpanzees (Langergraber, Mitani & Vigilant, 2007) and capuchins (Cebus capucinus; Perry, Manson, Muniz, Gros-Luis & Vigilant, 2008). Given the matrilineal structure of rhesus society, high levels of maternal kin affiliation may easily be explained by familiarity. Kin bias among paternal half-sisters may be the result of familiarity acquired through a cohort association or mediated by mothers spending time around a common male (i.e., paternal half-sisters may have increased exposure to each other if their mothers both spend time around the common father). Thus, these field studies appear to point toward a strong role for familiarity in kin recognition, but that does not rule out other sources of kin information.
Alternative sources of kin information, such as phenotypic similarity, may come into play when familiarity is absent. Experimental studies have controlled for familiarity effects by testing subjects who were unfamiliar with each other. An early experiment using infant pigtail macaques, Macaca nemestrina, found that unfamiliar peers preferred to sit closer to paternal half-siblings than to nonkin (Wu, Holmes, Medina, & Sackett, 1980). However, the mechanism for kin recognition is unclear as subjects were raised in peer groups without exposure to genetic relatives prior to testing. Attempts to replicate and extend these findings failed to find an effect for kinship (Fredrickson & Sackett, 1984; Sackett & Fredrickson, 1987). Baboons (Papio cynocephalus) who were raised apart from kin other than their mothers and subsequently introduced to a social group containing paternal kin also showed no evidence of kin biased social behavior (Erhart, Coelho, & Bramblett, 1997). Despite these negative results, other captive studies have provided clear positive evidence for the role of phenotypic similarity in kin recognition among nonhuman primates. For example, Parr and de Waal (1999) showed that chimpanzees can match kin pairs in facial photographs even of unfamiliar peers. Parr, Heintz, Lonsdorf, and Wroblewski (2010) recently extended these findings and showed that both chimpanzees and rhesus macaques trained on a match-to-sample task were able to match unfamiliar parent conspecifics with offspring. These findings demonstrate that in some situations, adult macaques, like chimpanzees and humans, are able to discriminate kin on the basis of phenotypic similarity in the absence of familiarity.
Phenotype matching can take many forms (i.e., odor, visual appearance, characteristic movement, vocal distinctiveness, etc.), but the face may be one readily available source of phenotypic similarity to which macaques are likely to attend. Rhesus macaques are known to respond to several types of social information found in the face. Macaques appear to respond instinctively to certain facial expressions, such as a threat face (Sackett, 1966), and infant macaques are sensitive to the gender of facial stimuli (Paukner, Huntsberry, & Suomi, 2009). Facial kin recognition in humans, evidence for similar skills in chimpanzees and macaques, and the social importance of the macaque face suggest that macaques may be sensitive to kin information found in the face.
The purpose of the current study was to explore the possibility of kinship information in the rhesus macaque face by comparing facial measurements of related and unrelated macaques. We sought to define discrete aspects of facial features based on distance measures taken from digital images of macaque faces and to use those measures to compare differences in the facial features of related and unrelated adult female rhesus macaques. If kinship information is contained in the physical features of adult macaque faces, facial measurements should vary with respect to kin status.
Method
Subjects
A digital camera was used to capture images of 63 adult female rhesus macaques (Macaca mulatta) aged 5 to 29 (mean age = 10.4) in their home run or outdoor colony. All images were frontal, full-face with neutral expressions, and standardized for interpupilar distance. Subjects were drawn from breeding groups consisting of 1 or 2 males and 10 to 12 females housed in indoor/outdoor runs and from a larger colony housed in an outdoor field station at the National Institutes of Health Animal Center in Poolesville, Maryland.
Procedure
An investigator blind to subject kin status used GNU Image Manipulation Program, version 2.6 to place measurement points and to calculate 10 vertical and two horizontal distances for each face (see Figure 1B). For measures having both a right-side and left-side value (eye height, etc.) the mean value of left and right measurements was used. Measurement points and distances were based on those used by Koba, Izumi, and Nakamura (2009).
Figure 1.
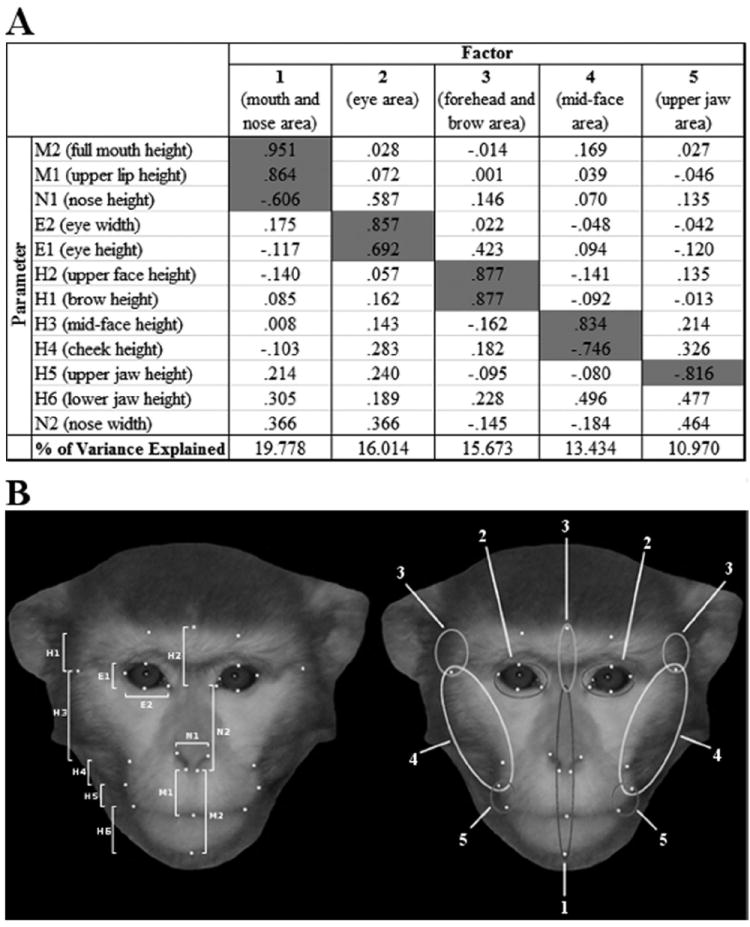
(A) Factor loadings for each component. Parameters used to interpret factors are highlighted. Parameter key: M = mouth, N = nose, E = eye, H = height. (B) Left: Measurement points and measures taken from each face. Right: Facial areas associated with each factor derived from the PCA.
Facial Measure Analysis
Measurements from each of the 63 facial photographs were submitted to a principal component analysis (PCA) to reduce the number of dependent measures into a smaller subset of orthogonal dependent variables. SPSS, version 17.0 was used for all statistical analysis. We used a varimax rotation and the Anderson-Rubin method of extraction to create factors with all variables contributing to all factors (see Figure 1). Factors with an eigenvalue greater than 1 were retained for subsequent analysis and loadings with an absolute value > 0.6 were used to assign labels to individual factors.
Facial Comparison Analysis
Subjects with at least one paternal half-sister in the pool of 63 images were included in the facial comparison analysis (N = 14). Kin status was determined by a review of colony birth records. Where records were uncertain, no judgment was made about paternity and the monkey was ruled out as a subject for kin analysis. Factors derived from the PCA were used to calculate 3 sets of difference scores by taking the difference between each subject and (a) a paternal half-sister, (b) an unrelated female randomly selected from all macaques of the same age as (a), and (c) an unrelated female randomly selected from all remaining macaques for all factors. We then used absolute values of all difference scores for subsequent analysis (effectively making difference scores distances between factors). This procedure resulted in a set of scores representing the overall differences in facial structure between related female macaques, a set of scores representing the overall differences in facial structure between unrelated female macaques of a similar age, and a set of scores representing the overall differences in facial structure between unrelated female macaques as a general group. The age-matched group was included in order to address concerns that facial similarity may be difficult to detect between faces that have a large age mismatch as facial appearance may continue to change throughout life and older faces may be more distinctive than younger faces.
Results
The PCA summarized the 12 facial measurements into five factors, which explained 75.9% of the variance in the facial measurements (see Figure 1A). Each factor was interpreted as follows: (1) the mouth and nose area, (2) the eye area, (3) the forehead and brow area, (4) the midface area, and (5) the upper jaw area. Figure 1B illustrates the interpretation of each factor.
A repeated-measures analysis of variance with comparison group (3) and factor (5) as within-subject variables showed a significant main effect for group, F(2, 26) = 4.13, p = .028, partial eta squared = 0.24, but no effect for factor, F(4, 52) = 1.64, p = .18, partial eta squared = 0.11, and no interaction, F(8, 104) = 0.26, p = .98, partial eta squared = 0.02. Follow-up comparisons with Fisher’s least significant difference tests showed that the related group had significantly lower difference scores than the age-matched control group (mean difference = −0.32, p = .024) and the random control group (mean difference = −0.311, p = .059) (see Figure 2).
Figure 2.
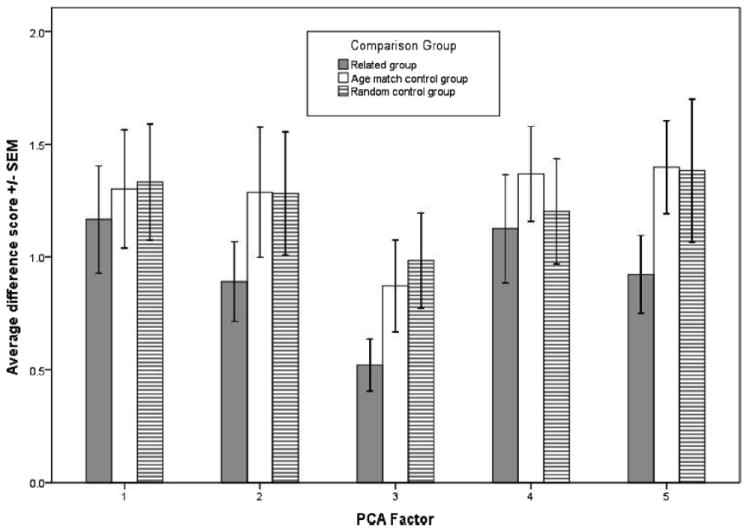
Difference scores for the related group are significantly smaller as compared with the age-matched control group (p = .024) and with the random control group (p = .059).
Discussion
Five measures of female macaque faces likely to capture variance in facial structure were derived from a PCA. Using these measures, adult female rhesus macaque faces were compared with the faces of kin and nonkin. The difference in these measures between related females was less than the difference between age-matched females and between random females. It, therefore, appears that related female macaque faces are more similar to each other than unrelated female macaque faces. The inclusion of an age-matched control group indicates that similarity between related faces cannot be simply ascribed to age-similarity.
Finding that similar genotypes produce similar phenotypes in this case is unsurprising, but nonetheless important. Many factors (dominant vs. recessive genes, gene-environment interactions, etc.) can influence genotypes to produce different and sometimes widely divergent phenotypes. Therefore, in order to make a case for kin recognition based on phenotypic similarity, it is essential to confirm the assumption that similar genotypes produce similar phenotypes. In addition, because our kin group was comprised of paternal half-sisters, our findings indicate that facial similarity in rhesus macaques may be useful for identifying kin relationships that are more distant than parent/offspring or full sibling.
As important as it may be to establish facial similarity between kin in rhesus macaques, it is only the first step in making a case for facial kin recognition. Our study provides no insight into whether or not macaques are sensitive to facial kin information or into what aspects of the face may be important to kin discrimination. Current research in human facial kin recognition may provide a model for future research in these areas. For example, Dal Martello and Maloney (2006) have found that the upper half of the human face is far more important for kin recognition than the lower half. The full face was found to be optimal for extraction of kin signals suggesting holistic facial information is important for kin recognition despite differing levels of importance for some facial regions. With these findings in mind, it would be interesting to explore whether the facial kin recognition abilities of nonhuman primates demonstrated by Parr and de Waal (1999) show a similar reliance on certain areas of the face over others. Though there was no significant group by factor interaction, our results may be helpful in focusing future research in this vein. Factor 2 (the eye area), Factor 3 (the forehead and brow area), and Factor 5 (the upper jaw area) appear to show the clearest distinction between the kin group and the two control groups (see Figure 2). The facial areas represented by these factors may be fruitful to consider when exploring what aspects of the macaque face are more or less important to kin discrimination.
In several ways, macaques appear to use face processing strategies that are similar to those used by humans (Dahl, Logothetis, & Hoffman, 2007; Dahl, Wallraven, Bülthoff, & Logothetis, 2009; Gothard, Brooks, & Peterson, 2009; Guo, Robertson, Mahmoodi, Tadmor, & Young, 2003). However, Parr, Heintz, and Pradhan (2008) found that rhesus macaques show several important deficits in facial processing as compared with humans and chimpanzees and concluded that macaques do not possess facial expertise in the same way that humans and chimpanzees do. Nevertheless, the overlap in some facial processing strategies between macaques and humans, the established social importance of the macaque face, and the presence of kin information in the macaque face all constitute strong circumstantial evidence that facial similarity plays a role in nonhuman primate kin recognition.
Interestingly, macaques in the Parr et al. (2010) study showed a strong bias toward male faces, performing the worst on matching mother-daughter trials, the best on father-son trials, and showing an overall advantage for father-offspring and parent-son trials. The authors hypothesized that male rhesus faces may be more distinctive than female faces, explaining the strong performance bias for male faces in the matching task. These results have important implications for the current study, which included only adult female faces. If male rhesus faces are in fact more distinctive than female rhesus faces, similarities (and therefore kin information) in male faces may be easier to detect than similarities in female faces. Nonetheless, as our results show, female macaque faces do contain kin information. Future research should explore whether macaques may attend differently to male and female faces and are, therefore, less sensitive to kin information in female faces, or whether the kin information available in female macaque faces differs in some crucial way from kin information present in male macaque faces.
Due to constraints placed on this study by the lineage of available subjects and in order to maintain a consistent definition of kin, only paternal half-sisters were included in the related group. Ideally, measurements could be taken from more closely related individuals who may show greater similarity in facial features. It is also possible that some variance in facial features relevant to kin status was not captured by the measures used here. Despite these limitations, the results presented here indicate that there may be features available in the facial morphology of adult female rhesus macaques, which could indicate kinship. The presence of kin signals in macaque facial structure and the demonstration by Parr et al. (2010) that rhesus macaques may be sensitive to these signals in some contexts, suggest that recognizing facial similarity may be one strategy that rhesus macaques use to discriminate kin.
Acknowledgments
This research was supported by the Division for Intramural Research, NICHD. We thank Consuel Ionica and Craig Abbott for help in data collection and analysis.
References
- Alvergne A, Huchard E, Caillaud D, Charpentier MJE, Setchell JM, Ruppli C, Raymond M, et al. Human ability to recognize kin visually within primates. International Journal of Primatology. 2009;30:199–210. doi: 10.1007/s10764-009-9339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier MJE, Peignot P, Hossaert-Mckey M, Wickings EJ. Kin discrimination in juvenile mandrills. Mandrillus sphinx Animal Behaviour. 2007;73:37–45. doi: 10.1016/j.anbehav.2006.02.026. [DOI] [Google Scholar]
- Dahl CD, Logothetis NK, Hoffman KL. Individuation and holistic processing of faces in rhesus monkeys. Proceedings of the Royal Society B. 2007;274:2069–2076. doi: 10.1098/rspb.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl CD, Wallraven C, Bülthoff HH, Logothetis NK. Humans and macaques employ similar face-processing strategies. Current Biology. 2009;19:509–513. doi: 10.1016/j.cub.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Dal Martello MF, Maloney LT. Where are kin recognition signals in the human face? Journal of Vision. 2006;6:1356–1366. doi: 10.1167/6.12.2. [DOI] [PubMed] [Google Scholar]
- Erhart EM, Coelho AM, Bramblett CA. Kin recognition by paternal half-siblings in captive Papio cynocephalus. American Journal of Primatology. 1997;43:147–157. doi: 10.1002/(SICI)1098-2345(1997)43:2<147∷AID-AJP4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Fredrickson WT, Sackett GP. Kin preference in primates (Macaca nemestrina): Relatedness or familiarity? Journal of Comparative Psychology. 1984;98:29–34. doi: 10.1037/0735-7036.98.1.29. [DOI] [Google Scholar]
- Gothard KM, Brooks KN, Peterson MA. Multiple perceptual strategies used by macaque monkeys for face recognition. Animal Cognition. 2009;12:155–167. doi: 10.1007/s10071-008-0179-7. [DOI] [PubMed] [Google Scholar]
- Guo K, Robertson RG, Mahmoodi S, Tadmor Y, Young MP. How do monkeys view faces? – A study of eye movements. Experimental Brain Research. 2003;150:363–374. doi: 10.1007/s00221-003-1429-1. [DOI] [PubMed] [Google Scholar]
- Kaminski G, Dridi S, Graff C, Gentaz E. Human ability to detect kinship in strangers’ faces: Effects of the degree of relatedness. Proceedings of the Royal Society B. 2009;276:3193–3200. doi: 10.1098/rspb.2009.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba R, Izumi A, Nakamura K. Sexual dimorphism in facial shapes and their discrimination in Japanese monkeys (Macaca fuscata) Journal of Comparative Psychology. 2009;123:326–333. doi: 10.1037/a0016219. [DOI] [PubMed] [Google Scholar]
- Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proceedings of the National Academy of Science U S A. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, de Waal FBM. Visual kin recognition in chimpanzees. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- Parr LA, Heintz M, Lonsdorf E, Wroblewski E. Visual kin recognition in nonhuman primates: (Pan troglodytes and Macaca mulatta): Inbreeding avoidance or male distinctiveness? Journal of Comparative Psychology. 2010;124:343–350. doi: 10.1037/a0020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Heintz M, Pradhan G. Rhesus monkeys (Macaca mulatta) lack expertise in face processing. Journal of Comparative Psychology. 2008;122:390–402. doi: 10.1037/0735-7036.122.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A, Huntsberry ME, Suomi SJ. Visual discrimination of male and female faces by infant rhesus macaques. Developmental Psychobiology. 2009;52:54–61. doi: 10.1002/dev.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S, Manson JH, Muniz L, Gros-Luis J, Vigilant L. Kin-biased social behaviour in wild adult female white-faced capuchins. Cebus capucinus Animal Behaviour. 2008;76:187–199. doi: 10.1016/j.anbehav.2008.01.020. [DOI] [Google Scholar]
- Rendall D. “Recognizing” kin: Mechanisms, media, minds, modules, and muddles. In: Chapais B, Berman CM, editors. Kinship and behavior in primates. New York, NY: Oxford University Press; 2004. pp. 295–316. [Google Scholar]
- Sackett GP. Monkeys reared in isolation with pictures as visual input: Evidence for an innate releasing mechanism. Science. 1966;154:1468–1473. doi: 10.1126/science.154.3755.1468. [DOI] [PubMed] [Google Scholar]
- Sackett GP, Fredrickson WT. Social preferences by pigtailed macaques: Familiarity versus degree and type of kinship. Animal Behaviour. 1987;35:603–606. doi: 10.1016/S0003-3472(87)80290-7. [DOI] [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology. 2006;61:183–195. doi: 10.1007/s00265-006-0249-2. [DOI] [Google Scholar]
- Smith K, Alberts SC, Altmann J. Wild female baboons bias their social behavior towards paternal half-sisters. Proceedings of the Royal Society B. 2003;270:503–510. doi: 10.1098/rspb.2002.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdig A. Paternal kin discrimination: The evidence and likely mechanisms. Biological Reviews. 2007;82:319–334. doi: 10.1111/j.1469-185X.2007.00011.x. [DOI] [PubMed] [Google Scholar]
- Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch FB. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proceedings of the National Academy of Science U S A. 2001;98:13769–13773. doi: 10.1073/pnas.241210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch FB. Affiliation and aggression among adult female rhesus macaques: A genetic analysis of paternal cohorts. Behaviour. 2002;139:371–391. doi: 10.1163/156853902760102717. [DOI] [Google Scholar]
- Widdig A, Streich WJ, Nürnberg P, Croucher PJP, Bercovitch FB, Krawczak M. Paternal kin bias in the agonistic interventions of adult female rhesus macaques (Macaca mulatta) Behavioral Ecology and Sociobiology. 2006;61:205–214. doi: 10.1007/s00265-006-0251-8. [DOI] [Google Scholar]
- Wu HMH, Holmes WG, Medina SR, Sackett GP. Kin preference in infant Macaca nemestrina. Nature. 1980;285:225–227. doi: 10.1038/285225a0. [DOI] [PubMed] [Google Scholar]


