Abstract
Objective
Prediabetic states are associated with accelerated atherosclerosis, but the availability of mouse models to study connections between these diseases has been limited. The aim of this study was to test the selective role of impaired insulin receptor/insulin receptor substrate-1 signaling on atherogenesis.
Methods and Results
To address the effects of impaired insulin signaling associated with hyperinsulinemia on atherosclerosis in the absence of obesity and hyperglycemia, we generated insulin receptor (Insr)/insulin receptor substrate-1 (Insr1) double heterozygous apolipoprotein (Apoe)-knockout mice (Insr+/−Irs1+/−Apoe−/−) mice. Insr+/−Irs1+/−Apoe−/− mice fed a Western diet for 15 weeks showed elevated levels of fasting insulin compared to Insr+/+Irs1+/+Apoe−/− mice. There were no significant differences in glucose, triglyceride, HDL, VLDL, cholesterol levels or free fatty acid in the plasma of Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice. Atherosclerotic lesions were increased in male (brachiocephalic artery) and female (aortic tree) Insr+/−Irs1+/−Apoe−/− compared to Insr+/+Irs1+/+Apoe−/− mice. Bone marrow transfer experiments demonstrated that nonhematopoietic cells have to be Insr+/−Irs1+/− to accelerate atherosclerosis. Impaired insulin signaling resulted in decreased levels of vascular phospho-eNOS, attenuated endothelium-dependent vasorelaxation and elevated VCAM-1 expression in aortas of Insr+/−Irs1+/−Apoe−/− mice. In addition, phospho-ERK and vascular smooth muscle cell proliferation were significantly elevated in aortas of Insr+/−Irs1+/−Apoe−/− mice.
Conclusion
These results demonstrate that defective insulin signaling is involved in accelerated atherosclerosis in Insr+/−Irs1+/−Apoe−/− mice by promoting vascular dysfunction and inflammation.
Keywords: atherosclerosis, insulin resistance, leukocytes, vascular biology
Cardiovascular disease due to accelerated atherosclerosis is widely recognized as the most common cause of mortality in diabetic patients worldwide.1 Atherosclerosis is a multi-factorial inflammatory disease of large and medium-sized arteries that is characterized by the formation of atherosclerotic lesions which can result in acute arterial thrombosis and ischemic injury of the dependent vascular bed.2 A growing body of evidence suggests a significant correlation between type 2 diabetes, the metabolic syndrome3 and atherosclerosis, but the molecular and pathophysiological links between these diseases are unclear. One of the approaches that has been taken to develop a model of the metabolic syndrome-accelerated atherosclerosis is to use atherogenic or diabetogenic diets in mice and then characterize influences of the different parameters of the metabolic syndrome on atherogenesis.4 However, concomitant elevated plasma lipid levels, hyperglycemia or obesity have complicated the interpretation of the results in these models.5
The role of insulin and insulin receptor (IR) signaling in the development and progression of atherosclerosis has been controversial6; however, recent data highlighted an atheroprotective role of IR signaling in macrophages via the reduction of ER stress-induced apoptosis.7 Additional studies with endothelial cell-specific conditional knockout mice of the IR gene (Insr) in apolipoprotein-E-deficient mice (Apoe−/−) mice revealed that complete loss of insulin signaling in endothelium accelerated atherosclerosis, suggesting an important role of insulin signaling in atherogenesis.8 The major intracellular substrates of the IR tyrosine kinase are IR substrates (Irs)-1 and -2.9,10 Alterations in the expression levels of the IR and IRS-1 have been reported in the onset of type 2 diabetes in animal models11 and humans with type 2 diabetes.12 Importantly, patients with impaired insulin signaling and associated metabolic syndrome begin to develop coronary artery disease before the onset of type 2 diabetes.13 To investigate the mechanisms of accelerated atherosclerosis under conditions of partially defective insulin signaling, we have chosen to use mice that are heterozygous for IR and IRS-1. Insr+/−Irs1+/− mice represent a polygenic model with a combination of 2 minor defects in the insulin signaling cascade that act synergistically to result in type 2 diabetes when used on a diabetogenic background such as C57BL/6.14 The human IRS1 gene is highly polymorphic and shows coding sequence variations in 10% to 20% of patients with type 2 diabetes.12,15 Importantly, partial defects in insulin signaling in Insr+/−Irs1+/− mice are comparable to what is seen in tissues of obese insulin-resistant16 and diabetic patients. 17–19 Heterozygosity in Insr+/−Irs1+/− mice results in the synergistic decrease of insulin signaling in skeletal muscle and liver by 60% compared to control mice, leads to a 60% to 70% reduction of insulin-stimulated tyrosine phosphorylation of IR and IRS-1, and reduced phosphorylation of IRS-2 in liver.14 Insr+/−Irs1+/− mice on a C57BL/6 background showed hyperinsulinemia, islet hyperplasia, and developed early hyperglycemia, with 85% of all mice becoming overtly diabetic after 6 months.14,20
To determine the effect of impaired insulin signaling on atherogenesis, we generated double heterozygous Insr+/−Irs1+/−Apoe−/− mice. Plasma levels of total cholesterol, LDL, and HDL cholesterol and fasted glucose were not different between Insr+/−Irs1+/−Apoe−/− and littermate (Insr+/+Irs1+/+Apoe−/−) controls. Insr+/−Irs1+/−Apoe−/− mice fed a WD developed accelerated atherosclerosis throughout the aortas (females) or within the brachiocephalic artery (males) when compared to Insr+/+Irs1+/+Apoe−/− mice. Bone marrow transfer experiments highlighted an important role of nonhematopoietic cells in accelerated atherosclerosis. Reduced insulin signaling in aortas of Insr+/−Irs1+/−Apoe−/− mice resulted in attenuated levels of p-eNOS, reduced endothelium-dependent vasorelaxation, and elevated levels of p-ERK with concomitant increased proliferation and migration of vascular smooth muscle cells (VSMCs). Taken together, these results demonstrate that selective inhibition of the insulin signaling pathway due to reduced IR/IRS1 expression resulted in accelerated atherosclerosis.
Materials and Methods
Mice
Insr+/−/Irs1+/− double heterozygous mice (on C57BL/6 background) were a kind gift of Dr. C.R. Kahn (Harvard Medical School, Boston, MA). Apoe−/− mice on the C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME) and crossed with Insr+/−Irs1+/− mice to obtain Insr+/−Irs1+/−Apoe−/− and matched control Insr+/+Irs1+/+Apoe−/− mice (Supplemental Figure I, available online at http://atvb.ahajournals.org). All mice were bred and maintained under specific pathogen-free conditions in the animal facilities of the University of Virginia and Eastern Virginia Medical School. Microsatellite marker screening have shown >98% of C57BL/6 background of Insr+/−Irs1+/−Apoe−/− mice (Charles River Laboratory., Wilmington, MA). Mice were fed a Western diet (WD, Harlan-Teklad, #88137, % by kcal: protein, 17.3%, carbohydrate, 48.5%, fat, 21%) for 15 weeks and were used for experiments at ages 23 to 25 weeks. All animal experiments were approved by the Institutional Animal Care and Use Committees.
Results
Metabolic Parameters in Insr+/−Irs1+/−Apoe−/− Mice
In preliminary experiments we compared Insr+/−Irs1+/−Apoe−/− mice with control littermates on a chow diet. Insr+/−Irs1+/−Apoe−/− mice at 61 weeks of age showed lower levels of LDL cholesterol than Insr+/+Irs1+/+Apoe−/− mice (270.5 ±65 and 604±54.7 mg/dL, respectively, n=4, P<0.01). Therefore, the chow diet model was not suitable for our study. To obtain similar lipid levels for the 2 groups, we chose to use WD fed mice. Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice were fed a WD for 15 weeks starting at 8 weeks of age. At 8 weeks of age, (before the start of dietary intervention), Insr+/−Irs1+/−Apoe−/− mice had slightly reduced body weight (Table). There were no significant differences between Insr+/−Irs1+/−Apoe−/− and matched Insr+/+Irs1+/+Apoe−/− male mice in free fatty acids (0.51 ±0.11 versus 0.50±0.08 mmol/L, respectively), total cholesterol, HDL and LDL cholesterol levels, triglycerides and body weight after 15 weeks on WD (Table). Fasting insulin levels were dramatically elevated in male Insr+/−Irs1+/−Apoe−/− compared to Insr+/+Irs1+/+Apoe−/− mice (1.6±0.5 versus 0.3±0.05 ng/mL, respectively, P<0.05, Figure 1A). We also detected a significant increase in insulin levels in fed (heterozygous: 2.8±0.9 versus controls: 0.4±0.09 ng/mL, P<0.05) and fasting (heterozygous: 1.4±0.3 versus controls: 0.2±0.03 ng/mL, P<0.01) female Insr+/−Irs1+/−Apoe−/− mice in comparison to littermate controls (Figure 1A). Thus, the absence of one copy of the Insr and Irs1 genes in Insr+/−Irs1+/−Apoe−/− mice on WD leads to hyperinsulinemia that is not associated with increased serum lipid levels when compared to normoinsulinemic Insr+/+Irs1+/+Apoe−/− mice.
Table. Body Weights, Total Cholesterol, Total Triglycerides, HDL and LDL Levels, and Blood Glucose in Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− Mice.
| Insr+/−Irs1+/−Apoe−/− (Female) | Insr+/−Irs1+/−Apoe−/− (Female) | Insr+/−Irs1+/−Apoe−/− (Male) | Insr+/−Irs1+/−Apoe−/− (Male) | |
|---|---|---|---|---|
| Body weight (23 wks of age), g | 22.9±0.4 | 23.7±0.5 | 30.4±1.0 | 32.9±1.2 |
| Body weight (8 wks of age), g | 16.2±0.6* | 18.0±0.3 | 21.3±1.0 | 23.8±0.7 |
| Total cholesterol, mg/dl | 925±75 | 1166±106 | 1191 ±201 | 1032±199 |
| Total triglycerides, mg/ml | 94±8 | 122±29 | 129±16 | 153±26 |
| HDL | 41±15 | 55±16 | 35±11 | 47±12 |
| VLDL | 887±83 | 1092±97 | 1067±216 | 961 ±198 |
| Fed glucose, mg/dl | 223±27 | 176±17 | 199±21 | 156±10 |
| Fasting glucose, mg/dl | 99.5±12 | 122±13 | 129±14 | 120±21 |
Data are presented as mean±SE for n=7–17 mice. Mice were fed with a Western diet for 15 wk and plasma of fasted 23 wk old female and male mice were analyzed for total cholesterol, triglycerides, HDL, VLDL, and glucose levels
P<0.05.
Insr indicates insulin receptor; Irs1, insulin receptor substrate-1; Apoe, apolipoprotein.
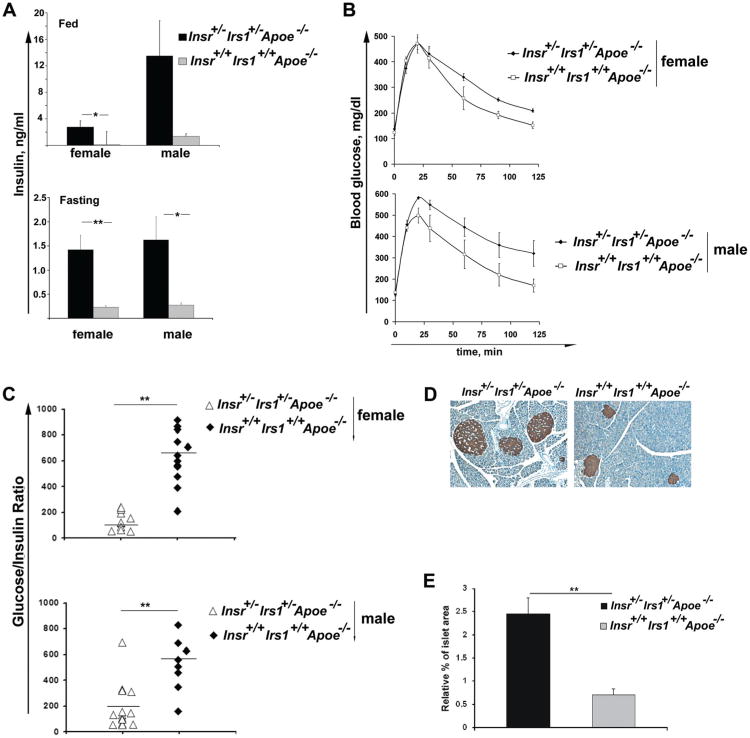
Figure 1.
Hyperinsulinemia in insulin receptor/insulin receptor substrate-1 double heterozygous apolipoprotein E (Insr+/−Irs1+/−Apoe−/−) mice. A, Blood insulin concentrations were determined in randomly fed and fasting mice of the indicated genotype using samples that were obtained from tail blood. Data represent the mean±SE for 7 to 13 mice for each genotype. *P<0.05, **P<0.01. B, Glucose tolerance tests. Mice (5-8 male and female mice for each genotype) were fasted overnight (16 hours) before the test. Glucose was injected IP (2g/kg) and blood samples were taken before and at 10, 20, 30, 60, 90, and 120 minutes after the injection of glucose. Blood glucose values (mean±SE) were plotted vs. time. Two-way ANOVA showed statistically significant differences between the 2 genotypes for female and male mice (P<0.05). C, Glucose/insulin ratio was calculated using fasting insulin and glucose levels. Each symbol represents one animal, horizontal bars represent means. **P<0.01. D, E, Insr+/−Irs1+/−Apoe−/− mice typically had larger pancreatic islets. D, Representative examples of islets stained for insulin (brown) were taken from pancreatic tissue. E, Islet area was calculated as fraction of total area each pancreatic section (n=7). **P<0.01.
Interestingly, fed and fasting glucose levels were similar between the groups (Table). Insr+/−Irs1+/−Apoe−/− mice were less glucose-tolerant than Insr+/+Irs1+/+Apoe−/− mice, as assessed by an intraperitoneal glucose tolerance test (Figure 1B). Insr+/−Irs1+/−Apoe−/− and controls had similar IP insulin tolerance tests at 23 weeks of age (data not shown). We next calculated the fasting glucose to insulin ratio that reflects insulin resistance under physiological conditions. Decreased glucose to insulin ratio in Insr+/−Irs1+/−Apoe−/− mice clearly indicated that both female and male Insr+/−Irs1+/−Apoe−/− mice were more insulin-resistant than the control groups (Figure 1C). Interestingly, unlike Insr+/−Irs1+/−C57BL/6 mice, Insr+/−Irs1+/−Apoe−/− mice did not develop type 2 diabetes with age or after a longer period of WD feeding and maintained normal blood glucose levels (data not shown).
Insr+/−Irs1+/−Apoe−/− Mice Demonstrate Pancreatic Islet Hyperplasia
To test whether hyperinsulinemia was accompanied by an increase in islet size, pancreatic sections from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice were analyzed by immunohistochemisty using an anti-insulin Ab. The islet size was increased 2- to 3-fold in both female and male Insr+/−Irs1+/−Apoe−/− as compared to Insr+/+Irs1+/+Apoe−/− mice (Figure 1D), indicating that Insr+/−Irs1+/−Apoe−/− mice actively compensate for insulin resistance by increasing β cell mass (Figure 1E). This may explain the ability of Insr+/−Irs1+/−Apoe−/− mice to control their blood glucose levels after prolonged high-fat feeding.
Increased Development of Atherosclerosis in Insr+/−Irs1+/−Apoe−/− Mice
To investigate the influence of impaired IR/IRS-1 signaling and associated hyperinsulinemia on atherosclerosis, we performed en face staining of the aortas. Female Insr+/−Irs1+/−Apoe−/− mice showed a doubling of the area occupied by lesions throughout the aortas in comparison to female Insr+/+Irs1+/+Apoe−/− mice (13.9±2.4% versus 7.0±1.0%, respectively) (Figure 2A, 2B). En face staining demonstrated no difference in the size of the atherosclerotic plaques throughout the aortas of male Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice (Figure 2C). The average lesion size of Insr+/−Irs1+/−Apoe−/− male mice and littermate controls was 8.5±2.9% and 8.4±3.0%, respectively. No lesions were observed within the aortas of either Insr+/−Irs1+/− or Insr+/+Irs1+/+ mice that carried wild-type alleles of the Apoe gene (Figure 2A).
Figure 2.
Increased atherosclerotic lesion size in insulin receptor/insulin receptor substrate-1 double heterozygous apolipoprotein E (Insr+/−Irs1+/−Apoe−/−) mice. A, Representative en face Sudan IV staining of aortas from 23- to 25-week-old female Insr+/−Irs1+/−Apoe−/−, Insr+/+Irs1+/+Apoe−/− and Insr+/−Irs1+/− mice. Lesion sizes from the aortas of female (B) and male (C)Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice (% of whole aorta). Each symbol represents 1 animal, horizontal bars represent means. *P<0.05. (D) Representative Movat staining of brachiocephalic artery from 23- to 25-week-old male Insr+/−Irs1+/−Apoe−/− (left) and Insr+/+Irs1+/+Apoe−/− (right) mice. E, Cross-sectional lesion area from the sections of brachiocephalic arteries of Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice (n=5-8 mice for each group). *P<0.05.
Lesions in the brachiocephalic artery were significantly increased in male Insr+/−Irs1+/−Apoe−/− mice compared with controls by 35% (P<0.05), (Figure 2D, 2E). Female Insr+/−Irs1+/−Apoe−/− mice showed no significant difference in cross-sectional lesion area (11.8±2.4 versus 8.4±1.0×104 μm2, P=0.09 respectively). Taken together, these data demonstrate that impaired IR/IRS1 signaling associated with hyperinsulinemia induces accelerated atherosclerosis in male (brachiocephalic area) and female Insr+/−Irs1+/−Apoe−/− mice (throughout the aortas), and these changes occurred without hyperglycemia or differences in circulating lipid levels.
Macrophages in Insr+/−Irs1+/−Apoe−/− Mice
To further analyze the roles of different cell types in increased atherosclerosis observed in Insr+/−Irs1+/−Apoe−/− mice, we first examined the phenotype of bone-marrow-derived macrophages in this model. There is some evidence that defective insulin signaling in macrophages could lead to a proinflammatory, lipid-laden macrophage phenotype that promotes atherogenesis.21 We therefore performed cholesterol efflux assays to measure the ability of Insr+/−Irs1+/−Apoe−/− macrophages to efflux cholesterol to HDL and to apolipoprotein A-I. The efflux of cholesterol to both HDL (Figure 3A) and apolipoprotein A-I (data not shown) was similar between Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− macrophages. There was also no significant difference in the survival of Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− macrophages in response to acLDL-treatment (Figure 3B). Taken together, these results suggest that the presence of 1 allele of Insr and Irs1 is sufficient to protect macrophages from the proatherogenic phenotype observed in Irs1-deficient macrophages.7
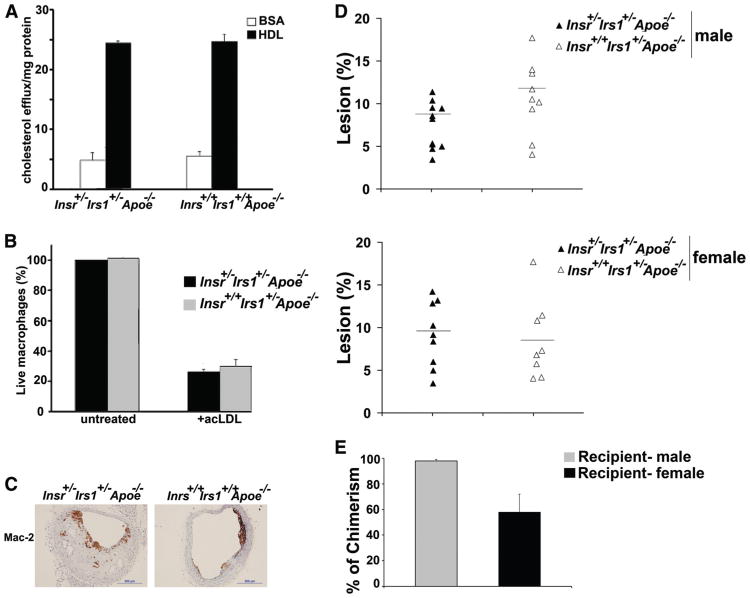
Figure 3.
Role of impaired insulin receptor/insulin receptor substrate-1 (IR/IRS-1) signaling in macrophage functions. A, Cellular cholesterol efflux measurements. [3H]cholesterol-labeled pooled thioglycolate-elicited peritoneal macrophages from females and males either Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− were used in a cholesterol efflux assay. Data represent the mean±SE of 3 to 5 mice from each group. B, Susceptibility of Insr+/−Irs1+/−Apoe−/− macrophages to acLDL-induced cell death. Pooled thioglycolate-elicited peritoneal macrophages from either Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− mice were incubated with or without acLDL (100 /ng/mL) at 37°C for 24 hours. Viability of macrophages was detected using Live/Dead Aqua staining. The percentage of live cells from untreated groups was set at 100% and the percentage of Live/Dead Aqua+ acLDL-treated macrophages was calculated (n=4–5 mice from each group). C, Paraffin-embedded sections from Insr+/−Irs1+/−Apoe−/− mice and littermate controls were stained with anti-Mac-2 Ab. Representative staining is shown. D, At 6 to 7 weeks of age Apoe−/− mice were irradiated and injected with bone marrow (BM) cells from either Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− mice into the tail vein and fed WD for 15 weeks starting 5 weeks after reconstitution. Percentage of lesion in aortas obtained from en face analysis of female (n=8) and male and male (n=8) Apoe−/− mice receiving BM of Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− mice. Each data point represents the value from a single mouse, horizontal bars represent means. E, The percent of chimerism of mice that received Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− BM was determined by performing quantitative PCR. Genomic DNA isolated from the peripheral blood mononuclear cells of recipient mice of the opposing sex was assayed for the presence of the Y chromosome and the Ct values were normalized to the total mass of DNA, determined by GAPDH. The data are expressed as mean±SE.
Next, to examine effects of impaired insulin signaling on macrophage content in atherosclerosis-prone vessels, serial sections of brachiocephalic arteries from Insr+/−Irs1+/−Apoe−/− and control Insr+/+Irs1+/+Apoe−/− male mice were stained for Mac-2 (Figure 3C), and the chemokines CXCL1 and CCL2. To account for the difference in the lesion size of the analyzed plaques, we calculated the ratio of the positively stained area of the lesion to the total plaque area and expressed the results as a percentage. We found no difference in the Mac-2+ area in the brachiocephalic artery (6.7±2.1% versus 10.4±2.2% for Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe+/− mice, respectively). There were also no significant differences in the expression of CXCL1 and CCL2 between Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs+/+Apoe−/− mice (data not shown).
Bone Marrow Chimeras
To further investigate whether the expression level of IR and IRS-1 on macrophages and other bone-marrow-derived cells was important in the development of accelerated atherosclerosis in Insr+/−Irs1+/−Apoe−/− mice, bone marrow (BM) cells isolated from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice, were injected IV into lethally irradiated Apoe−/− mice. Mice were placed on WD 5 weeks after BM transfer. After 15 weeks on WD, we found no difference in body weight, plasma insulin, and blood glucose levels between mice transplanted with Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− BM cells (Supplemental Table). Although Apoe−/− mice that received Insr+/−Irs1+/−Apoe−/− BM cells showed no elevated levels of insulin or glucose, we nevertheless analyzed atherosclerosis in those recipients to dissect a potential role of partially insulin-signaling deficient BM cells in atherosclerosis. There was no difference in lesion size throughout the aortas between the animals receiving Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− BM cells (Figure 3D, 3E). We also analyzed the plaque burden within the brachiocephalic arteries of BM-transplanted Apoe−/− mice. We found no statistically significant difference between recipient mice that received Insr+/−Irs1+/−Apoe−/− or Insr+/+Irs1+/+Apoe−/− BM cells (data not shown). Together, these results suggest that nonhematopoietic cells are at least partially responsible for accelerated atherosclerosis in Insr+/−Irs1+/−Apoe−/− mice. Hematopoetic chimerism in peripheral blood cells was on average 77%, indicating substantial repopulation of BM-derived cells by donor tissue (Figure 3E). Although recipient female mice showed a slightly lower percentage of chimerism, the analysis of chimerism in individual mice showed no correlation between the levels of chimerism and percentage of plaque area (data not shown).
Reduced IR/IRS-1 Expression Results in Altered ERK/eNOS Signaling and Impaired Endothelium-Dependent Vasorelaxation in the Aortas of Insr+/−Irs1+/−Apoe−/− Mice
The initial study that characterized insulin signaling in Insr+/−Irs1+/− mice demonstrated that heterozygosity for Insr and Irs1 genes caused reduced IR and IRS-1 protein expression in proportion to gene dosage in Insr+/−Irs1+/− mice.14 To confirm a proportional decrease of heterozygous genes in Insr+/−Irs1+/−Apoe−/− mice, we examined the expression of IR in aortas isolated from Insr+/−Irs1+/−Apoe−/− mice and Insr+/+Irs1+/+Apoe−/− mice by Western blot analysis. As expected, we found that the heterozygosity for Insr gene resulted in a ≈50% reduction in IR and p-IR protein expression in aortas of Insr+/−Irs1+/−Apoe−/− mice (Supplemental Figure II).
To determine how reduced IR/IRS-1 levels affect insulin signaling and vascular functions, we first focused on the expression of antiatherogenic endothelial nitric oxide (NO) synthase (eNOS)22,23 that plays an essential role in NO production and vasodilation. The p-eNOS/eNOS ratio was reduced in aortic homogenates obtained from Insr+/−Irs1+/−Apoe−/− mice under basal, unstimulated conditions compared with the p-eNOS/eNOS ratio from Insr+/+Irs1+/+Apoe−/− mice (Figure 4A). Importantly, insulin stimulation increases eNOS phosphorylation in Insr+/+Irs1+/+Apoe−/−, but not significantly in Insr+/−Irs1+/−Apoe−/− mice.
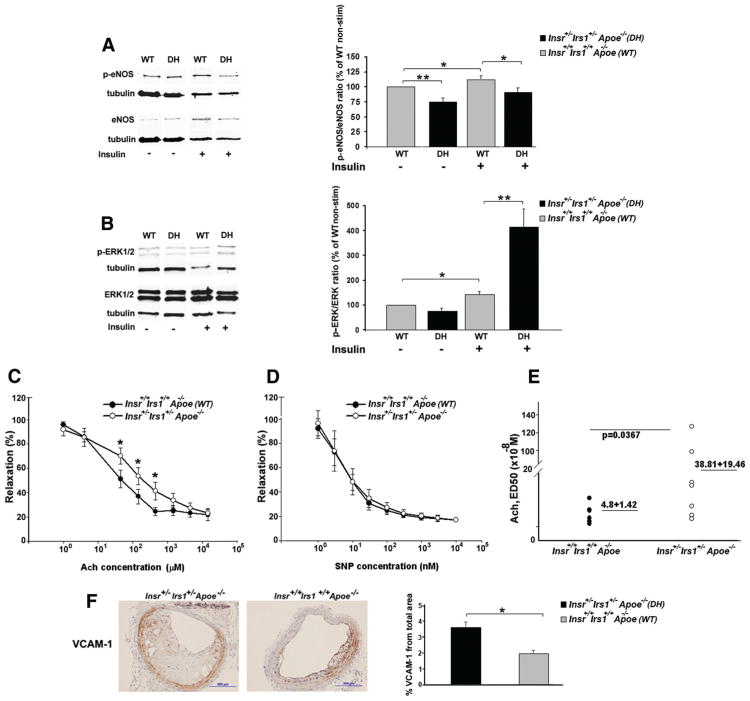
Figure 4.
Endothelial dysfunction in insulin receptor/insulin receptor substrate-1 double heterozygous apolipoprotein E (Insr+/−Irs1+/−Apoe−/−) mice. A, Impaired eNOS phosphorylation in aortic homogenates generated from Insr+/−Irs1+/−Apoe−/− mice. Representative Western blot of basal and insulin-stimulated p-eNOS and eNOS expression from individual aortas of Insr+/−Irs1+/−Apoe−/− (DH) and Insr+/+Irs1+/+Apoe−/− (WT) mice (n=8–10). Bars represent the ratio of p-eNOS/e-NOS from Insr+/−Irs1+/−Apoe−/− (insulin-stimulated and unstimulated) and Insr+/+Irs1+/+Apoe−/− (insulin-stimulated) mice relatively to the ratio of unstimulated Insr+/+Irs1+/+Apoe−/− aortas (as 100%). B, Western blots from lysate of whole aortas show expression of p-ERK and ERK from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice. Bar chart shows the ratios of p-ERK/ERK in Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− (unstimulated as 100%) mice (n=6–8 mice for each genotype). C, D, Vasodilator responses in aortic rings from Insr+/−Irs1+/−Apoe−/− and Insr+/−Irs1+/−Apoe−/− mice (n=6-7). (C) Ach and (D) sodium nitroprusside (SNP)-induced vasorelaxation in Insr+/−Irs1+/−Apoe−/− aortic rings treated with 3 /nmol/L phenylephrine. Results are represented as mean±SEM (E) Mean ED50 for each individual mouse is shown. Horizontal bars show mean. F, Representative staining of brachiocephalic arteries from Insr+/−Irs1+/−Apoe−/− and littermate controls (n=3–7) mice for VCAM-1 (brown) is shown. Bars show percentage of VCAM-1+ staining relative to the total area of the atherosclerotic vessels. *P<0.05, **P<0.01.
We next focused on the MAP/ERK1/2 pathway that plays an important role in the inhibition of eNOS phosphorylation.6 Although basal ratios of p-ERK/ERK were reduced in the aortas of Insr+/−Irs1+/−Apoe−/− mice, insulin stimulation resulted in significantly increased pERK/ERK ratio of insulin-stimulated pERKs in Insr+/−Irs1+/−Apoe−/− aortas compared with Insr+/+Irs1+/+Apoe−/− aortas (Figure 4B).
To investigate the impact of impaired insulin signaling on aortic relaxation in Insr+/−Irs1+/−Apoe−/− mice, vasodilation in response to the endothelium-dependent mediator acetylcholine (Ach) and the endothelium-independent dilator sodium nitroprusside was measured in aortic rings precon-stricted with phenylephrine. Aortic rings from the thoracic aortas of Insr+/−Irs1+/−Apoe−/− of female and male mice showed attenuated vasodilation in response to Ach compared to Insr+/+Irs1+/+Apoe−/− mice (Figure 4C). Importantly, the average Ach ED50 in heterozygous aortas was significantly higher compared to controls, indicating reduced sensitivity to acetylcholine-induced vasorelaxation. (Figure 4E) In contrast, there was no significant alteration in VSMC vasorelaxation in the response to sodium nitroprusside (Figure 4D).
To further assess endothelial status in aortas, we examined VCAM-1 expression within the brachiocephalic arteries from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice. The immunostaining revealed strong expression of VCAM-1 within the VSMCs, media, intima, and occasionally within the core of the lesions in the Insr+/−Irs1+/−Apoe−/− mice (Figure 4F). When VCAM-1 expression was visualized in the same areas of the brachiocephalic arteries in Insr+/+Irs1+/+Apoe−/− mice (Figure 4F), the percentage of the VCAM-1-positive area was significantly higher in the brachiocephalic arteries of Insr+/−Irs1+/−Apoe−/− compared to Insr+/+Irs1+/+Apoe−/− control mice (Figure 4F).
Reduced Expression of IR and IRS-1 Resulted in the Activation of Aortic Vascular Cells
Having identified an important role of IR/IRS-1-dependent insulin signaling in the regulation of the AKT/eNOS and ERK pathways, we next sought to determine whether increased phosphorylation of ERK in Insr+/−Irs1+/−Apoe−/− cells is also accompanied by vascular activation, in particularly proliferation and migration of VSMCs. VSMCs were isolated from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− aortas and labeled with CFSE for the detection of proliferating cells. We detected increased proliferation of CFSE-labeled Insr+/−Irs1+/−Apoe−/− VSMCs compared to Insr+/+Irs1+/+Apoe−/− VSMCs in response to cell growth media (Figure 5A and B). Interestingly, we detected no difference in platelet-derived growth factor (PDGF)-induced migration but noticed a decrease in fetal bovine serum (FBS)-dependent migration of VSMCs isolated from Insr+/−Irs1+/−Apoe−/− aortas compared to Insr+/+Irs1+/+Apoe−/− isolated VSMCs (Figure 5C), suggesting that FBS-derived factors other than PDGF affect migration of Insr+/−Irs1+/−Apoe−/− VSMCs. To examine the arterial content of VSMCs in vivo, serial sections of brachiocephalic arteries from male and female Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice were stained with antibodies against smooth muscle cell α-actin (Figure 5D). The VSMC-positive area was significantly increased in the atherosclerosis-prone areas of Insr+/−Irs1+/−Apoe−/− brachiocephalic arteries (Figure 5D, E).
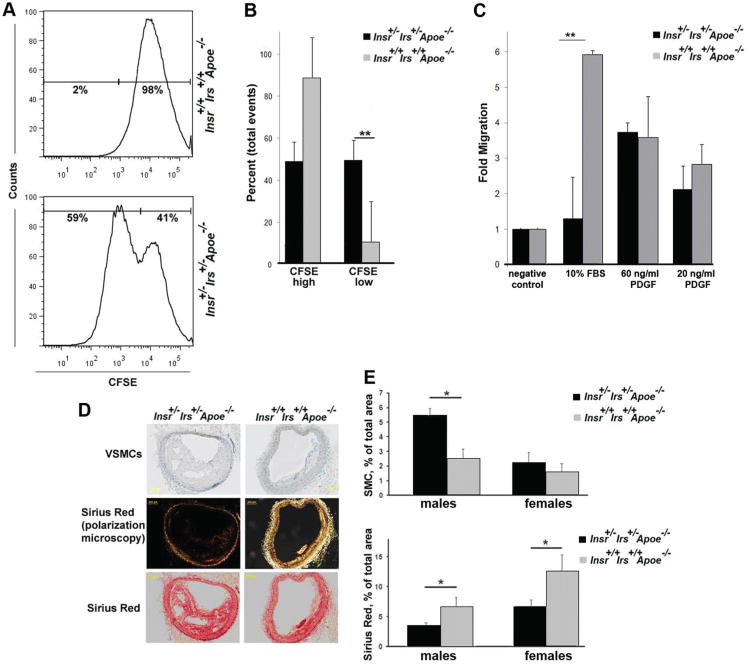
Figure 5.
Elevated vascular smooth muscle cell (VSMC) activation in insulin receptor/insulin receptor substrate-1 double heterozygous apolipoprotein E (Insr+/−Irs1+/−Apoe−/−) mice. A, B, VSMC proliferation. VSMCs were labeled with carboxyfluorescein diacetate-succinimidyl ester (CFSE) and recultured for 4 days. On the fourth day, the CFSE-labeled VSMCs were analyzed to flow cytometry. A, Representative histogram. B, Average percentage of CFSEhigh and CFSElow-divided VSMCs as determined by FACS (n=4). C, VSMC migration. Serum starved primary Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− VSMCs were assayed for differences in migratory capacity via Boyden chamber assays (n=5). D, Paraffin-embedded sections (n=3–7) were stained with anti-α-actin Ab to detect VSMCs or Sirius Red. Representative staining from one mouse is shown. E, Percentage of VSMC+ or Sirius Red+ staining relative to the total area of the atherosclerotic vessels.
To further define the phenotype of atherosclerotic plaques, we performed Sirius Red staining to examine maturation of collagen. Age-matched 21-week-old Insr+/−Irs1+/−Apoe−/− mice had increased plaque burden compared to littermate controls, but collagen maturation in brachiocephalic arteries from Insr+/−Irs1+/−Apoe−/− mice was decreased compared to Insr+/+Irs1+/+Apoe−/− mice (Figure 5D and 5E). These results suggest that impaired insulin signaling activates vascular aortic wall and decreases the presence of mature collagen fibers in atherosclerotic plaques.
Discussion
Epidemiological evidence shows a strong association between states of insulin resistance and coronary artery disease, peripheral artery disease and cerebrovascular dysfunction.24 Our study clearly demonstrates that partial deficiency of IR and IRS-1 already results in the development of accelerated atherosclerosis and highlights an important role of insulin signaling in vascular cells even in the setting of globally attenuated IR/IRS-1 expression.
To investigate the impact of impaired insulin signaling on atherosclerosis development, we generated Insr+/−Irs1+/−Apoe−/− mice. Insr+/−Irs1+/−Apoe−/− mice developed clear hyperinsulinemia but had similar levels of total cholesterol, triglycerides, LDL, HDL, and glucose compared to Insr+/+Irs1+/+Apoe−/− mice. It is interesting that despite higher insulin levels, Insr+/−Irs1+/−Apoe−/− mice were able to keep triglycerides and cholesterol at the same levels as control mice. Perhaps more subtle qualitative changes in the composition of VLDL, LDL, or HDL, such as changes in the free fatty acid composition or proteomic composition of these particles might exist in Insr+/−Irs1+/−Apoe−/− mice. The majority of Insr+/−Irs1+/− mice on the C57BL/6 background developed diabetes at 6 months of age.14,25 Intriguingly, Insr+/−Irs1+/−Apoe−/− mice remained nondiabetic, suggesting an ameliorating effect on glucose homeostasis by the combined lack of Apoe and partial IR/IRS-1 deficiency. This is supported by recent data that ApoE deficiency abrogates the insulin resistance and improves blood glucose in a mouse model of type 2 diabetes (MKR/Apoe−/− mice) via reduced triacylglycerol content in liver and reduced fat accumulation in liver and adipose tissues.26 Interestingly, Insr+/−Irs1+/−Apoe−/− mice developed marked β cell hyperplasia. The mechanisms of relative protection of β cells and functions of the liver and adipose tissue in Insr+/−Irs1+/−Apoe−/− mice remain unclear.
Insr+/−Irs1+/−Apoe−/− mice showed more prevalent atherosclerosis in the brachiocephalic artery, whereas female Insr+/−Irs1+/−Apoe−/− mice developed larger atherosclerosis throughout the aortas after 15 weeks WD feeding. Lesion distribution throughout the aorta in both Ldlr−/− and Apoe−/− mice is known to show gender-dependent differences.27 The influence of gender on atherosclerosis is complex and involves hormone-specific and immune-specific responses in males and females.27 More detailed analysis of the immune cell composition within the aortas may aid in understanding this phenomenon. Cardiovascular disease remains a major unresolved issue for women,28 and female patients with diabetes and atherosclerosis have significantly worse outcomes, possibly reflecting more extensive diffuse atherosclerotic burden, consistent with what we observed in this study.29
In a previous study, Ldlr−/− mice synthesizing only apoB100 were crossed with transgenic mice overexpressing insulin-like growth factor-II.30 Insulin-like growth factor-II/Ldlr−/−ApoB100/100 mice demonstrated insulin resistance, hyperglycemia, mild hyperinsulinemia, and concomitant increase in lesion calcification within advanced lesions. This is the first model that mimics accelerated atherosclerosis in a type 2 diabetes-like background without lipid changes. The Insr+/−Irs1+/−Apoe−/− mouse model described here shows that insulin resistance promotes atherosclerosis even in the “prediabetic” state.
There is a growing body of evidence demonstrating altered functions of macrophages at the onset of type 2 diabetes.21 Insr−/− macrophages have impaired cholesterol efflux, are prone to cholesterol-induced apoptosis, and induce more complex advanced necrotic lesions.7 Macrophages from Insr+/−Irs1+/−Apoe−/− mice show no alterations in cholesterol efflux to HDL or survival in the presence of oxLDL, indicating that macrophages with partial deficiency in Insr/Irs1 are less affected than Insr−/− macrophages. Further studies will be needed to investigate possible variations in insulin signaling cascade that might lead to different macrophage phenotypes in those models.
One of the early events in atherogenesis appears to be an induction of vascular dysfunction and the activation of aortic vasculature that lead to platelet aggregation and leukocyte adhesion.2 IR deficiency in vascular endothelial cells increases atherosclerosis development in Apoe−/− mice via the impairment of endothelium-dependent vasodilation, induction of VCAM-1, and elevated leukocyte adhesion to endothelium.8 Our BM transplantation experiments support this motion and indicate that nonhematopoietic cells play an essential role in the induction of accelerated atherosclerosis in Insr+/−Irs1+/−Apoe−/− mice.
Interactions of insulin with IRs activates phosphatidylinositol 3-kinase (PI[3]K)/AKT signaling cascade and subsequently results in the induction of the active eNOS that plays an atheroprotective role.31 Insulin also activates the mitogenic MAP kinase-dependent pathway that participates in cell growth and migration. Our study conclusively demonstrates the essential role of insulin signaling in the maintenance of the fine balance between the PI(3)K/eNOS and MAP/ERK1/2 pathways. To test whether partially deficient insulin signaling results in endothelial dysfunction, we investigated endothelium-dependent vasorelaxation of Insr+/−Irs1+/−Apoe−/− aortic rings and found that attenuated expression of IR and IRS-1 significantly decreased endothelial-dependent vasorelaxation reflecting diminished eNOS activity and NO production. NO has several antiatherogenic activities,32 including attenuation of leukocyte-endothelial cell interactions via the regulation of adhesion molecule expression. Indeed, as a confirmation of attenuated p-eNOS/eNOs activities, the expression of 1 of the key leukocyte adhesion molecules, VCAM-1, was also increased in brachiocephalic arteries of Insr+/−Irs1+/−Apoe−/− mice. Consistent with this data, Madonna et al recently demonstrated that prolonged exposure of human umbilical vein endothelial cells to high insulin concentrations, similar as seen in diabetic patients, downregulates the PI(3)K/eNOS pathway and upregulates VCAM-1 expression in vitro.33
Partial inactivation of Irs2 in Apoe−/− mice results in accelerated atherosclerosis via increased modified LDL uptake in macrophages, diminished aortic AKT2, and Ras expression and enhanced CCL2 expression.34 Our results show that partial deficiency in IR/IRS-1 leads to aortic vascular dysfunction and accelerated atherosclerosis with a dominant role of nonhematopoietic cells in these processes. Thus, even partial deficiency in insulin signaling is sufficient to promote accelerated atherosclerosis under dyslipidemic conditions.
Insulin signaling in VSMCs initiates important cellular events such as proliferation and migration. Insulin-stimulated VSMC mitogenesis is mainly controlled through the MAPK pathway; however, the PI(3)K/AKT pathway also contributes to maintaining VSMC quiescence.35 Partial deficiency in IR/IRS-1 signaling induced elevated levels of pERK1/2, concomitant proliferation of VSMCs isolated from the aortas of Insr+/−Irs1+/−Apoe−/− mice and a significant increase in the numbers of VSMCs within the brachiocephalic arteries of Insr+/−Irs1+/−Apoe−/− mice. Recently, it has been reported that deficiency in AKT-1 reduces PDGF- and FBS-induced VSMC migration and survival.36 Interestingly, we detected no difference in migration between Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− VSMCS toward PDGF but found reduced migration of Insr+/−Irs1+/−Apoe−/− toward FBS suggesting attenuated migration of VMSCs to other FBS-derived chemotactic factors rather than PDGF.
In conclusion, these results suggest that impaired IR/IRS-1 signaling provokes accelerated atherogenesis via increased vascular dysfunction. Thus, impaired insulin signaling observed in patients with metabolic syndrome and type 2 diabetes might be an important component in the mechanism that increases the relative risk of cardiovascular diseases in these patients.
Supplementary Material
Supplemental Table I Average body weights, total cholesterol and glucose in Apoe−/− mice transplanted with Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− bone marrow after 15 weeks on western diet.
Supplemental Fig. I. Breeding scheme used to (A) generate and (B) maintain Insr+/−Irs1+/−Apoe−/− (denoted as +/−,+/−,−/−) mice and Insr+/+Irs1+/+Apoe−/− (denoted as +/+,+/+,−/−) littermate control mice. (C) DNA extracted from tail biopsies of Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice were subjected to PCR analysis.
Supplemental Fig. II. Basal and insulin-stimulated IR expression in aortic homogenates from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice. Mean levels of IR expression for each individual aortic sample (n=5-6 aortas) was normalized to the tubulin. IR expression in aortas from Insr+/+Irs1+/+Apoe−/− was set as 100% and the percentage of IR expression in Insr+/−Irs1+/−Apoe−/− mice was calculated. *P<0.05, **P<0.01
Acknowledgments
We would like to thank Dr Zarbock for advice with bone marrow transfer experiments.
Sources of Funding: This work was supported by NIH HL58108 (K.L.), PO1 HL55798 (K.L., E.G., J.N., C.C.H. and S.S.), the University of Virginia Diabetes and Endocrine Research Center Animal Characterization Core Facility NIH DK063609 (S.R.K.) and NIH DK55240 (J.N.).
Footnotes
Disclosures: None.
Contributor Information
Elena V. Galkina, Departments of Microbiology and Molecular Cell Biology, Eastern Virginia Medical School, Norfolk VA
Matthew Butcher, Departments of Microbiology and Molecular Cell Biology, Eastern Virginia Medical School, Norfolk VA.
Susanna R. Keller, Departments of Diabetes and Hormone Center of Excellence, University of Virginia, Charlottesville, VA
Matthew Goff, Departments of Microbiology and Molecular Cell Biology, Eastern Virginia Medical School, Norfolk VA.
Anthony Bruce, Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, VA.
Hong Pei, Departments of La Jolla Institute for Allergy and Immunology, La Jolla, CA.
Ian J. Sarembock, Departments of Ohio Heart and Vascular Center, Cincinnati, OH
John M. Sanders, Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, VA
Melissa H. Nagelin, Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, VA
Suseela Srinivasan, Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, VA.
Rohit N. Kulkarni, Departments of Joslin Diabetes Center, Harvard Medical School Boston, Boston, MA
Catherine C. Hedrick, Departments of La Jolla Institute for Allergy and Immunology, La Jolla, CA
Frank A. Lattanzio, Departments of Physiological Sciences, Eastern Virginia Medical School, Norfolk VA
Anca D. Dobrian, Departments of Physiological Sciences, Eastern Virginia Medical School, Norfolk VA
Jerry L. Nadler, Departments of Medicine and Strelitz Diabetes Center, Eastern Virginia Medical School, Norfolk VA
Klaus Ley, Departments of La Jolla Institute for Allergy and Immunology, La Jolla, CA.
References
- 1.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105:2231–2239. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic Syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadan-Diehl C, Goldberg IJ. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100:1415–1427. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 5.Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–249. doi: 10.1016/j.atherosclerosis.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 7.Han S, Liang CP, Vries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 10.Sun XJ, Crimmins DL, Myers MG, Jr, Miralpeix M, White MF. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993;13:7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White MF, Kahn CR. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992;90:1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest. 1995;95:2195–2204. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am. 2008;37:603–621. doi: 10.1016/j.ecl.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 15.Laakso M, Malkki M, Kekalainen P, Kuusisto J, Deeb SS. Insulin receptor substrate-1 variants in non-insulin-dependent diabetes. J Clin Invest. 1994;94:1141–1146. doi: 10.1172/JCI117429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar RS, Gorden P, Roth J, Kahn CR, De MP. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976;58:1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolterman OG, Gray RS, Griffin J, Burstein P, Insel J, Scarlett JA, Olefsky JM. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981;68:957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince MJ, Tsai P, Olefsky JM. Insulin binding, internalization, and insulin receptor regulation in fibroblasts from type II, non-insulin-dependent diabetic subjects. Diabetes. 1981;30:596–600. doi: 10.2337/diab.30.7.596. [DOI] [PubMed] [Google Scholar]
- 19.Olefsky JM, Kolterman OG, Scarlett JA. Insulin action and resistance in obesity and noninsulin-dependent type II diabetes mellitus. Am J Physiol. 1982;243:E15–E30. doi: 10.1152/ajpendo.1982.243.1.E15. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni RN, Almind K, Goren HJ, Winnay JN, Ueki K, Okada T, Kahn CR. Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes. 2003;52:1528–1534. doi: 10.2337/diabetes.52.6.1528. [DOI] [PubMed] [Google Scholar]
- 21.Liang CP, Han S, Senokuchi T, Tall AR. The macrophage at the crossroads of insulin resistance and atherosclerosis. Circ Res. 2007;100:1546–1555. doi: 10.1161/CIRCRESAHA.107.152165. [DOI] [PubMed] [Google Scholar]
- 22.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 23.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg IJ, Dansky HM. Diabetic vascular disease: an experimental objective. Arterioscler Thromb Vasc Biol. 2006;26:1693–1701. doi: 10.1161/01.ATV.0000231521.76545.f6. [DOI] [PubMed] [Google Scholar]
- 25.Xue B, Kim YB, Lee A, Toschi E, Bonner-Weir S, Kahn CR, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B deficiency reduces insulin resistance and the diabetic phenotype in mice with polygenic insulin resistance. J Biol Chem. 2007;282:23829–23840. doi: 10.1074/jbc.M609680200. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima Y, Chen J, Sun H, Lann D, Hajjar RJ, Yakar S, Leroith D. Apolipoprotein E deficiency abrogates insulin resistance in a mouse model of type 2 diabetes mellitus. Diabetologia. 2009;52:1434–1441. doi: 10.1007/s00125-009-1378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia. 2005;48:856–861. doi: 10.1007/s00125-005-1730-6. [DOI] [PubMed] [Google Scholar]
- 29.Natali A, Vichi S, Landi P, Severi S, L'Abbate A, Ferrannini E. Coronary atherosclerosis in Type II diabetes: angiographic findings and clinical outcome. Diabetologia. 2000;43:632–641. doi: 10.1007/s001250051352. [DOI] [PubMed] [Google Scholar]
- 30.Heinonen SE, Leppanen P, Kholova I, Lumivuori H, Hakkinen SK, Bosch F, Laakso M, Yla-Herttuala S. Increased atherosclerotic lesion calcification in a novel mouse model combining insulin resistance, hyperglycemia, and hypercholesterolemia. Circ Res. 2007;101:1058–1067. doi: 10.1161/CIRCRESAHA.107.154401. [DOI] [PubMed] [Google Scholar]
- 31.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsui M, Shimokawa H, Otsuji Y, Yanagihara N. Pathophysiological relevance of NO signaling in the cardiovascular system: novel insight from mice lacking all NO synthases. Pharmacol Ther. 2010;128:499–508. doi: 10.1016/j.pharmthera.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Madonna R, De CR. Prolonged exposure to high insulin impairs the endothelial PI3-kinase/Akt/nitric oxide signalling. Thromb Haemost. 2009;101:345–350. [PubMed] [Google Scholar]
- 34.Gonzalez-Navarro H, Vinue A, Vila-Caballer M, Fortuno A, Beloqui O, Zalba G, Burks D, Diez J, Andres V. Molecular mechanisms of atherosclerosis in metabolic syndrome: role of reduced IRS2-dependent signaling. Arterioscler Thromb Vasc Biol. 2008;28:2187–2194. doi: 10.1161/ATVBAHA.108.175299. [DOI] [PubMed] [Google Scholar]
- 35.Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52:2562–2569. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Hernando C, Jozsef L, Jenkins D, Di LA, Sessa WC. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:2033–2040. doi: 10.1161/ATVBAHA.109.196394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I Average body weights, total cholesterol and glucose in Apoe−/− mice transplanted with Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− bone marrow after 15 weeks on western diet.
Supplemental Fig. I. Breeding scheme used to (A) generate and (B) maintain Insr+/−Irs1+/−Apoe−/− (denoted as +/−,+/−,−/−) mice and Insr+/+Irs1+/+Apoe−/− (denoted as +/+,+/+,−/−) littermate control mice. (C) DNA extracted from tail biopsies of Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice were subjected to PCR analysis.
Supplemental Fig. II. Basal and insulin-stimulated IR expression in aortic homogenates from Insr+/−Irs1+/−Apoe−/− and Insr+/+Irs1+/+Apoe−/− mice. Mean levels of IR expression for each individual aortic sample (n=5-6 aortas) was normalized to the tubulin. IR expression in aortas from Insr+/+Irs1+/+Apoe−/− was set as 100% and the percentage of IR expression in Insr+/−Irs1+/−Apoe−/− mice was calculated. *P<0.05, **P<0.01