Abstract
The oxidative dearomatization of resorcinol derivatives, which are outfitted with a lactic acid derived chiral tether, and mitigated by hypervalent iodine derivatives of PhIO, affords stable chiral cyclohexadienones as useful building blocks for the construction of many highly functionalized chiral six and seven-membered ring systems. Herein, we report a multitude of remarkable and unexpected diastereoselective transformations stemming from these cyclohexadienone adducts.
Keywords: Dearomatization, Cyclohexadienone, Hypervalent iodine, Lactone, Vinylogous ester
1. Introduction
Despite many advances in asymmetric organic reaction processes, only a handful of chemical methods have emerged to provide enantiomerically enriched cyclohexadienones.1 Few are catalytic. None are general. This is surprising because chiral cyclohexadienones offer attractive entry points to a number of complex non-racemic natural products. One needs to look no further than the early racemic syntheses of ryanodol, calicheamicinone, and ovalicin to be seduced by the utility of chiral cyclohexadienones. However, after reflecting and studying the processes that deliver these building blocks, we have come to appreciate the thorny issues of product stability and utility, which have prevented real progress. Any new dearomatization process should be judged just as much, if not more so, in these realms as in their overall diastereomeric or enantiomeric selectivity.
Diastereoselective dearomatization processes are inherently useful. In total syntheses of epoxysorbicillinol,2 bisorbicillinol,2 rishirilide,3 and the cleroindicins,4 as well as for other putative natural products,5 we have confronted the issues of cyclohexadienone stability and subsequent utility head-on. Herein, we recount this diastereoselective dearomatization process, and report for the first time some of the remarkable reactivity we have observed amongst the resulting cyclohexadienone products.
2. The plan
Early on we speculated that a catalytic enantioselective method offered limited synthetic potential because its efficient use would be restricted by cyclohexadienone reactivity to circumstances where the target molecule contained a single stereocenter.1 Therefore, our formulative ideas centered upon developing more utilitarian diastereoselective dearomatization processes.6 We imagined some hypervalent I(III) reagent prompted oxidative dearomatization involving an unprecedented ortho-cyclization of a lactic acid derived tether should produce a family of sturdy cyclohexadienones capable of further regioselective and diastereoselective reactions.7 Low yields (<70%) have long plagued the synthesis of cyclohexadienones. This stems from the reactivity of the reaction intermediates and the respective products. A workaround solution, which we were resolved to not use, has been the deployment of the oxidative dearomatization at a very early stage, when starting material is overly abundant, or very near the end, when the target compound can be easily reached. However, these solutions fail to capitalize on the malleability intrinsic to the cyclohexadienones product themselves. Fortunately, widespread use of hypervalent iodine oxidants,8 particularly I(III) derivatives of iodosyl benzene (Ph–I═O),9 have supplanted Pb(IV), Tl(III), and DDQ as oxidants. These hypervalent iodine reagents have led to improved yields, because these non-toxic I(III) reagents rarely participate in undesirable side-reactions with the intermediate phenonoxonium cation.10
We anticipated that the δ-lactone formed by our proposed oxidation would further stabilize the cyclohexadienone so that the enone, vinylogous ester, and ketone functionality could be manipulated in both chemoselective and stereoselective manners to address a number of natural products, in spite of the inescapable fact that the cyclohexadienone product would display five trigonal sp2 carbon atoms. We therefore set out to build several of these scaffolds so that we might determine their subsequent reactivity, which we expected to be nucleus dependent. For example, o-quinol derivatives are susceptible to dimerization,1b,d whereas their p-quinol counterparts succumb to single electron transfer reduction and rearomatization.11 While none of these issues can be avoided altogether, we imagined that some electronic and structural features could be introduced into the phenol and tether functionalities to dissuade some of undesired reactivities in the resulting cyclohexadienone adduct. An added benefit of our plan was that the oxygen atom, which would be installed during the dearomatization reaction, would be already protected. This increased the efficiency of any future synthetic plans by expanding the repertoire of accessible chemical transformations and obviating the need for subsequent hydroxyl protection.
3. Diastereoselective dearomatization with I(III) reagents; the affects of reaction conditions and substrate substituents on the overall yield and diastereoselectivity
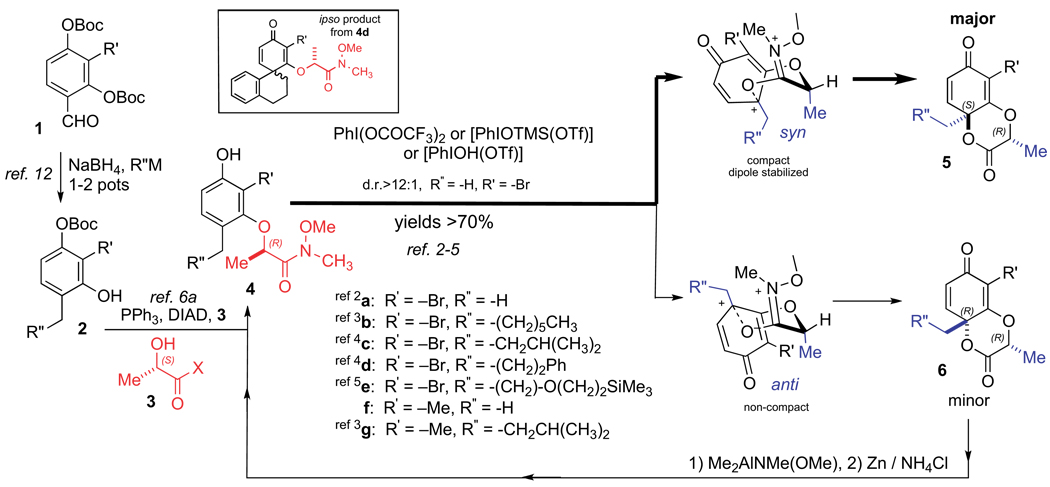
Our studies have principally utilized two 2,4-dihydroxybenzaldehydes 1 (R′=–Me, –Br, Scheme 1). Some time ago, we reported a method for the straightforward conversion of these kinds of aldehydes into their corresponding 2-bromo- and 2-methyl-resorcinol derivatives 2 so as to display differing 4-alkyl substituents (a–g).12 Before the development of our mild process, which involves o-quinone methide (o-QM) generation and consumption, these types of mono-protected 4-alkyl resorcinols were relatively inaccessible. The unprotected phenol product participates in an SN2 reaction under Mitsunobu conditions with the secondary alcohol within various amides 3, which are derived from (S)-methyl lactate.13 Subsequent Boc deprotection with ZnBr2 in CH3NO2 or in situ with aqueous LiOH at the conclusion of the Mitsunobu reaction affords the respective non-racemic mono-‘protected’ resorcinols 4 with enantiomeric excess of greater than 99%, demonstrated by chiral GC comparison of a derivative to a racemic standard.6a
Scheme 1.
Diastereoselective dearomatization of resorcinol derivatives with [PhIOTMS(OTf)].
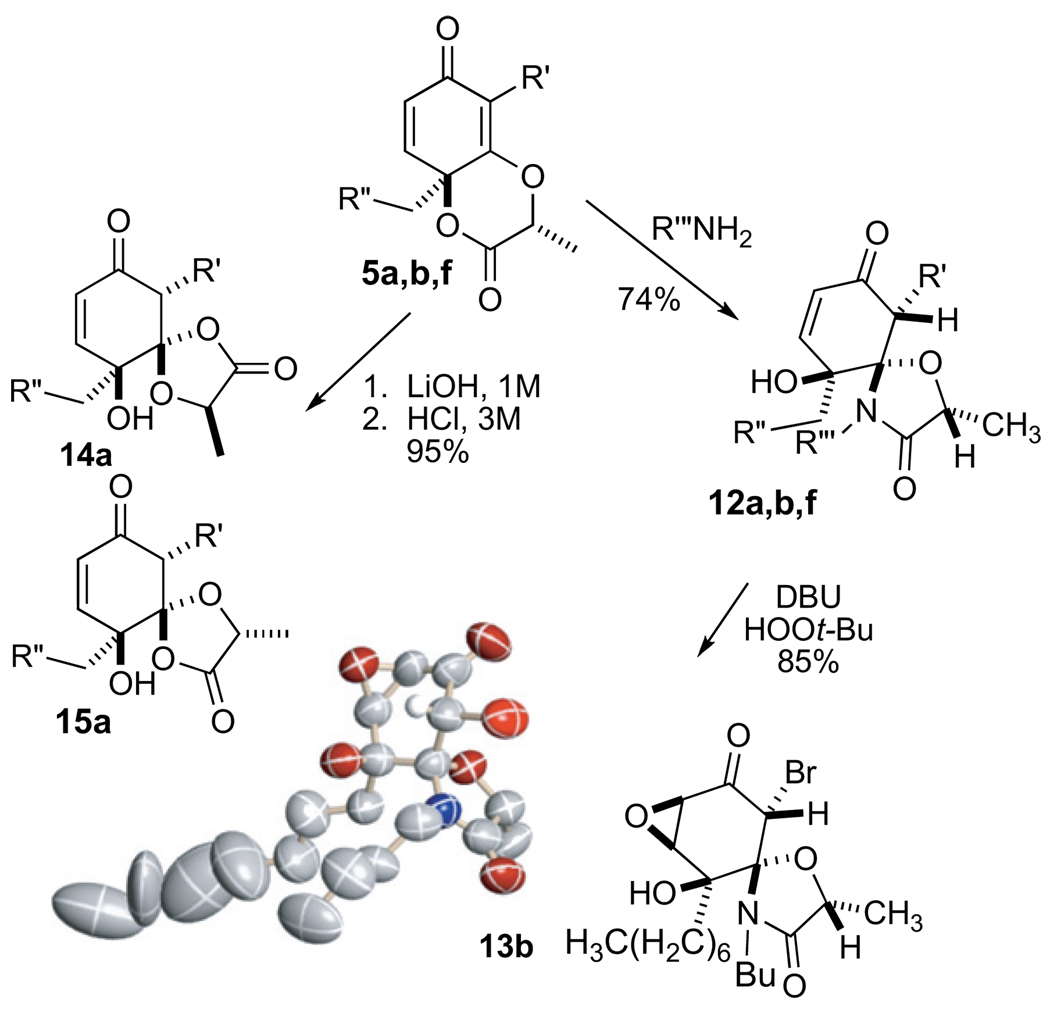
Oxidation of these substrates using various PhI(III) species afford the corresponding lactones 5 as the major product (dr>12:1). In some instances, where the R″ substituent is fairly small (R″=–H), a minor amount (<7%) of the lactones 6 can be observed. However, this unwanted diastereomer is easily removed by column chromatography and it can even be re-introduced into the synthetic stream by formation of the corresponding Weinreb amide followed by the appropriate reduction. Given the structure of the major product, we speculate that it arises from the compact, dipole stabilized transition-state. From this transition-state, the Me– of the lactate residue, and the R″CH2– substituent emerge on the same face (syn) with respect to the newly forming 1,4-di-oxygenated ring. On the other hand, for the minor diastereomer 6, the Me– and the R″CH2– substituent emerge on the opposite face (anti). The X-ray crystal structures for 5a and ent-5a have been previously reported.4
After much experimentation with the oxidative dearomatization of resorcinol 4d (R″=–(CH2)2Ph), which can undergo either ipso or ortho-cyclization (Scheme 1, inset shows the ipso product), we believe that we have successfully ascertained the optimum combination of reagents and substituents for the formation of the desired 1,4-dioxan-2-one scaffold 5.3 Dilute conditions (0.05 M) and very polar solvents, particularly nitromethane (CH3NO2), proved best in most instances. A bromine or alkyl substituent at the 2-position of the resorcinol also appeared to facilitate the dearomatization process, and increase the diastereoselectivity. Remarkably, the lactate tether motif not only resulted in diastereoselection, it also improved yields and it provided a more stable cyclohexadienone adduct than the corresponding glycolate derived tethers. In addition, we found that the transformation can be carried out at much higher concentrations (0.3 M) in methylene chloride when the oxidation is carried out with either (1.1 equiv PhIO, 0.1 equiv TMSOTf) or (1.1 equiv PhIO and 1.1 equiv TfOH).3,5 These oxidants, which are generated in situ, prevent any undesired participation of the ligand from the hypervalent iodine species with the intermediate phenoxonium cation.14
4. Some surprising reactivity differences amongst epimeric chiral cyclohexadienone adducts
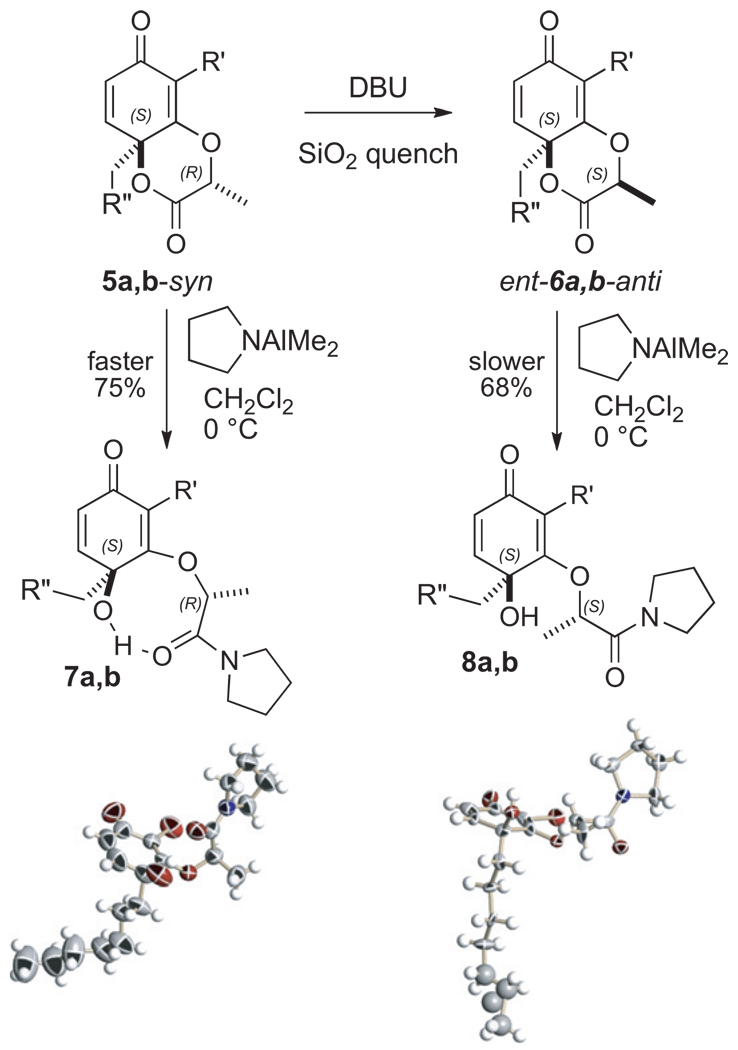
The reactivity of the chiral cyclohexadienones 5 was next studied. Remarkably, all attempts to epoxidize cyclohexadienones resembling 5 under standard basic conditions failed.15 In our experience, we found that as long as the tertiary alcohol is protected, then the resorcinol derived cyclohexadienones are quite stubborn toward epoxidation.6 On the other hand, cyclohexadienones derived from phenols usually undergo epoxidation, even if their tertiary alcohol is protected.16 The conditions we used for the attempted epoxidation, however, resulted in formation of the ent-6 epimer. Indeed, independent treatment of the syn-δ-lactone 5a (0.04 M in THF, 1.0 equiv DBU) caused rapid epimerization of the tether methyl residue to produce, upon quenching with silica gel, the corresponding anti-δ-lactone ent-6a (Scheme 2). We speculate the axial hydrogen atom is unusually acidic because of the attached vinylogous ester oxygen atom and its zwitterionic nature. Moreover, in the product 6a, both of the sp3 substituents in the 1,4-dioxan-2-one reside in a thermodynamically preferred anti 1,4-diaxial orientation. This anti-arrangement thwarts further access to the lactone carbonyl and many of the reactions that will be subsequently described (Scheme 6) for type 5-system do not necessarily apply to the corresponding 6-system. For example, compounds 6a,b are a great deal less reactive with aluminum amides than their corresponding diastereomer 5a,b. The relative and absolute stereochemistry of the two pyrrolidine products have been confirmed by X-ray analysis. In the case of the cyclohexadienone 7b, the amide carbonyl is positioned in an eight-membered ring so as to form a hydrogen bond with the neighboring tertiary alcohol. In the cyclohexadienone 8b, however, the carbonyl turns away from the tertiary alcohol. Again, this minor structural difference profoundly influences the subsequent chemistry. The enone 8b smoothly undergoes syn selective anionic epoxidation (t-BuOOH, DBU), whereas the enone 7b proves unreactive.
Scheme 2.
Epimers displaying different reactivity.
Scheme 6.
Unexpected reductions in rigid cyclohexadienones.
5. Remarkable electrocylization discovered
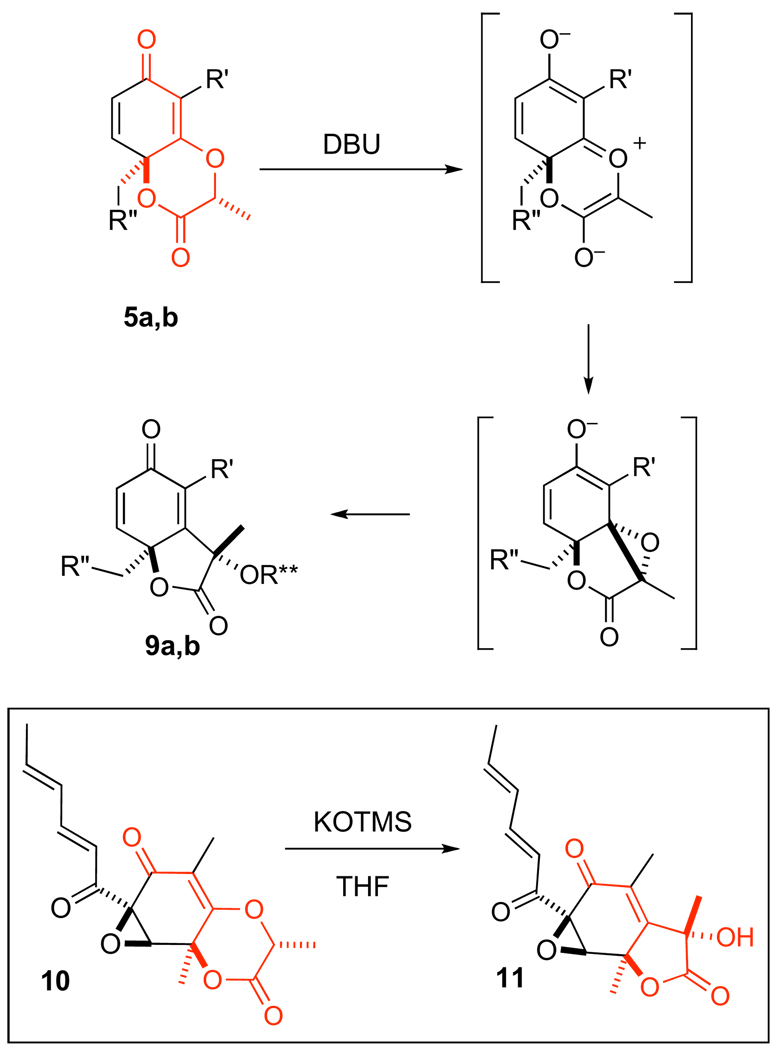
Even more interesting is the stereospecific electrocyclization that we observe upon treatment of the lactone 5 (0.1 M in THF) with or DBU. We postulated this reaction, which resembles a Favorskii rearrangement, proceeds through the anion shown to afford the γ-lactone 9 (Scheme 3). Again, the transformation is somewhat structure dependant. In the case of 5b, where [R″=–(CH2)5CH3], the rearrangement product (R**=H) emerges quite cleanly (>85% yield). However, when R″=–H as in 5a, compound 9 emerges (R**=–H), along with a dimeric product where the tertiary alcohol has participated in a stereoselective 1,4-addition with the enone of the rearrangement product (1:3 ratio, monomer–dimer). Coincidently, this rearrangement does not readily occur with either the thermodynamic diastereomer 6b, or the corresponding lactones displaying a glycolate derived tether (not shown), suggesting that the release of steric strain may play a role in the transformation. Our observation of this rearrangement in other cores, such as compound 10 and 18a, which converts to 11 with a variety of bases,17 leads us to believe that neither an electron deficient R′ residue nor an enone are critical for this rearrangement process. However, the vinylogous ester and the syn configured 1,4-dioxan-2-one seem to be essential motifs.
Scheme 3.
An unexpected rearrangement.
6. Functional groups interconversion involving the lactone and vinylogous ester moieties
Owing to the conjoined 1,4-dioxan-2-one and vinylogous ester, compound 5 undergoes many other unusual functional group interconversions, several of which prove diastereoselective. Some time ago, we found that the glycolate lactone derivatives corresponding to 5 (not shown) undergo saponification and diastereoselective lactonization to afford a spirolactone.2 Further saponification cleaves the spirolactone to reveal the carbonyl moiety that was previously masked as the vinylogous ester and thereby removes the tether. In the case of the lactic acid cyclohexadienone derivatives, such as 5, however, the reaction is less synthetically useful. Saponification and subsequent lactonization now proceeds to a mixture of γ-spirolactone adducts (14 and 15) possessing dissimilar reactivity (2:1 ratio, 95% overall yield). Compound 14, for example, undergoes an E2 elimination rather than saponification upon treatment with aqueous base to return the starting lactone 5, whereas compound 15 converts under similar conditions into the corresponding β-diketone (not shown). Compound 5 does, however, undergo a highly diastereoselective transformation to afford the lactam 12 upon the addition of a primary amine (74% yield for 12a, Scheme 4). Amines R‴=–Bn and –Bu have both been tested.15 The resulting spirocyclic lactam 12 forms as a single diastereomer and undergoes further stereoselective epoxidation to produce the anticipated syn-epoxy-alcohol 13 (85% yield). X-ray analysis of 13 has secured the stereochemistry of the cyclization despite some variability throughout the lipophyllic side chain. Yields are lower for cyclohexadienones when the R′=alkyl residues (12f) as opposed to R′=–Br; it therefore appears that this lactamization is facilitated, at least some degree, by an electron deficient R′ residue.
Scheme 4.
Remarkable functional group interconversions of the vinylogous ester in type 5 cyclohexadienones.
7. Reactions of the cyclohexadienone scaffold 5
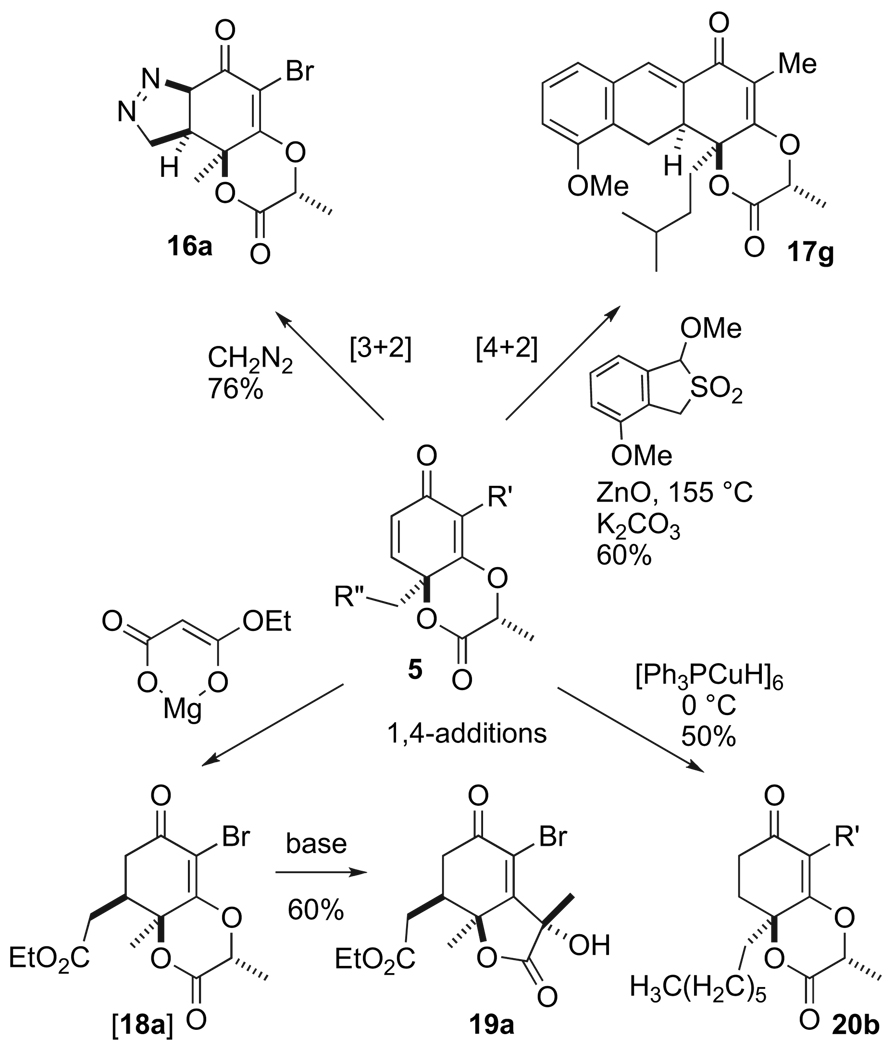
Because of a pseudo equatorial arrangement of the C–O bond on the cyclohexadienone, as opposed to a pseudo axial orientation, the bicyclic compound 5 enjoys enhanced chemical and thermal stability as compared to a rudimentary p-quinol acetate cyclohexa-2,5-dienone adduct. Compound 5 is far less susceptible to single electron transfer and elimination. Moreover, because of the pseudo axial –CH2R″ residue, many reactions of the bicyclic compound 5 prove stereoselective. For example, compound 5a undergoes a selective [3+2] cycloaddition with diazomethane at 0 °C in diethyl ether to afford the tetrahydro-3H-indazole 16a (76% yield, Scheme 5).18 On the other hand, the enone 5g proceeds in a [4+2] cycloaddition at 155 °C in a sealed tube containing toluene and the masked Durst–Charlton dimethide19 to afford the tricyclic ring system 17g as a single diastereomer.17 Type 5 enones can also participate in 1,4-conjugate additions. For example, addition of the magnesium enolate20 to 5a followed by decarboxylation results in the formation of the ester 18a, which under basic conditions undergoes further rearrangement to afford 19a as a single diastereomer. However, 1,4-conjugated addition of alkyl and vinyl cuprates is not as useful. In most instances the cyclohexadienone succumbs to single electron transfer and elimination to return an aromatic product (not shown). For example, addition of Stryker’s reagent ([Ph3PCuH]6) to compound 5b affords the anticipated reduced product 20b (50% yield) along with about a 40% yield of the corresponding 2-(R)-(2-bromo-6-heptyl-3-hydroxyphenoxy)-propanoic acid (not shown). We therefore chose to next examine other potentially chemoselective and stereoselective reduction procedures to better understand and unleash the utility of this new chiral building block.
Scheme 5.
Some stereoselective [3+2] and [4+2] additions and some 1,4-conjugate additions to the enone of adduct 5.
8. Reductions of the cyclohexadienone 5 and related scaffolds
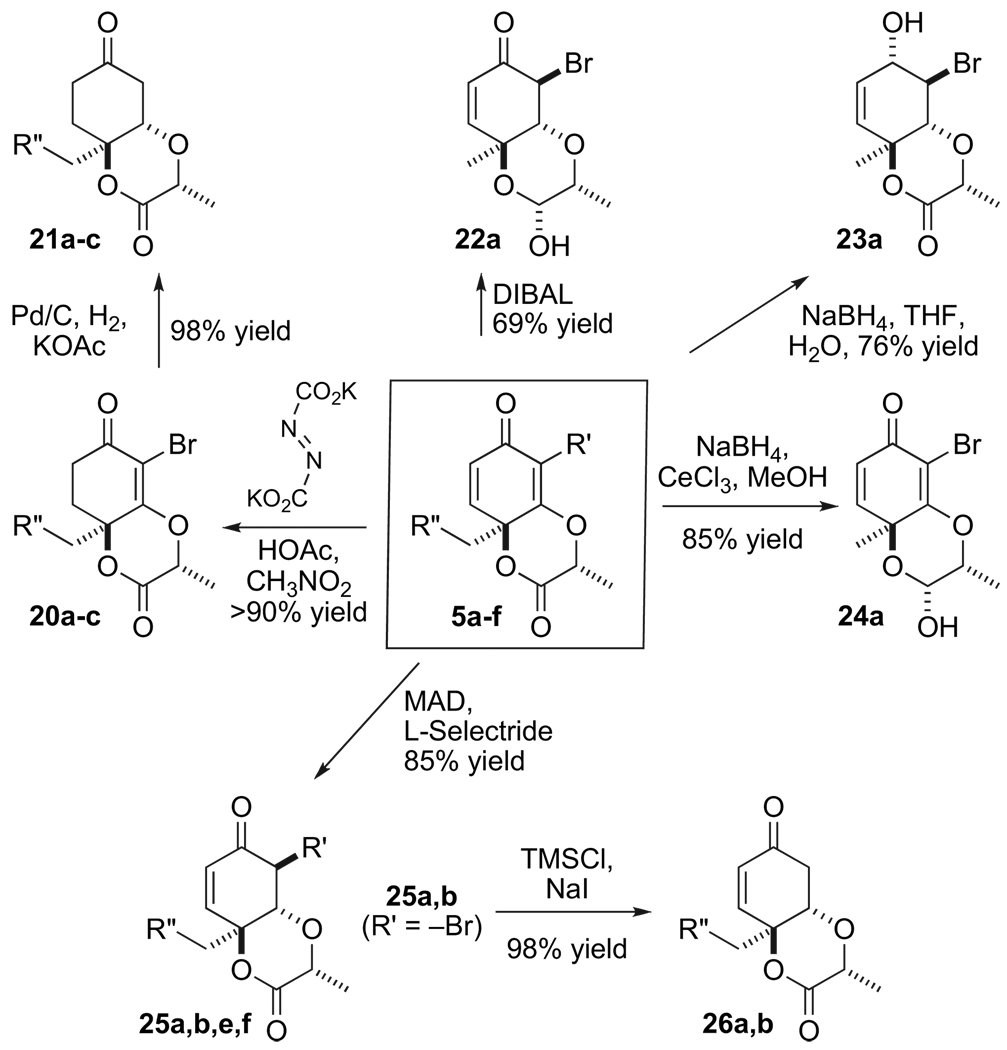
Despite its structural enhancements and rigidity, type 5 cyclohexadienones still performed erratically in most transition metal mediated procedures. Thus, most hydrogenation conditions are accompanied with a fair amount of rearomatization.6a After considerable experimentation and newly found appreciation of Corey’s ovalicin work, we chose to subject the enone 5 (0.07 M in CH3NO2) to a source of diimide, generated from potassium azodicarboxylate by the addition of 3 equiv of acetic acid.21 The reduction smoothly afforded the vinylogous ester 20 (Scheme 6). With one of the double bonds removed, the vinylogous ester undergoes subsequent reduction with palladium/carbon and hydrogen to smoothly afford the ketone 21. In addition, the bromine atom can be removed from 20 by standard conditions to afford its hydrido counterpart 21. The vinylogous ester of 5 (0.1 M in toluene) at −78 °C succumbs to a diastereoselective reduction with methylaluminum bis(2,6-di-tert-butyl-4-methylphenoxide) (MAD) (2.2 equiv) and L-Selectride (1.1 equiv) to afford the trans fused system 25 in 85% yield.22 Lower yields are obtained in systems where R′=–Me residue 25f, however the reaction is still quite useful. The bromide in scaffolds, such as 25a can be removed by treatment with TMSI (2 equiv) or PPh3 and methanol to furnish the enone 26 in 98% yield.15 Hydrogenation of either compound 20 or 25 affords the expected ketone 21. On the other hand, we found that addition of DIBAL (2 equiv) to 5a (0.04 M in toluene at −78 °C) results in the lactol 22a in 69% yield. Presumably, this transformation proceeds by sequential stereoselective reduction of the vinylogous ester, followed by the lactone. Surprisingly, the use of sodium borohydride (10 equiv) with 5a (0.04 M in THF/H2O at 0 °C) reduces the vinylogous ester, followed by 1,2-reduction of the enone to thereby afford compound 23a in >75% yield. More remarkably still, compound 5a (0.04 M in MeOH at −78 °C) in the presence of cerium trichloride (1.2 equiv) undergoes reduction of the lactone with sodium borohydride (2 equiv) to produce the lactol 24a. This outcome was certainly not anticipated from Luche reduction conditions.
9. Other useful transformations of scaffolds derived from the cyclohexadienone 5
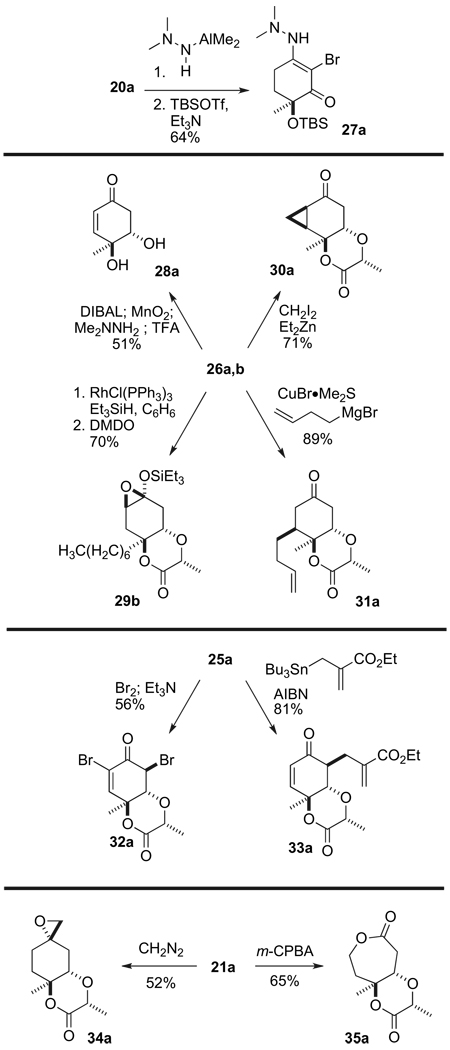
We have developed several methods for removal of the tether, that is, derived from methyl lactate and directs the stereochemical formation of the tertiary alcohol in the cyclohexa-2,5-dienone. The simplest is treatment of fused compounds, such as 21, with KOTMS. Saponification of the lactone causes a ring flip and concomitant β-elimination of the lactate derived functionality.6a However, we have also developed processes that enables us to retain the oxygen atom by severing the C–O bond from both the lactone and its corresponding hemiketal by formation of various hydrazone derivatives.3,4 For example, treatment of 20a (0.05 M in CH2Cl2 at 0 °C) with excess Me2AlNHNMe2 (3 equiv) affords a single vinylogous hydrazide. Subsequent silylation facilitating isolation as compound 27a. Reduction of the enone carbonyl and lactone functionalities in 26a, followed by allylic MnO2 oxidation and treatment of the corresponding lactol with H2NNMe2 followed by TFA affords the diol 28a by a similar C–O fragmentation mechanism.
The rigid enone 26 also participates in many other selective transformations. For example, it undergoes 1,4-addition of hydride mediated by rhodium to afford the corresponding silyl enol ether. Further epoxidation with DMDO affords compound 29b. The enone in 26 also undergoes stereoselective cyclopropanation under Furukawa modified Simmons–Smith conditions to produce compound 30a (Scheme 7). The conjugate addition of homo-allyl cuprate (0.04 M in THF at −78 °C) to afford 31a is similarly diastereoselective. We speculated that the outstanding stereoselectivity in all of these reactions is due to the steric encumbrance of the angular –CH2R‴ residue.
Scheme 7.
Some other useful transformations.
The equatorial bromide compound 25, formed by L-Selectride reduction, also undergoes selective reactions. For example, bromination (1 equiv of Br2; 1.5 equiv Et3N) of enone 25 (0.05 M in CH2Cl2 at 0 °C) proceeds as expected to produce the vinyl bromide 32a. On the other hand, treatment of 25a (0.02 M in benzene at 80 °C) with AIBN and the allyl tin species shown (2 equiv) affords a single diastereomer of the unsaturated ester 33a in greater than 80% yield.
The fully reduced scaffold 21 also undergoes some unexpectedly reactions. For example, in the presence of CH2N2, 21a affords the spiroepoxide 34a, rather than the intended ring expanded product (not shown). Surprisingly, migration proceeds in only one direction upon treatment of 21a with m-CPBA, to afford the seven-membered lactone 35a as a single regioisomer. This selective ring expansion further illustrates the remarkable and unexpected selectivity of these cylcohexadienones and their derivatives.
10. Conclusion
In closing, we believe that the dearomatization process, that is, mitigated by hypervalent iodine derivatives of PhIO, that afford chiral cyclohexadienones, such as 5 from the corresponding methyl lactate 3 and the resorcinol 2 compounds, displays tremendous potential. The cyclohexadienone 5, that is, formed is a useful building block for both six and seven-membered rings as well as for acyclic arrays. Moreover, its epimer 6 is expected to afford a very different reactivity profile because its lactone carbonyl is better protected by the anti-3,5-alkyl substituents of the corresponding 1,4-dioxan-2-one. We therefore believe that the 5-pot protocol used to construct 5 in either optical antipode with various hypervalent iodine reagents might indeed find many good applications in future enantioselective syntheses.
11. Experimental procedures and spectral data for representative scaffolds 2–9, 11, 19–36
11.1. General techniques
In reactions, where water was not present as solvent, reagent, or by-product, vessels were flame-dried under a slow nitrogen flow. A slight positive pressure of dry nitrogen was maintained via rubber septum seal during the course of the reaction. Diethyl ether, tetrahydrofuran, benzene, and toluene were distilled from sodium and benzophenone. Dichloromethane and nitromethane were distilled from calcium hydride. 1H NMR and 13C NMR spectra were recorded at 400 MHz and 100 MHz, respectively, and in CDCl3 unless otherwise noted. Chromatography purification was performed using 40–63 µm silica gel.
11.2. Scaffold 4
To a stirred solution of triphenyl phosphine (6.49 g, 24.8 mmol) in THF (60 mL) at 0 °C was added diisopropyl azodicarboxylate (4.9 mL, 24.9 mmol), dropwise over 1 min. The resulting suspension was stirred from 0 °C to rt for 1 h. The mixture was re-cooled to 0 °C, and a solution of 3 (5.01 g, 16.5 mmol) in THF (40 mL) was added via a cannula, followed by a solution of 2a (2.20 g, 16.5 mmol) in THF (25 mL). The reaction was stirred at 0 °C to rt for 12 h. It was concentrated under reduced pressure and crude residue was purified by column chromatography (20% EtOAc–hexanes) to afford a light yellow oil.
The above product was redissolved in CH3NO2 (125 mL) and ZnBr2 (14.3 g, 63.4 mmol) was added. The reaction was stirred at rt for 5 h, then quenched with 1 Maq HCl and diluted with EtOAc. The organic layer was washed twice with 1 M citric acid, twice with deionized water, once with brine, and dried (Na2SO4). The mixture was filtered, concentrated, and the crude residue was purified by chromatography (30% EtOAc–hexanes) to afford 4a as a white solid (4.11 g, 78% yield over two steps).
11.2.1. Compound 4a
1H NMR δ (multiplicity, J (Hz), integration): 7.02 (d, 8.5, 1H), 6.74 (d, 8.4, 1H), 5.55 (s, 1H), 5.14 (d, 6.4, 1H), 3.59 (s, 3H), 3.23 (s, 3H), 2.28 (s, 3H), 1.55 (d, 6.6, 3H); 13C NMR δ 172.4, 153.6, 152.1, 130.4, 124.7, 111.7, 105.9, 75.6, 61.6, 32.5, 18.1, 16.7; IR (thin film): 3242, 2934, 1651, 1423, 1177, 1076, 1038, 987 cm−1; HRMS (ESI) calcd for C12H16NO4BrNa+: 340.0154, found 340.0159; mp=127–128 °C.
11.2.2. Compound 4f
Yield 75%. 1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 6.83 (d, 8.2, 1H), 6.55 (d, 8.1, 1H), 5.54 (s, 1H), 4.88 (br q, 6.0, 1H), 3.49 (s, 3H), 3.2 (s, 3H), 2.21 (s, 3H), 2.19 (s, 3H), 1.49 (d, 6.6, 3H); 13C NMR δ: 155.8, 153.4, 128.3, 122.9, 118.0, 111.0, 74.5, 61.5, 32.5, 18.2, 16.8, 10.1.
11.3. Scaffold 5
A solution of 4a (0.363 g, 1.33 mmol) previously dried under vacuum (48 h at 0.07 mmHg), in CH3NO2 (12 mL) was cooled to 0 °C and [bis(trifluoroacetoxy)iodo]benzene (0.738 g, 1.72 mmol) was added in one portion. The reaction mixture was stirred at 0 °C for 15 min, and gradually turned light yellow. Deionized water (20 mL) was added followed by CH2Cl2 (20 mL) and it was stirred vigorously at 0 °C for 15 min. Upon initial addition of water the solution turned dark green, then faded to yellow after several minutes. The aqueous layer was extracted with CH2Cl2 four times and the combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude product was purified by flash chromatography (30% EtOAc–hexanes) to afford 5a (0.224 g, 75% yield) as an off-white solid.
11.3.1. Compound 5a
1H NMR δ (multiplicity, J (Hz), integration): 6.84 (d, 10.0, 1H), 6.36 (d, 10.0, 1H), 4.99 (q, 7.2, 1H), 1.88 (d, 7.2, 3H), 1.85 (s, 3H); 13C NMR δ: 179.4, 166.5, 162.1, 143.1, 127.1, 107.0, 76.6, 74.4, 29.9, 19.5; IR (thin film): 2924, 1759, 1668, 1607, 1261, 1049 cm−1; HRMS (EI) calcd for 271.9684, found 271.9689; mp=111–114 °C.
11.3.2. Compound 5b–d
See Ref. 6a.
11.3.3. Compound 5e
See Ref. 4.
11.3.4. Compound 5f
Yield 70%. 1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 6.79 (d, 10.0, 1H), 6.24 (d, 10.0, 1H), 4.79 (q, 7.0, 1H), 1.87 (s, 3H), 1.81 (d, 7.0, 3H), 1.79 (s, 3H); 13C NMR (125 MHz) δ: 186.5, 167.6, 160.0, 143.0, 128.1, 119.2, 75.5, 73.9, 29.4, 19.4, 7.6.
11.3.5. Compound 5g
See Ref. 3.
11.4. Scaffold 6
This minor diastereomer formed during the synthesis of 5 was isolated by chromatography (30% EtOAc–hexanes). Alternatively, ent-6a can be obtained from the following procedure: to a solution of 5a (0.020 g, 0.073 mmol) in THF (2 mL) at 0 °C was added DBU (0.012 mL, 0.079 mmol). The reaction was stirred for 30 s, quenched with silica gel, and immediately concentrated under reduced pressure and purified by flash chromatography (30% EtOAc–hexanes) to afford ent-6a (0.018 g, 90% yield) as a light yellow oil.
11.4.1. Compound 6a
1H NMR δ (multiplicity, J (Hz), integration): 6.80 (d, 10.0, 1H), 6.30 (d, 10.0, 1H), 5.20 (q, 6.5, 1H), 1.88 (s, 3H), 1.78 (d, 6.7, 3H); 13C NMR δ: 179.2, 166.3, 162.6, 142.3, 126.7, 102.2, 77.0, 73.1, 27.0, 18.3; IR (thin film): 2923, 1767, 1666, 1603, 1286, 1248. 1055 cm−1; HRMS (EI) calcd for : 271.9684, found: 271.9693.
11.5. Scaffold 7
To a solution of pyrrolidine (0.0034 mL, 0.041 mmol) in CH2Cl2 (0.5 mL) cooled to 0 °C was added AlMe3 (0.0175 mL, 0.040 mmol, 2.3 M in hexanes), and the resulting solution was stirred at 0 °C for 25 min. A solution of dienenone 5a (0.010 g, 0.036 mmol) in CH2Cl2 (0.5 mL) was added to the aluminum amide. The reaction was allowed to slowly warm to rt and was stirred for 2.5 h. It was quenched with aq 1 M Rochelle’s salt, diluted with CH2Cl2, and stirred vigorously for 30 min. The aqueous layer was extracted four times with CH2Cl2. The combined organic layers were dried (Na2SO4), concentrated, and the crude residue was purified by chromatography (40% EtOAc–hexanes) to afford 7a as a light yellow oil (0.009 g, 75% yield).
11.5.1. Compound 7a
1H NMR δ (multiplicity, J (Hz), integration): 6.84 (d, 9.9, 1H), 6.48 (s, 1H), 6.17 (d, 9.9, 1H), 6.08 (q, 6.7, 1H), 3.61–3.48 (m, 2H), 3.44–3.34 (m, 2H), 2.01 (quintet, 6.4, 2H), 1.87 (m, 6.4, 2H), 1.65 (d, 6.7, 3H), 1.58 (s, 3H); 13C NMR δ: 181.2, 174.0, 170.1, 151.2, 123.6, 106.8, 75.5, 71.8, 46.9, 46.5, 27.0, 26.3, 23.9, 18.6; IR (thin film): 3252, 2977, 1664, 1630, 1583, 1458, 1186, 1026; HRMS (EI) calcd for : 343.0419, found: 343.0423.
11.5.2. Compound 7b
Yield 72%. 1H NMR δ (multiplicity, J (Hz), integration): 6.76 (d, 10.1, 1H), 6.54 (s, 1H), 6.24 (d, 9.9, 1H), 6.03 (q, 6.8, 1H), 3.60–3.48 (m, 2H), 3.44–3.33 (m, 2H), 2.07–1.98 (m, 2H), 1.89–1.78 (m, 2H), 1.65 (d, 6.6, 3H), 1.30–1.00 (m, 12H), 0.87 (t, 6.8, 3H).
11.6. Scaffold 8
The procedure for scaffold 7 was used, except the reaction was allowed to stir for 6 h following the addition of dienone 6. The reaction generally and gave slightly lower yields (68%).
11.6.1. Compound 8a
1H NMR δ (multiplicity, J (Hz), integration): 6.84 (d, 10.0, 1H), 6.25 (d, 10.0, 1H), 5.66 (q, 6.8, 1H), 3.70–3.41 (m, 4H), 2.05 (quin, 6.7, 2H), 1.93 (quin, 6.7, 2H), 1.59 (s, 3H), 1.53 (d, 6.8, 3H).
11.6.2. Compound 8b
Yield 64%. 1H NMR δ (multiplicity, J (Hz), integration): 6.77 (d, 9.8, 1H), 6.32 (d, 9.8, 1H), 6.19 (s, 1H), 5.54 (q, 6.83, 1H), 3.70–3.40 (m, 4H), 2.10–1.78 (m, 2H), 1.71–1.56 (m, 2H), 1.53 (d, 6.84, 3H), 1.34–1.17 (m, 12H), 0.86 (t, 6.83, 3H); IR (thin film): 3254, 2929, 1665, 1631, 1584, 1187 cm−1.
11.7. Scaffold 9
To a solution of 5a (0.020 g, 0.073 mmol) in THF (2 mL) at 0 °C was added DBU (0.012 mL, 0.079 mmol). The reaction was stirred for 30 min, then concentrated under reduced pressure and purified by flash chromatography (30% EtOAc–hexanes) to afford 9a as a light yellow oil (0.017 g, 88% yield). For the synthesis of 9b, BnNMe3OH (40% wt in methanol, 1.0 equiv) was used instead of DBU, and the reaction was quenched with silica gel upon completion by TLC analysis.
11.7.1. Compound 9a (dimer)
1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 6.80 (d, 10.3, 1H), 6.14 (d, 10.3, 1H), 5.15 (s, 1H), 4.24 (d, 10.1, 1H), 2.95 (d, 9.9, 1H), 2.65 (s, 1H), 1.93 (s, 3H), 1.81 (s, 3H), 1.79 (s, 3H), 1.68 (s, 3H); 13C NMR (125 MHz) δ: 188.0, 185.2, 173.6, 167.6, 152.6, 145.7, 130.2, 120.0, 89.2, 85.9, 85.5, 81.2, 74.4, 58.5, 55.3, 54.1, 32.2, 27.4, 20.9, 20.5; IR (thin film): 3464, 2925, 1794, 1757, 1695, 1380, 1120, 1072; HRMS (ESI) calcd for C20H18O8Br2Na+: 566.9260, found: 566.9257.
11.7.2. Compound 9b (R**=H)
Yield 79%. 1H NMR δ (multiplicity, J (Hz), integration): 7.2 (d, 10.0, 1H), 6.42 (d, 10.0, 1H), 2.61 (s, 1H), 2.21 (m, 1H), 2.0 (m, 1H), 1.98 (s, 3H), 1.38–1.17 (m, 10H), 0.86 (t, 6.8, 3H).
11.7.3. Compound 9c (R**=H)
Yield 80%. 1H NMR δ (multiplicity, J (Hz), integration): 7.18 (d, 9.9, 1H), 6.44 (d, 9.9, 1H), 2.71 (s, 1H), 2.21 (dt, 4.5, 13.4, 1H), 2.01 (dt, 4.8, 12.4, 1H), 1.97 (s, 3H), 1.51 (sep, 6.7, 1H), 1.20 (m, 1H), 1.13 (m, 1H), 0.85 (t, 6.7, 6H).
11.8. Scaffold 11
To a solution of 1017 (0.012 g, 0.038 mmol) in THF (1 mL) was added KOTMS (0.0072 g, 0.057 mmol) and the resulting orange slurry was stirred for 25 min. The reaction was quenched with TFA (one drop), concentrated, and purified by column chromatography (20% EtOAc–hexanes) to afford 11 (0.009 g, 75% yield).
11.8.1. Compound 11
1H NMR δ (multiplicity, J (Hz), integration): 7.38 (dd, 9.8, 15.2, 1H), 6.41–6.21 (m, 3H), 3.96 (s, 1H), 2.05 (s, 3H), 1.90 (d, 6.8, 3H), 1.78 (s, 3H), 1.73 (s, 3H).
11.9. Scaffold 12
To a solution of 5a (0.0500 g, 0.183 mmol) in CH2Cl2 (3 mL) cooled to 0 °C was added BnNH2 (0.022 mL, 0.201 mmol). The solution was stirred at 0 °C to rt for 14 h, concentrated and purified by chromatography (40% EtOAc–hexanes) to afford 12a (0.026 g, 74% yield based on consumed starting) and recovered 5a (0.025 g, 50% recovery).
11.9.1. Compound 12a (R”’=Bn)
1H NMR δ (multiplicity, J (Hz), integration): 7.38 (m, 5H), 6.58 (d, 10.0, 1H), 6.18 (d, 10.0, 3H), 5.71 (s, 1H), 5.20 (d, 15.3, 1H), 4.77 (q, 6.8, 1H), 4.02 (d, 15.3, 1H), 1.46 (d, 6.8, 3H), 1.27 (s, 3H); 13C NMR δ: 188.6, 175.6, 148.9, 137.5, 129.3, 128.7, 128.3, 127.6, 99.8, 75.8, 73.9, 60.9, 47.0, 23.6, 18.4; IR (thin film): 3380, 2924, 1697, 1095, 703; HRMS (ESI): calcd for C17H18NO4BrNa+: 402.0311, found: 402.0300.
11.9.2. Compound 12b (R‴=Bu)
This compound was characterized as is epoxide derivative 13b.
Compound 12f (R‴=Bn): 48% yield. 1H NMR δ (multiplicity, J (Hz), integration): 7.44–7.32 (m, 5H), 6.54 (d, 10.0, 1H), 6.06 (d, 10.0, 1H), 5.11 (d, 14.6, 1H), 4.54 (q, 6.8, 1H), 4.20 (d, 14.8, 1H), 3.52 (q, 7.0, 1H), 1.46 (d, 6.7, 3H), 1.24 (s, 3H), 1.01 (d, 6.8, 3H).
11.10. Scaffold 13
To a solution of 12b (0.0047 g, 0.019 mmol) in CH2Cl2 (0.5 mL) was added t-BuOOH (0.0040 mL, 0.038 mmol) and DBU (cat.). The solution was stirred at rt for 45 h, concentrated, and purified by chromatography (40% EtOAc–hexanes) to afford 13b (0.0041 g, 85% yield).
11.10.1. Compound 13b
1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 4.67 (d, 1.7, 1H), 4.34 (q, 6.9, 1H), 3.75 (m, 1H), 3.63 (m, 2H), 3.55 (d, 3.7, 1H), 1.97 (m, 1H), 1.85 (m, 1H), 1.75 (m, 1H), 1.50–1.70 (m, 4H), 1.45–1.23 (m, 12H), 0.97 (t, 7.2, 3H), 0.90 (t, 6.8, 3H); 13C NMR (125 MHz) δ: 197.6, 172.6, 102.9, 94.6, 91.7, 74.3, 54.4, 51.7, 42.3, 31.8, 31.6, 31.0, 29.2, 29.2, 22.8, 22.1, 20.6, 18.4, 14.3, 13.9; IR (thin film): 2930, 1718, 1413, 1374 cm−1; HRMS (EI) calcd for C20H32NO5Br+: 446.1542, found: 446.1559.
11.11. Scaffold 14,15
To a solution of 5a (0.0730 g, 0.267 mmol) in THF was added LiOH (0.30 mL, 0.30 mmol, 1 M in H2O). The solution was stirred for 10 min, then HCl (0.30 mL, 0.90 mmol, 3 M in H2O) was added stirring was continued for 1 h. The THF was removed under reduced pressure; the aq solution was further diluted and extracted five times with EtOAc. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude residue was a 2:1 mixture of 14a and 15a (0.0690 g, 95% NMR yield). Chromatography generally gave some separation and low isolated yields, however an analytically pure sample was obtained by flash chromatography (50% EtOAc–hexanes) to afford 14a and a mixture of 14a and 15a (0.0140 g, 20% isolated yield).
11.11.1. Compound 14a
1H NMR δ (multiplicity, J (Hz), integration): 6.87 (d, 10.3, 1H), 6.18 (d, 10.3, 1H), 5.01 (s, 1H), 4.90 (q, 6.8, 1H), 1.583 (s, 3H), 1.580 (d, 6.8, 1H); 13C NMR δ: 187.0, 172.5, 152.2, 126.2, 111.1, 74.1, 73.3, 60.5, 22.6, 17.9; IR (thin film): 3461, 2925, 1803, 1697, 1271, 1204, 1003; HRMS (ESI) calcd for C10H11O5BrNa+ 312.9692, found 312.9694.
11.11.2. Compound 15a
1H NMR δ (multiplicity, J (Hz), integration): 6.85 (d, 10.3, 1H), 6.20 (d, 10.3, 1H), 5.05 (s, 1H), 4.70 (q, 6.84, 1H), 1.60 (s, 3H), 1.59 (d, 6.8, 3H).
11.12. Scaffold 16
To a solution of 5a (0.0050 g, 0.018 mmol) in ether (1 mL) at 0 °C was added diazomethane (0.27 M in Et2O, 1.0 mL, 0.27 mmol) and the resulting solution was stirred for 12 h, gradually turning colorless. The reaction was concentrated and purified by crystallization from CH2Cl2–hexanes to afford 16a (0.0043 g, 76% yield).
11.12.1. Compound 16a
1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 5.86 (dd, 2.5, 9.4, 1H), 5.24 (dd, 9.5, 18.5, 1H), 5.12 (q, 7.4, 1H), 4.41 (ddd, 2.5, 9.7, 18.4, 1H), 2.98 (q, 9.4, 1H), 1.94 (s, 3H), 1.86 (d, 7.4, 3H); 13C NMR (125 MHz) δ: 177.8, 165.6, 162.2, 103.6, 92.0, 81.0, 80.5, 72.8, 41.0, 33.4, 19.6; IR (thin film): 2988, 2942, 1759, 1672, 1597, 1273, 1211, 1035 cm−1; HRMS (ESI) calcd for C11H11N2O4NaBr+: 336.9800, found: 336.9798.
11.13. Scaffold 17
A mixture of dienone 5g (0.080 g, 0.30 mmol), sulfone (0.87 g, 0.38 mmol), zinc oxide (0.081 g, 1.0 mmol), and K2CO3 (0.138 g, 1.00 mmol), in toluene (4 mL) was heated in a sealed tube to 155 °C for 8–10 h. The reaction was worked up with 2 M aq HCl and extracted with EtOAc. The combined organic layers were washed with water, brine, dried (Na2SO4), and concentrated.
The crude material was taken up in CHCl3 and treated with TFA (0.0093 mL, 0.125 mmol). The solution was stirred at rt for 4 h, concentrated and purified by chromatography (20% EtOAc–hexanes) to afford 17g as a light yellow oil (0.067 g, 60% yield).
11.13.1. Compound 17g
1H NMR δ (multiplicity, J (Hz), integration): 7.65 (d, 2.9, 1H), 7.20 (t, 7.7, 1H), 7.00 (d, 7.4, 1H), 6.95 (d, 7.4, 1H), 4.87 (q, 7.1, 1H), 3.88 (s, 3H), 3.46 (m, 1H), 3.27 (m, 1H), 2.67 (t, 16.6, 1H), 2.12 (m, 1H) 1.98 (s, 3H), 1.96 (m, 1H), 1.84 (d, 7.1, 3H), 1.53 (sep, 6.6, 1H), 0.88 (m, 6H).
11.14. Scaffold 18,19
To a cooled solution of mono-ethylmalonate (0.0043 mL, 0.036 mmol) in THF (0.5 mL) at 0 °C was added t-BuMgCl (0.83 M in THF, 0.089 mL, 0.072 mmol). The solution was stirred at 0 °C to rt for 10 h, and the residual solvent was removed by evaporation under a gentle flow of N2. A solution of dienone 5a (0.0050 g, 0.018 mmol) in DMF (0.5 mL) was added to the malonate salt and the solution was stirred at 60 °C for 2 h. The reaction was quenched with 1 M aq HCl and diluted with Et2O. The organic layer was washed twice with water, once with brine, dried (Na2SO4), and concentrated. The crude material was purified by chromatography (30% EtOAc–hexanes) to afford 19a as a colorless oil (0.0039 g, 60% yield).
11.14.1. Compound 19a
1H NMR δ (multiplicity, J (Hz), integration): 4.14 (q, 7.0, 1H), 3.13 (m, 1H), 2.95 (m, 3H), 2.74 (s, 1H), 2.57 (dd, 15.8, 5.4, 1H), 2.20 (dd, 15.8, 9.0, 1H), 1.95 (s, 3H), 1.82 (s, 3H).
11.15. Scaffold 20
To a solution of dienone 5a (0.199 g, 0.729 mmol) in CH3NO2 (10 mL) at 0 °C was added potassium azodicarboxylate (1.706 g, 8.78 mmol). Acetic acid (1.50 mL, 26.2 mmol) was added dropwise via a syringe pump over 4 h while the temperature was maintained at 0 °C. The reaction mixture was immediately diluted with EtOAc, filtered through Celite, and washed successively with satd aq NaHCO3, water, and brine. The organic layer was dried (Na2SO4), and concentrated. It was occasionally necessary to re-subject the crude material to additional potassium azodicarboxylate (1.137 g, 5.85 mmol) and acetic acid (1.00 mL, 17.5 mmol). The crude product was purified by crystallization from EtOAc–hexanes to afford 20a (0.190 g, 95% yield). The diimide reduction proved superior over previously reported use of [Ph3PCuH]6 for 20b,17 and Rh–alumina hydrogenation to afford 20c.6a
11.15.1. Compound 20a
1H NMR δ (multiplicity, J (Hz), integration): 5.09 (q, 7.3, 1H), 2.88 (ddd, 2.4, 4.8, 17.5, 1H), 2.56 (ddd, 5.7, 8.3, 17.7, 1H), 2.40 (dt, 4.1, 13.1, 1H), 2.53 (ddd, 2.1, 5.6, 12.8, 1H), 1.87 (d, 7.3, 3H), 1.83 (s, 3H); 13C NMR δ: 188.9, 166.7, 164.5, 105.6, 79.7, 73.4, 34.3, 34.0, 19.7; IR (thin film) 2941, 1751, 1670, 1600, 1274, 1188, 1038 cm−1; HRMS (EI) calcd for : 273.9841, found: 273.9832; mp=116–118 °C.
11.16. Scaffold 21
To a solution of enone 20a (0.057 g, 0.207 mmol) in EtOAc (3 mL) in 50 mL flask was added Pd/C (10%, 0.011 g, 0.010 mmol) followed by KOAc (0.034 g, 0.313 mmol). The flask was purged with H2 for several minutes, then stirred under a H2 balloon for 4 h. The reaction mixture was filtered through a pad of Celite, and concentrated. The crude material was purified by chromatography (30% EtOAc–hexanes) to afford 21a as a colorless oil (0.040 g, 0.203 mmol, 98% yield).
11.16.1. Compound 21a
1H NMR δ (multiplicity, J (Hz), integration): 4.46 (q, 7.0, 1H), 3.84 (dd, 10.2, 1H), 2.79 (ddd, 15.8, 5.6, 2.3, 1H), 2.56 (dd, 16.7, 5.1, 1H), 2.43 (dd, 13.1, 15.4, 1H), 2.38 (dd, 14.0, 7.3, 1H), 2.17 (ddd, 13.4, 8.8, 1.9, 1H), 1.93 (dd, 13.9, 5.6, 1H), 1.62 (s, 3H), 1.61 (d, 7.0, 3H); 13C NMR δ: 205.14, 170.1, 81.63, 75.16, 73.15, 43.85, 37.74, 31.67, 18.59, 18.38; IR (thin film) 2988, 2943, 2880, 1731, 1373, 1257. 1124 cm−1; HRMS (CI) calcd for : 199.0970, found: 199.0968.
11.16.2. Compound 21c
Please see Ref. 6a.
11.17. Scaffold 22
To a solution of dienone 5a (0.0500 g, 0.183 mmol) in toluene (5 mL) cooled to −78 °C was added DIBAL (1 M in hexanes, 0.366 mL, 0.366 mmol). The solution was stirred at −78 °C for 15 min. It was quenched with 1 M aq Rochelle’s salt, diluted in EtOAc, quickly warmed to rt and stirred vigorously for 30 min. The aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The residue was purified by flash chromatography (30% EtOAc–hexanes) to yield 22a as a colorless oil (0.035 g, 0.127 mmol, 69% yield).
11.17.1. Compound 22a
1H NMR δ (multiplicity, J (Hz), integration): 7.04 (d, 10.0, 1H), 6.05 (d, 10.1, 1H), 4.89 (t, 8.0, 1H), 4.41 (d, 12.4, 1H), 3.92 (d, 12.4 Hz, 1H), 3.46 (dq, 6.2, 8.2, 1H), 2.76 (d, 8.0, 1H), 1.56 (s, 3H), 1.39 (d, 6.2, 3H); 13C NMR δ: 190.4, 153.6, 126.6, 94.0, 82.5, 78.7, 76.0, 51.7, 19.1, 16.7; IR (thin film) 3450, 2980, 2935, 1691, 1105, 1080 cm−1; HRMS (ESI) calcd for C10H13BrO4Na+: 300.9868, found: 300.9879.
11.18. Scaffold 23
Dienone 5a (0.021 g, 0.077 mmol) was dissolved in THF–H2O (5/1, 1.8 mL) and cooled to 0 °C. NaBH4 (0.0029 g, 0.77 mmol) was allowed to stand in air for several minutes until it appeared slightly moist, at which time it was added to the dienone solution. Addition of dry NaBH4 generally resulted in a mixture of products, including over-reduction. The reaction was stirred for 5 min at 0 °C, quenched with saturated NH4Cl, and diluted in EtOAc. The aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude material was purified by flash chromatography (40% EtOAc–hexanes) to give 23a as colorless oil (0.016 g, 0.059 mmol, 76% yield).
11.18.1. Compound 23a
1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 5.90 (dd, 2.3, 10.2, 1H), 5.73 (dd, 2.7, 10.3, 1H), 4.65 (m, 1H), 4.49 (q, 7.0, 1H), 3.96 (dd, 8.0, 12.0, 1H), 3.82 (d, 12.0, 1H), 2.65 (d, 4.5, 1H), 1.66 (d, 7.0, 3H), 1.55 (s, 3H); 13C NMR (125 MHz) δ: 170.1, 130.7, 128.8, 81.6, 75.6, 75.1, 67.5, 54.2, 24.2, 18.6; IR (thin film) 3435, 2986, 2938, 2872, 1744, 1275, 1227, 1047 cm−1; HRMS (ESI) calcd for C10H13BrO4Na+: 298.9889, found: 298.9894.
11.19. Scaffold 24
A suspension of anhydrous CeCl3 (0.010 g, 0.046 mmol) and MeOH (1 mL) was cooled to −78 °C. Dienone 5a (0.01 g, 0.037 mmol) was added, followed by NaBH4 (0.0018 g, 0.048 mmol). The temperature was maintained at −78 °C and additional NaBH4 (0.0018 g, 0.048 mmol) was added every hour until the reaction was complete by TLC. The reaction was normally completed within 2–3 h. The reaction was quenched with satd aq NH4Cl, and diluted in EtOAc. The aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude material was purified by flash chromatography (30% EtOAc–hexanes) to give 24a as colorless oil (0.009 g, 85% yield).
11.19.1. Compound 24a
1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 6.82 (d, 9.9, 1H), 6.25 (d, 9.9, 1H), 5.19 (m, 1H), 4.01 (q, 6.5, 1H), 2.96 (s, 1H), 1.71 (s, 3H), 1.59 (d, 6.5, 3H); 13C NMR (125 MHz. CDCl3) δ: 180.6, 168.9, 147.0, 125.9, 105.5, 92.7, 80.0, 73.6, 27.2, 17.1; IR (thin film) 3398, 2986, 2935, 1659, 1591, 1027, 1055; HRMS (ESI) calcd for C10H11BrO4Na+: 296.9732, found: 296.9737.
11.20. Scaffold 25
A solution of BHT (1.88 g, 8.54 mmol) in toluene (15 mL) was cooled to 0 °C and AlMe3 (25% in hexanes, 1.85 mL, 4.26 mmol) was added. The resulting mixture of MAD was stirred at 0 °C to rt for 1 h, then cooled to −78 °C. In a separate flask, dienone 5a was dissolved in toluene (5 mL), and the solution was added via cannula to the MAD at −78 °C, immediately turning deep purple. The reaction was stirred for 5 min, then L-Selectride (1 M in THF, 2.1 mL, 2.1 mmol) was added and the solution was stirred for another 5 min at −78 °C. The reaction mixture was quenched with 1 M Rochelle’s salt (10 mL), diluted with EtOAc (20 mL), and quickly warmed by hand to rt. It was stirred vigorously for 1 h. The aqueous layer was extracted with EtOAc four times and the combined organic layers were washed with brine (5 mL), dried (Na2SO4), and concentrated. The crude material was purified by flash chromatography (100% hexanes to 30% EtOAc–hexanes) to give 25a as white solid (0.410 g, 1.49 mmol, 77% yield).
11.20.1. Compound 25a
1H NMR δ (multiplicity, J (Hz), integration): 7.08 (d, 10.2, 1H), 6.17 (d, J=10.0 Hz, 1H), 4.63 (q, 7.0, 1H), 4.50 (d, 11.9, 1H), 4.13 (d, 11.9, 1H), 1.69 (d, 7.0, 3H), 1.66 (s, 3H); 13C NMR δ: 188.7, 168.9, 149.6, 127.6, 80.8, 79.8, 74.6, 50.4, 22.5, 18.4; IR (thin film) 2923, 1747, 1693, 1271, 1225, 1182, 1070 cm−1; HRMS (EI) calcd for C10H11BrO4Na+: 296.9732, found: 296.9737.
11.20.2. Compound 25b
Yield 85%. 1H NMR δ (multiplicity, J (Hz), integration): 7.10 (d, 10.1, 1H), 6.18 (dd, 10.1, 0.8, 1H), 4.60 (q, 6.6, 1H), 4.58 (d, 11.8, 1H), 4.14 (d, 11.8, 1H), 2.13 (t, 9.8, 3H), 1.66 (d, 7.1, 3H), 1.4–1.2 (m, 11H), 0.87 (t, 6.9, 3H). 13C NMR δ: 188.6, 169.1, 149.6, 127.9, 83.0, 80.5, 74.5, 50.0, 37.2, 31.8, 30.1, 29.1, 22.8, 22.7, 18.4, 14.2.
11.20.3. Compound 25e
See Ref. 4.
11.20.4. Compound 25f
Yield 62%. 1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 6.96 (d, 10.1, 1H), 6.01 (d, 10.1, 1H), 4.52 (q, 7.0, 1H), 3.75 (d, 12.1, 1H), 2.41 (dq, 12.5, 6.4, 1H), 1.64 (d, 7.0, 3H), 1.63 (s, 3H), 1.31 (d, 6.7, 3H); 13C NMR (125 MHz) δ: 197.8, 169.9, 148.7, 128.8, 81.0, 79.9, 74.3, 44.6, 22.1, 18.5, 11.1.
11.21. Scaffold 26
To a solution of bromide 25a (0.230 g, 0.842 mmol) in CH3CN (15 mL) was added a solution of NaI (0.379 g, 2.53 mmol) in CH3CN (5 mL), followed by TMSCl (0.215 mL, 1.68 mmol). The resulting orange solution was stirred at rt for 3 h then quenched with saturated aq Na2S2O3. The colorless solution was diluted with EtOAc, and the aqueous layer was extracted with EtOAc three times. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude material was purified by flash chromatography (30% EtOAc–hexanes) to afford 26a as a colorless oil (0.162 g, 98% yield).
11.21.1. Compound 26a
1H NMR δ (multiplicity, J (Hz), integration): 7.01 (d, 10.0, 1H), 6.02 (d, 10.0, 1H), 4.54 (q, 7.0, 1H), 4.17 (dd, 5.6, 13.1, 1H), 2.91 (dd, 5.6, 17.5, 1H), 2.49 (dd, 13.1, 17.5, 1H), 1.61 (m, 6H); 13C NMR δ 149.6, 129.3, 80.8, 74.6, 40.3, 21.7, 18.5; IR (thin film) 2981, 2934, 1747, 1688, 1273, 1111; HRMS (CI) calcd for : 197.0813, found: 197.0810.
11.21.2. Compound 26b
Yield 98%. 1H NMR (500 MHz) δ (multiplicity, J (Hz), integration): 7.03 (d, 10.1, 1H), 6.03 (dd, 10.1, 0.9, 1H), 4.52 (q, 7.1, 1H), 4.20 (dd, 12.9, 5.7, 1H), 2.89 (ddd, 17.5, 5.7, 0.9, 1H), 2.58 (dd, 17.5, 12.9, 1H), 2.03 (t, 12.3, 1H), 1.76 (t, 13.2, 1H), 1.61 (d, 6.9, 3H), 1.40–1.20 (m, 10H), 0.88 (t, 6.8, 3H); 13C NMR (125 MHz. CDCl3) δ: 195.1, 169.9, 149.6, 129.6, 82.8, 75.2, 73.9, 39.9, 36.3, 31.9, 30.2, 29.2, 22.9, 22.8, 18.5, 14.3.
11.22. Scaffold 27
A solution of N,N-dimethylhydrazine (0.021 mL, 0.27 mmol) in CH2Cl2 (1.0 mL) was cooled to 0 °C and AlMe3 (25% in hexanes, 0.110 mL, 0.253 mmol) was added dropwise. The solution was stirred and allowed to slowly warm to rt for 1 h. The solution was cooled to 0 °C, and to it was added the vinylogous ester 20a (0.023 g, 0.084 mmol) as a solution in CH2Cl2 (0.5 mL). The reaction was stirred from 0 °C to rt for 4 h. The reaction was quenched with 1 M aq Rochelle’s salt, diluted with EtOAc and stirred vigorously for 1 h. The aqueous layer was extracted four times with EtOAc, and the combined organic layers were washed with brine, dried (Na2SO4), and concentrated. To facilitate isolation, the product was derivatized as the silyl ether.
The crude product was dissolved in CH2Cl2 (1.5 mL) and Et3N (0.105 mL, 0.753 mmol) was added, followed by TBSOTf (0.115 mL, 0.501 mmol). Stirring was continued at rt for 40 min, then the reaction was quenched with satd aq NaHCO3 and diluted in CH2Cl2. The aqueous layer was extracted four times with CH2Cl2 and the combined organics were washed with brine, dried (Na2SO4), and concentrated. The residue was purified by flash chromatography (20% EtOAc–hexanes) to afford 27a as a colorless oil (0.020 g, 64% yield over two steps).
11.22.1. Compound 27a
1H NMR δ (multiplicity, J (Hz), integration): 5.70 (s, 1H), 2.66–2.57 (m, 1H), 2.32 (dt, 17.5, 4.9, 1H), 1.81 (m, 7H), 1.49–1.43 (m, 1H), 1.39 (s, 3H), 1.00 (s, 9H), 0.24 (s, 3H), 0.30 (s, 3H); 13C NMR δ: 187.9, 159.5, 92.4, 75.9, 48.1, 36.8, 26.5, 25.7, 24.4, 19.0, −2.1, −3.1; IR (thin film) 2926, 2971, 2854, 1581, 1387 cm−1; HRMS (ESI) calcd for C15H29BrN2O2Si+: 399.1073, found: 399.1090.
11.23. Scaffold 28
To a solution of 26a (0.098 g, 0.499 mmol) in toluene (7 mL) cooled to −78 °C was added DIBAL (1 M in hexanes, 2.50 mL, 2.50 mmol) and the solution was stirred for 1 h. The reaction mixture was quenched with 1 M aq Rochelle’s salt, diluted with EtOAc, quickly warmed to rt and stirred vigorously for 45 min. The aqueous layer was extracted with EtOAc four times. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude reaction material was sufficiently pure for subsequent chemistry.
To a solution of the above crude product in CH2Cl2 (10 mL) was added active precipitated MnO2 (1.063 g, 12.2 mmol) and the solution was stirred at rt for 16 h. The reaction mixture was filtered through Celite and concentrated. The crude material was sufficiently pure for subsequent chemistry.
To a solution of the crude reaction material in CH2Cl2 (5 mL) was added H2NNMe2 (0.025 mL, 0.549 mmol). The solution was stirred at rt for 4 h, and concentrated under reduced pressure to afford the hydrazone, which was taken on crude in the following sequence.
The hydrazone (0.064 g, 0.566 mmol) was dissolved in CHCl3 (5 mL). TFA (five drops) was added and the reaction was stirred at rt for 9 h. It was concentrated and the residue was purified by chromatography (5% MeOH–CH2Cl2) to provide 28a as a colorless oil (0.036 g, 51% yield over four steps).
11.23.1. Compound 28a
1H NMR δ (multiplicity, J (Hz), integration): 6.85 (d, 10.1, 1H), 5.94 (d, 10.2, 1H), 4.14 (dd, 5.1, 12.1, 1H), 2.83 (dd, 5.1, 17.0, 1H), 2.45 (dd, 12.1, 16.9, 1H), 1.9 (br s, 2H), 1.43 (s, 3H); 13C NMR δ: 197.5, 154.6, 128.4, 73.8, 73.4, 43.3, 20.7; IR (thin film) 3409, 2954, 2921, 1666, 1047 cm−1; HRMS calcd for : 143.0708, found: 143.0705.
11.24. Scaffold 29
To a solution of 26b (57.9 mg, 0.207 mmol) in degassed benzene (4 mL, 0.05 M) was added triethylsilane (0.33 mL, 2.07 mmol) and RhCl(PPh3)3 (19.0 mg, 0.0207 mmol) and the solution was stirred for 2 h. The solvent was removed by evaporation under a gentle flow of N2 and the residue was taken up in hexanes. The catalyst was removed by filtration through a cotton pad and the solution was concentrated under reduced pressure. The crude oil was dissolved in CH2Cl2 (0.5 mL) and cooled to 0 °C. A solution of DMDO (0.04 M in acetone, 3 mL, 0.62 mmol) was added and the reaction was stirred at 0 °C for 1 h. The reaction mixture was dried (Na2SO4) and concentrated. The residue was purified by alumina chromatography (5% EtOAc–pet. ether) to afford of 29b (0.0598 g, 70% yield).
11.24.1. Compound 29b
1H NMR δ (multiplicity, J (Hz), integration): 4.28 (q, 6.9, 1H), 3.62 (dd, 5.8, 11.1, 1H), 3.14 (dd, 1.1, 5.5, 1H), 2.59 (dd, 1H, 5.8, 14.1, 1H), 2.46 (dd, 1H, 5.7, 15.4, 1H), 2.08 (dd, 11.1, 14.1, 1H), 1.93 (t, 9.5, 3H), 1.79 (dd, 1.8, 15.4, 1H), 1.4–1.2 (m, 18H), 0.96 (m, 9H), 0.89 (t, 7.1, 3H), 0.63 (m, 6H).
11.25. Scaffold 30
To a solution of enone 26a (0.0050 g, 0.025 mmol) in toluene (1.0 mL) at 0 °C was added Et2Zn (1 M in hexanes, 0.052 mL, 0.052 mmol) followed by CH2I2 (0.0045 mL, 0.050 mmol). The reaction was stirred at 0 °C to rt for 40 h. Pyridine (0.050 mL) was added and the solution was stirred for 10 min, then diluted with EtOAc and worked up with H2O. The aqueous layer was extracted four times with EtOAc. The combined organic layers were washed with brine, dried (Na2SO4), and concentrated. The crude product was purified by chromatography (30% EtOAc–hexanes) to afford 30a as a colorless oil (0.0038 g, 71% yield).
11.25.1. Compound 30a
1H NMR δ (multiplicity, J (Hz), integration): 4.46 (q, 7.0, 1H), 4.44 (dd, 10.8, 7.2, 1H), 3.77 (d, 4.1, 1H), 3.49 (d, 4.2, 1H), 2.90 (dd, 19.4, 7.2, 3H), 2.29 (dd, 19.3, 10.8, 3H), 1.58 (d, 7.0, 3H), 1.29 (m, 2H).
11.26. Scaffold 31
Freshly prepared homoallylic magnesium bromide (0.153 mmol) in THF (0.5 mL) was added to CuBr · Me2S (0.12 g, 0.058 mmol), instantly forming a dark purple solution. HMPA (0.054 mL, 0.31 mmol) was added to the cuprate and the solution was cooled to −78 °C. In a separate flask, enone 26a (0.0080 g, 0.041 mmol) was dissolved in THF (0.5 mL) and TMSCl (0.032 mL, 0.25 mmol) was added. The enone solution was added via a cannula to the cuprate solution and the reaction was stirred at −78 °C for 1.5 h. Et3N (0.035 mL, 0.25 mmol) was added to the solution, and it was warmed to rt, diluted with Et2O, and worked up with H2O. The organic layer washed twice with water, once with brine, dried (Na2SO4), and concentrated. It was purified by chromatography (20% EtOAc–hexanes) to afford 31a (0.0080 g, 89% yield).
11.26.1. Compound 31a
1H NMR δ (multiplicity, J (Hz), integration): 5.73 (m, 1H), 5.01 (m, 2H), 4.43 (q, 7.0, 1H), 3.99 (dd, 5.8, 12.6, 1H), 2.79 (dd, 5.8, 15.4, 1H), 2.54 (d, 4.3, 2H), 2.46 (dd, 12.5, 15.4, 1H), 2.16 (m, 2H), 1.96 (m, 2H), 1.68 (s, 3H), 1.60 (d, 7.0, 3H).
11.27. Scaffold 32
To a solution of 25a (0.0740 g, 0.269 mmol) in CH2Cl2 (4.0 mL) at 0 °C was added a solution of Br2 (0.043 g, 0.269 mmol) in CH2Cl2 (1.0 mL) dropwise over 1 h using a syringe pump. The solution was stirred for an additional 1 h and allowed to gradually warm to rt, as the solution turned from light orange to colorless. The solution was re-cooled to 0 °C and Et3N was added (0.056 mL, 0.40 mmol). The reaction was stirred for an additional 30 min and quenched with 1 M aq HCl. The aqueous layer was extracted with CH2Cl2 four times. The combined organic layers were dried (Na2SO4) and concentrated. The crude product was purified by chromatography (10% EtOAc–hexanes) to afford 32a (0.053 g, 56% yield).
11.27.1. Compound 32a
1H NMR δ (multiplicity, J (Hz), integration): 7.52 (s, 1H), 4.64 (q, 7.0, 1H), 4.58 (d, 12.0, 1H), 4.16 (d, 12.0, 1H), 1.68 (m, 6H).
11.28. Scaffold 33
To a solution of enone 25a (0.0050 g, 0.018 mmol) and ethyl 2-((tributylstannyl)methyl)acrylate (0.015 g, 0.037 mmol) in benzene (1.0 mL) was added AIBN (0.00060 g, 3.6 µmol) and the mixture was heated to 80 °C for 12 h. The solvent was removed under reduced pressure and the residue was purified by chromatography (30% EtOAc–hexanes) to afford 34a (0.0045 g, 81% yield).
11.28.1. Compound 33a
1H NMR δ (multiplicity, J (Hz), integration): 6.96 (d, 10.2, 1H), 6.20 (d, 1.1, 1H), 6.00 (d, 10.2, 1H), 5.64 (d, 1.1, 1H), 4.36 (q, 7.0, 1H), 4.22 (m, 2H), 3.90 (d, 11.6, 1H), 3.01 (m, 1H), 2.74 (m, 2H), 1.61 (m, 6H), 1.32 (t, 7.2, 3H).
11.29. Scaffold 34
Compound 21a (0.00236 g, 0.0119 mmol) was dissolved in a solution of CH2N2 (0.27 M in ether, 1 mL, 0.27 mmol). The mixture was stirred at 0 °C to rt for 4 h, or until the yellow solution turned colorless. Additional CH2N2 solution (1 mL) was added and the process was repeated twice. After the third portion of CH2N2 had turned colorless, the solution was concentrated and the residue was purified by chromatography to afford 34a as a colorless oil (0.00131 g, 52% yield).
11.29.1. Compound 34a
1H NMR δ (multiplicity, J (Hz), integration): 4.45 (q, 7.0, 1H), 3.90 (dd, 12.4, 4.8, 1H), 2.71 (m, 2H), 1.96 (m, 4H), 1.56 (m, 5H), 1.46 (s, 3H); 13C NMR (125 MHz) δ: 170.8, 82.6, 76.1, 73.9, 56.9, 52.6, 35.7, 33.0, 29.8, 18.7, 18.5.
11.30. Scaffold 35
To a solution of 21a (0.00570 g, 0.0289 mmol) in CH2Cl2 (1.0 mL) was added m-CPBA (0.0200 g, 0.111 mmol) followed by NaHCO3 (0.0045 g, 0.054 mmol). The solution was stirred at rt for 2 days. Additional m-CPBA (0.030 g, 0.174 mmol) and NaHCO3 (0.0060 g, 0.072 mmol) were added and stirring was continued for 24 h. The reaction was quenched with satd aq Na2S2O3 and diluted with CH2Cl2. The organic layer was washed with aq NaHCO3 and the aqueous layer was extracted with CH2Cl2 four times. The combined organic layers were dried (Na2SO4) and concentrated. The crude material was purified by chromatography (40% EtOAc–hexanes) to afford 35a (0.00401 g, 65% yield).
11.30.1. Compound 35a
1H NMR δ (multiplicity, J (Hz), integration): 4.47 (q, 7.0, 1H), 4.35 (ddd, 2.6, 5.1, 13.9, 1H), 4.18 (dd, 10.7, 13.8, 1H), 3.83 (5.1, 8.6, 1H), 2.85 (m, 2H), 2.17 (m, 2H), 1.58 (m, 6H); 13C NMR δ: 170.2, 169.4, 83.5, 77.4, 73.9, 63.9, 38.8, 36.7, 19.1, 18.3; IR (thin film): 2923, 1734, 1286, 1258, 1143, 1086 cm−1; HRMS (EI): calcd for : 215.0919, found: 215.0914.
Acknowledgements
The National Institutes of Health (R01-GM064831-09) is acknowledged for their support of oxidative dearomatization chemistry and their role in society is and appreciated.
References and notes
- 1.(a) Zhu J, Grigoriadis NP, Lee JP, Porco JA., Jr J. Am. Chem. Soc. 2005;127:9342. doi: 10.1021/ja052049g. [DOI] [PubMed] [Google Scholar]; (b) Dong S, Zhu J, Porco JA., Jr J. Am. Chem. Soc. 2008;130:2738. doi: 10.1021/ja711018z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dohi T, Maruyama A, Takenaga N, Senami K, Minamitsuju Y, Fujioka H, Caemmerer SB, Kita Y. Angew. Chem., Int. Ed. 2008;47:3787. doi: 10.1002/anie.200800464. [DOI] [PubMed] [Google Scholar]; (d) Quideau S, Lyvinec G, Marguerit M, Bathany K, Ozanne-Beaudenon A, Buffeteau T, Cavagnat D, Chénedé A. Angew. Chem., Int. Ed. 2009;48:4605. doi: 10.1002/anie.200901039. [DOI] [PubMed] [Google Scholar]; (e) Uyanik M, Yasui T, Ishihara K. Angew. Chem., Int. Ed. 2010;49:2175. doi: 10.1002/anie.200907352. [DOI] [PubMed] [Google Scholar]
- 2.Pettus LH, Van De Water RW, Pettus TRR. Org. Lett. 2001;6:905. doi: 10.1021/ol0155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mejorado LH, Pettus TRR. J. Am. Chem. Soc. 2006;128:15625. doi: 10.1021/ja062987w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenderski TA, Huang S, Pettus TRR. J. Org. Chem. 2009;74:4104. doi: 10.1021/jo900401k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoarau C, Pettus TRR. Org. Lett. 2006;8:2843. doi: 10.1021/ol061000s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Mejorado LH, Hoarau C, Pettus TRR. Org. Lett. 2004;6:1535. doi: 10.1021/ol0498592. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cha JY, Huang Y, Pettus TRR. Angew. Chem., Int. Ed. 2009;48:9519. doi: 10.1002/anie.200904716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magdziak D, Meek S, Pettus TRR. Chem. Rev. 2004;104:1383. doi: 10.1021/cr0306900. [DOI] [PubMed] [Google Scholar]
- 8.(a) Kita Y, Takada T, Tohma H. Pure Appl. Chem. 1996;68:627. [Google Scholar]; (b) Varvoglis A. Synthesis. 1984:709. [Google Scholar]; (c) Moriarty RM, Prakash O. Acc. Chem. Res. 1986;19:244. [Google Scholar]
- 9.(a) Takada T, Tajima R, Ando W. J Org. Chem. 1983;48:4764. [Google Scholar]; (b) Fox AR, Pausacker KH. J. Chem. Soc. 1957:295. [Google Scholar]
- 10.Tamura Y, Takura T, Haruta J, Kita Y. J. Org. Chem. 1987;52:3927. [Google Scholar]
- 11.Braun NA, Ciufolini MA, Peters K, Peters E-M. Tetrahedron Lett. 1998;39:4667. [Google Scholar]
- 12.(a) Van De Water RW, Magdziak DJ, Chau JN, Pettus TRR. J. Am. Chem. Soc. 2000;122:6502. [Google Scholar]; (b) Jones RM, Van De Water RW, Lindsey CC, Pettus TRR. J. Org. Chem. 2001;66:3435. doi: 10.1021/jo001752e. [DOI] [PubMed] [Google Scholar]; (c) Hoarau C, Pettus TRR. Synlett. 2003:127. doi: 10.1055/s-2003-36234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Patterson I, Wallace DJ, Cowden CJ. Synthesis. 1998:639. [Google Scholar]; (b) Less SL, Leadlay PF, Dutton CJ, Staunton J. Tetrahedron Lett. 1996;37:3519. [Google Scholar]
- 14.Van De Water RW, Hoarau C, Pettus TRR. Tetrahedron Lett. 2003;44:5109. [Google Scholar]
- 15.Hoarau C. Ph.D. Dissertation. Santa Barbara, CA: University of California; 2005. [Google Scholar]
- 16.For an example, see: Wipf P, Kim Y, Jahn H. Synthesis. 1995:1549.
- 17.Mejorado LH. Ph.D. Dissertation. Santa Barbara, CA: University of California; 2006. [Google Scholar]
- 18.Rosenstock B, Gais H-J, Herrmann E, Raabe G, Binger P, Freud A, Wedemann P, Krüger C, Lindner HJ. Eur. J. Chem. 1998:257. [Google Scholar]
- 19.(a) Durst T, Kozma EC, Charlton JL. J. Org. Chem. 1985;50:4829. [Google Scholar]; (b) Wang J, Pettus TRR. Tetrahedron Lett. 2004;45:5895. [Google Scholar]
- 20.Bohlmann F, Suding H. Liebigs Ann. Chem. 1985:160. [Google Scholar]
- 21.Hamersma JW, Snyder EI. J. Org. Chem. 1965;30:3985. [Google Scholar]
- 22.Doty BJ, Morrow GW. Tetrahedron Lett. 1990;43:6125. [Google Scholar]