Abstract
The hydrothermal vents on the East Scotia Ridge are the first to be explored in the Antarctic and are dominated by large peltospiroid gastropods, stalked barnacles (Vulcanolepas sp.) and anomuran crabs (Kiwa sp.) but their food webs are unknown. Vent fluid and macroconsumer samples were collected at three vent sites (E2, E9N and E9S) at distances of tens of metres to hundreds of kilometres apart with contrasting vent fluid chemistries to describe trophic interactions and identify potential carbon fixation pathways using stable isotopes. δ13C of dissolved inorganic carbon from vent fluids ranged from −4.6‰ to 0.8‰ at E2 and from −4.4‰ to 1.5‰ at E9. The lowest macroconsumer δ13C was observed in peltospiroid gastropods (−30.0‰ to −31.1‰) and indicated carbon fixation via the Calvin-Benson-Bassham (CBB) cycle by endosymbiotic gamma-Proteobacteria. Highest δ13C occurred in Kiwa sp. (−19.0‰ to −10.5‰), similar to that of the epibionts sampled from their ventral setae. Kiwa sp. δ13C differed among sites, which were attributed to spatial differences in the epibiont community and the relative contribution of carbon fixed via the reductive tricarboxylic acid (rTCA) and CBB cycles assimilated by Kiwa sp. Site differences in carbon fixation pathways were traced into higher trophic levels e.g. a stichasterid asteroid that predates on Kiwa sp. Sponges and anemones at the periphery of E2 assimilated a proportion of epipelagic photosynthetic primary production but this was not observed at E9N. Differences in the δ13C and δ34S values of vent macroconsumers between E2 and E9 sites suggest the relative contributions of photosynthetic and chemoautotrophic carbon fixation (rTCA v CBB) entering the hydrothermal vent food webs vary between the sites.
Introduction
Deep-sea hydrothermal vents are chemically reducing habitats occurring on mid-ocean and back-arc spreading centres, seamounts, volcanic hotspots and off-axis ridge settings [1], [2], [3]. They are distinct from the surrounding deep sea with respect to environmental conditions, the energy sources sustaining life and their biological communities [4], [5]. High densities of organisms are found to thrive at the interface where hot, mineral-rich fluids discharge from the seafloor and mix with colder, oxygenated seawater. The hot fluids emitted from the seafloor may differ in pH and are enriched in reduced gases (e.g. H2S, CH4, H2) and metals (e.g. Fe2+, Cu, Mn) relative to seawater [6]. Microorganisms oxidise the reduced species in vent fluids and utilise the energy released to fix CO2 or other single carbon compounds (e.g. CO, CH4) into cellular material [7]. This results in microbial chemosynthesis replacing photosynthetic primary production at the base of the food chain [7].
Sulfide oxidation appears to be the principal energy acquisition pathway, which microorganisms use to drive carbon fixation [3], [7], [8]. The most important carbon fixation pathways at the base of the metazoan hydrothermal vent food webs are the Calvin-Benson-Bassham (CBB) and reductive tricarboxylic acid (rTCA) cycles [9], [10], [11]. Methane oxidation (methanotrophy) is a further carbon fixation process at hydrothermal vents with CH4 of thermogenic, biogenic or magmatic origin available depending on the host substrate [3], [12]. Epipelagic photosynthetic primary production may also provide some nutrition to vent macroconsumers, although the relative contribution to vent fauna is thought to be negligible [12], [13]. Macroconsumers utilise the vent organic carbon through endo- and episymbiotic relationships, consumption of free-living microorganisms either from various surfaces or the water column and indirectly through predation and scavenging [14], [15], [16].
The relative contributions of different carbon sources and complexity of hydrothermal vent food webs vary globally depending on the species present, the geological host substrate and the vent fluid chemistry [17], [18], [19]. The first Antarctic hydrothermal vent communities were discovered recently on the East Scotia Ridge (ESR), a back-arc spreading centre in the Atlantic sector of the Southern Ocean [20], [21]. The two basalt-hosted vent fields occur on the ridge segments E2 and E9, which lack the characteristic alvinocarid shrimps, bathymodiolid mussels and siboglinid worms found at Atlantic, Indian and Pacific hydrothermal vents, respectively [21]. Instead, biomass at the ESR vents is dominated by anomuran crabs (Kiwa sp.), stalked barnacles (Vulcanolepas sp.) and large peltospiroid gastropods [22], indicating a new biogeographic province [21]. Furthermore, there are differences in the end-member vent fluid chemistry between the E2 and E9 vent fields as well as within field between northern (E9N) and southern (E9S) areas of E9 [21].
Stable isotopes of carbon (13C/12C expressed as δ13C), nitrogen (15N/14N expressed as δ15N) and sulfur (34S/32S expressed as δ34S) have been used to examine hydrothermal vent community trophodynamics [23], [24]. δ13C can be used to characterise the various carbon sources utilised by vent macroconsumers [25]. This is done by comparing the expected carbon fractionation between dissolved inorganic carbon (DIC) and the macroconsumer’s tissue. Enzymatic reactions catalysed by the ribulose-1,5-biphosphate carboxylase/oxygenase form I (RuBisCO form I) of the CBB cycle (22‰ to 30‰: [26], [27], [28]) exhibit greater fractionation than those of the rTCA cycle (2‰ to 14‰: [29], [30], [31]). Once organic material is incorporated into the macroconsumer food web, carbon trophic discrimination (Δ13C) is small, ranging from 0 to 1.5‰ between the food source and consumer [32]. δ34S also identifies energy sources (sulfur trophic discrimination, −1‰ to 2‰: [32]). The large difference in δ34S between seawater sulphate and sulfides at hydrothermal vents [33] results in organic matter of photosynthetic (∼16‰ to 19‰) and chemosynthetic (−9‰ to 10‰) origin having distinctive δ34S values [34], [35]. The greater trophic discrimination (2‰ to 5‰) in δ15N between consumer and food source provides information on the trophic position of an organism relative to a primary consumer [32]. Therefore, the isotopic value of a vent macroconsumer is the product of the following factors: (1) the inorganic substrate and its isotopic value used by the chemoautotroph; (2) the isotopic discrimination processes occurring during metabolic reactions involving inorganic substrates to create organic compounds (e.g. CBB or rTCA cycles) by the chemoautotroph; (3) food source-macroconsumer trophic interactions (e.g. endosymbiont-host, predator-prey) that occur as a function of (1) and (2); and (4) the physiology associated with the macroconsumer’s isotopic trophic discrimination.
The goal of the present research was to investigate intra- and inter-site patterns in the trophic assemblages of macroconsumers occurring at hydrothermal vents on the ESR using δ13C, δ34S and δ15N. Specifically, the aims were to: (1) compare δ13CDIC among vent sites and thus establish difference in the isotopic inorganic substrates used by chemoautotrophs; (2) compare δ13C, δ15N and δ34S between vent and benthic non-vent fauna to assess any photosynthetic inputs into the hydrothermal vent food web; (3) investigate differences in trophic structures among the three sites; and (4) assess which species are driving any differences in trophic structure. The investigation provides a unique opportunity to examine differences in trophic structure at the scale of tens of metres to 100s of kilometres in a newly discovered hydrothermal vent biogeographical province.
Materials and Methods
Ethics Statement
Permits for the fieldwork were granted by the United Kingdom Foreign and Commonwealth Office. This study met the ethical requirements of the affiliated research institutions for research utilising animal tissues. No animal husbandry or laboratory controlled experiments were part of the research that required permits from the UK Home Office. The fish were collected at a water depth of 2500 m, which meant that they were dead when they arrived on deck as a result of changes in pressure. This was the case with the majority of the animals dissected within this study. The research also adhered to the Inter Ridge code of conduct for sampling hydrothermal vents (http://www.interridge.org/IRStatement).
Study Sites
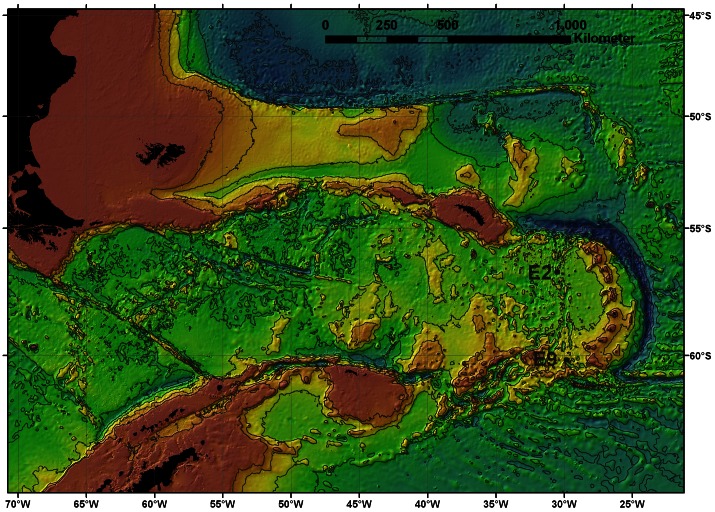
The E2 and E9 vent fields are situated approximately 440 km apart at 56° 05.35′S, 30° 19.20′W and 60° 02.50′ S, 29° 58.93′ W, respectively (Fig. 1). E2 is at a depth of ∼2600 m and seafloor topography is complex with a series of terraced features and lobed pillow basalts filling a major north-south steep-sided fissure [21]. The main high-temperature and diffusive venting occurred at an intersection between this fissure and an east-west running fault or scarp [21]. E9 was located at ∼2400 m depth and its topography was relatively flat with sheet lava, a series of lava drain back features and collapsed pillow basalts. A series of north-south fissures were found with venting mainly occurring on the most western [21], [22]. The end-member fluid chemistry exiting chimneys differed between the northern and southern sections of E9 [21], therefore E9N and E9S are here considered to be separate sites. Ambient seabed water temperatures were 0.0°C at E2 and between −0.1°C and −1.3°C at E9 [21].
Figure 1. Bathymetric map illustrating positions of the E2 and E9 vent sites (black circles).
The vent sites are located at the northern and southern ends of the East Scotia Ridge (ESR), located in the Atlantic sector of the Southern Ocean. The map shows the position of the ESR in relation to South America and the Antarctic Peninsula.
Sample Collection and Ship-board Processing
Samples were collected onboard the R.R.S James Cook during the 2010 austral summer (7 January to 21 February) using the remotely operated vehicle (ROV) Isis. High temperature and diffuse flow fluids were collected for DIC using titanium samplers, equipped with an inductively coupled link high temperature sensor. The nozzle of the titanium sampler was inserted into the chimney orifice for high temperature fluid samples and once the temperature reading became stable the fluid was collected. For diffuse flow samples, a circular titanium housing was placed over the area of diffuse venting to minimise the entrainment of seawater. Once the diffuse flow was visible exiting the top of the housing, the titanium sampler was inserted into the opening and the diffuse flow sample was collected once the temperature reading was stable. On board, an aliquot for stable isotope analysis of DIC was sampled to exclude air and poisoned with mercury chloride.
Vent macroconsumers were collected by suction sampler or scoop with species separated into a series of acrylic chambers or perspex boxes to avoid predation or contamination. Six species were collected at all three sites. No female Kiwa sp. were collected from E9N or E9S. Fish and pycnogonids were collected using large collapsible and small metal baited traps deployed from the ROV. Non-vent macroconsumers were collected from metres to tens of metres away from active venting where there were no obvious signs of hydrothermal influence, i.e. no bacterial mat, and where temperature was consistent with local Antarctic bottom water. Non-vent samples were collected on separate dives from those for vent fauna to avoid contamination. Only one non-vent species was collected from E2 and sampling was limited to the areas adjacent to E9N because of ROV operational time constraints. Potential food sources were collected by scraping material from rocks collected by ROV manipulators and epibionts from the ventral setae of the decapod Kiwa sp. Particulate suspended material was collected from the acrylic chambers, which was sampled incidentally during faunal collection. Samples were sorted on board to the lowest possible taxonomic resolution. The majority of the vent species are undescribed to date.
Faunal samples were frozen at −80°C whole or after dissection, depending on their size, for stable isotope analysis. Muscle was removed from the chelipeds of Kiwa sp., foot dissected from Peltospiroidea sp., tube feet removed from the asteroids Stichasteridae sp. and Freyella cf fragilissima and tentacles removed from the anemones. Legs were removed from the pycnogonids Colossendeis cf. concedis and C. cf. elephantis, while Sericosura spp. was sampled whole. The gastropods Provannidae sp. 1 and 2, Lepetodrilus sp., and juvenile Peltospiroidea sp. (<7 mm shell length), and the stalked barnacle Vulcanolepas sp. were removed from their shells and sampled whole. White muscle tissue was dissected from the anterior dorso-lateral region of the zoarcid fish.
Sample Processing Onshore
Each end-member and diffuse flow DIC sample was prepared for isotopic analysis by removing a 1 mL water sample and transferring it into a separate vial. The headspace was flushed with helium, phosphoric acid was injected into the vial and then the contents were vortex mixed. The samples were then left to react for 24 hours to ensure complete conversion of all DIC to CO2 for isotopic analysis. The CO2 was then analysed by continuous-flow isotope ratio mass spectrometry (IRMS) using a Europa Scientific 20–20 IRMS by Iso-Analytical (Crewe, United Kingdom). Samples were run in duplicate and the mean is reported. An internal reference gas (IA-R060, δ13C = −36.08‰ ± SD 0.13) was used to determine the δ13CDIC values and is traceable to the International Atomic Energy Agency standard, NBS-19. Concentrations of CH4 in the water samples were insufficient for isotope analysis.
Faunal tissue samples were freeze dried and ground to a homogenous powder using a pestle and mortar. Aliquots of fauna, particulate suspended material and material scraped from rocks were tested for carbonates prior to analysis with 0.1 N HCl. If the sample effervesced, this indicated carbonates were present and it was subsequently acidified by further addition of HCl until the effervescence ceased. Samples were re-dried at 50°C for 48 hours. If the sample did not effervesce, no acidification was carried out. Aliquots for δ13C analysis were not lipid extracted. Any confounding lipid effects due to metabolic processes would not affect the interpretation of the ultimate carbon sources of the vent fauna described by δ13C because of the large differences in the δ13C values of trophic end-members.
Approximately 0.7 mg of powder was weighed into a tin capsule for carbon and nitrogen IRMS. For sulfur, 2 mg of sample and 4 mg of the catalyst vanadium pentoxide were weighed into each tin capsule. Dual stable carbon and nitrogen isotope ratios were measured by continuous-flow IRMS using a Costech Elemental Analyser interfaced with Thermo Finnigan Delta Plus XP (Natural Environment Research Council, Life Sciences Mass Spectrometry Facility, SUERC, East Kilbride, United Kingdom). Two laboratory standards were analysed for every ten samples in each analytical sequence. These alternated between paired alanine standards, differing in δ13C and δ15N, and an internal laboratory gelatin standard. Sulfur was analysed by Iso-Analytical using a SERCON Elemental Analyser coupled to a Europa Scientific 20–20 IRMS. Laboratory standards of barium sulphate (two sets of differing δ34S) and silver sulfide were used for calibration and drift correction. An internal standard of whale baleen was used for quality control (n = 28, 16.34‰ ± SD 0.21). Stable isotope ratios were expressed in delta (δ) notation as parts per thousand/permil (‰). All internal standards are traceable to the following international standards: v-PDB (Pee Dee Belemnite), AIR (atmospheric nitrogen) and NBS-127 (barium sulphate), IAEA-S-1 (silver sulfide) and IAEA-SO-5 (barium sulphate). An external reference material of freeze dried and ground deep-sea fish white muscle (Antimora rostrata) was also analysed (δ13C, n = 24, −18.94‰ ± SD 0.09; δ15N, n = 24, 13.11‰ ± SD 0.38; δ34S, n = 30, 18.20‰, ± SD 0.59).
Data Analysis
Data were assessed for normality using a Shapiro-Wilk test before statistical tests examining spatial patterns in trophic structure and species stable isotope values. Homogeneity, or otherwise, of variances is ecologically informative, for example in identifying distinct energy sources at the base of the food web [36]. Inter-site differences in trophic structure were examined using a Fligner-Killeen test for homogeneity of variance to assess differences in the spread of the mean stable isotope values of each species. Inter-site differences in species were analysed using a one-way ANOVA followed by Tukey’s honest significant difference (HSD) when variance was homogeneous among sites. Welch’s ANOVA followed by t-tests were used when there was heterogeneity of variance among sites because it uses adjusted degrees of freedom to protect against Type I errors when variances are unequal [37]. A Bonferroni correction (p = 0.05/n) was used for multiple comparisons. When data were not normally distributed, a two sample Wilcoxon test was used. All statistics were preformed in R version 12.13.1 [38].
Results
Dissolved Inorganic Carbon Stable Isotope Values
Mean (± SD) δ13CDIC of high temperature and diffuse flow fluids are summarised in Table 1. δ13CDIC of high temperature samples collected at two E2 locations were −4.7‰ (±0.0) (max temperature 351.0°C) and −2.5‰ (±0.1) (max temperature 323.0°C). At E9N, δ13CDIC from separate orifices of the same chimney structure were −4.6‰ (±0.0) (max temperature 380.2°C) and −4.5‰ (±0.0) (max temperature 357.0°C). No high temperature fluids were collected from E9S for δ13CDIC analysis because the pressure was too high within the titanium samples to safely and accurately collect a representative sample. Diffuse flow samples from amongst Kiwa sp. and anemones, at E2, had δ13CDIC values of 0.8‰ (±0.1) (max temperature 19.9°C) and 0.2‰ (±0.2) (max temperature 3.5°C), respectively. A single diffuse flow sample collected from amongst an aggregation of Kiwa sp. at E9N had a δ13CDIC value of 1.5‰ (±0.1) (max temperature 12.6°C). At E9S, diffuse flow samples from amongst Kiwa sp. had a δ13CDIC value of 0.9‰ (±0.1) (max temperature 19.9°C), while a sample taken from a mixed aggregation of Kiwa sp. and peltospiroid gastropods had δ13CDIC value of 0.1‰ (±0.1) (max temperature 5.0°C).
Table 1. δ13C values of dissolved inorganic carbon (DIC) sampled from high temperature and diffuse flow venting from the E2 and E9 ridge segments of the East Scotia Ridge, Southern Ocean.
| Site | Temperature (°C) | δ13C DIC |
| E2 | 351.0 | −4.7 (0.0) |
| 323.0 | −2.5 (0.1) | |
| 19.9 | 0.8 (0.1) | |
| 3.5 | 0.2 (0.2) | |
| E9N | 380.2 | −4.7 (0.0) |
| 357.0 | −4.7 (0.0) | |
| 12.6 | 1.5 (0.1) | |
| E9S | 19.9 | 0.9 (0.1) |
| 5.0 | 0.1 (0.1) |
Standard deviations are in parentheses.
Comparison between Vent and Benthic Non-vent Macro-consumers at E9N
At E9N, mean δ13C and δ15N values of vent fauna overlapped with non-vent benthic fauna (Welch’s t-test, δ13C DF = 10.59, t = 0.66, p = 0.52; Welch’s t-test, δ15N DF = 10.42, t = −0.30, p = 0.76; Fig. 2, Tables 2 & 3) while mean δ34S values differed between non-vent benthic fauna and vent fauna (Welch’s t-test, DF = 12.56, t = −9.08, p<0.01) (Fig. 3, Tables 2 & 3).
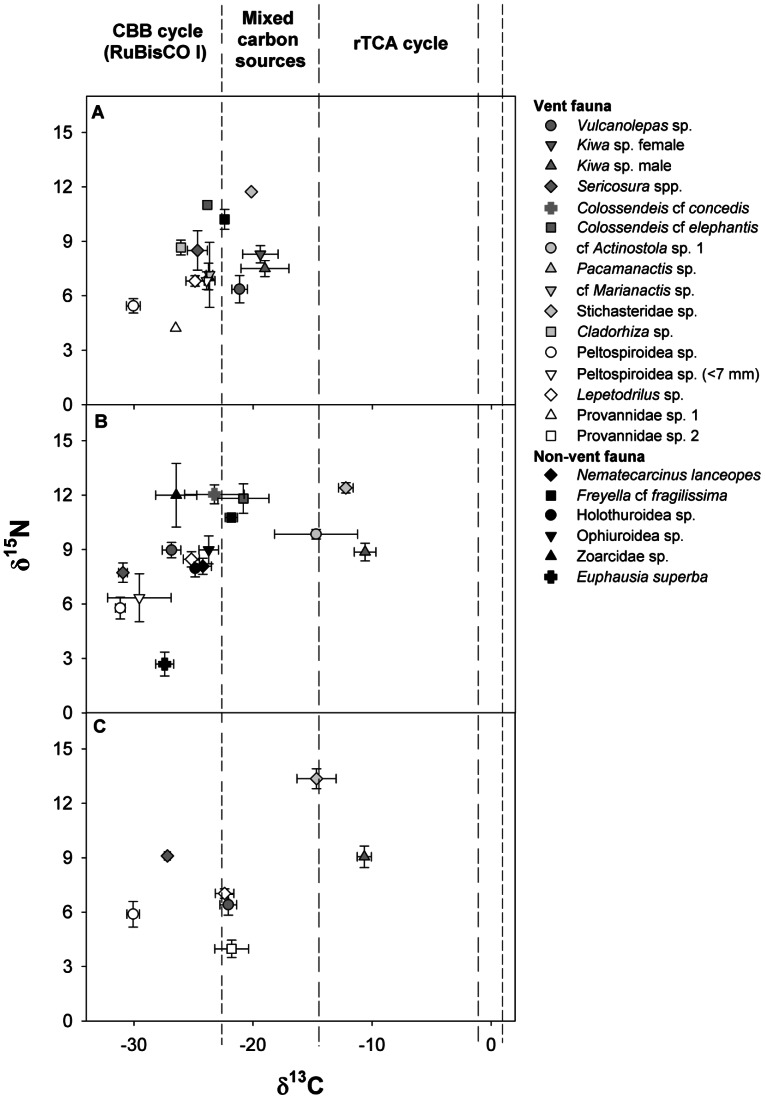
Figure 2. δ13C and δ15N values of macroconsumers collected from the East Scotia Ridge, Southern Ocean.
The values represent means (± standard deviations) for hydrothermal vent and non-vent macroconsumers from the three sample sites: (a) E2, (b) E9N and (c) E9S. Dashed vertical lines represent potential ranges of δ13C values indicative of carbon sources sustaining macroconsumers at the ESR: triple dashed line represents the Calvin-Benson-Bassham (CBB) cycle utilising form I RuBisCO, double dashed line represents the reductive tricarboxylic acid (rTCA) cycle, mixed carbon sources occur between the triple and double dashed line and the continuous dashed line represents the approximate δ13C values of the dissolved inorganic carbon from the diffuse flow areas.
Table 2. Mean δ13C, δ15N and δ34S values (‰) of non-vent deep-sea fauna collected from the E2 and E9 ridge segments of the East Scotia Ridge, Southern Ocean. Standard deviations are in parentheses.
| Taxonomic group | Species | Site | N | δ13C | δ 34S | δ 15N |
| Crustacea | ||||||
| Decapoda | Nematocarcinus lanceopes | E9 | 5 | −24.2 (0.7) | 18.9 (0.5) | 8.1 (0.4) |
| Euphausia superba | E9 | 3 | −27.4 (0.8) | 19.0 (0.1) | 2.7 (0.7) | |
| Echinodermata | ||||||
| Asteroidea | Freyella cf fragilissima | E2 | 3 | −22.4 (0.3) | 18.0 (0.8) | 10.2 (0.6) |
| Freyella cf fragilissima | E9 | 2 | −21.8 (0.5) | 17.5 (0.2) | 10.8 (0.2) | |
| Holothuroidea | Holothuroidea sp. | E9 | 3 | −24.9 (0.0) | 18.3 (0.6) | 8.0 (0.4) |
| Ophiuroidea | Ophiuroidea sp. | E9 | 3 | −23.7 (0.8) | 17.6 (0.9) | 9.0 (0.8) |
| Vertebrata | ||||||
| Osteichthys | Zoarcidae sp. | E9 | 4 | −26.5 (1.7) | 15.7 (0.4) | 12.0 (1.8) |
Table 3. Mean δ13C, δ15N and δ34S values (‰)of hydrothermal vent fauna collected from the E2 and E9 ridge segments of the East Scotia Ridge, Southern Ocean.
| Taxonomic group | E2 | E9N | E9S | |||||||||
| N | δ13C | δ34S | δ15N | N | δ13C | δ34S | δ15N | N | δ13C | δ34S | δ15N | |
| Cirripedia | ||||||||||||
| Vulcanolepas sp. | 22 | −21.1 (0.6) | 8.2 (1.0) | 6.3 (0.7) | 23 | −26.9 (0.8) | 11.0 (0.8) | 9.0 (0.4) | 23 | −22.1 (0.8) | 5.4 (1.1) | 6.4 (0.6) |
| Decapoda | ||||||||||||
| Kiwa sp. female | 20 | −19.4 (1.5) | 3.9 (1.3) | 8.2 (0.5) | 0 | – | – | – | 0 | – | – | – |
| Kiwa sp. Male | 18 | −19.0 (2.0) | 3.0 (1.2) | 7.5 (0.5) | 22 | −10.6 (0.9) | 4.0 (0.7) | 8.9 (0.5) | 30 | −10.7 (0.6) | 2.4 (0.9) | 9.1 (0.6) |
| Pycnogonida | ||||||||||||
| Sericosura spp. | 6 | −24.7 (0.9) | 11.9 (0.4) | 8.5 (1.3) | 9 | −30.9 (0.5) | 6.8 (0.8) | 7.7 (0.5) | 2 | −27.2 (0.3) | 14.9 (0.3) | 9.1 (0.1) |
| Colossendeis cf concedis | 0 | – | – | – | 6 | −23.3 (2.5) | 10.9 (1.4) | 12.1 (0.5) | 0 | – | – | – |
| Colossendeis cf elephantis | 1 | −23.8 | 14.9 | 11.0 | 3 | −20.8 (2.2) | 8.5 (1.6) | 11.8 (0.8) | 0 | – | – | – |
| Anthozoa | ||||||||||||
| cf Actinostola sp. 1 | 0 | – | – | – | 4 | −14.7 (3.5) | 10.3 (0.4) | 9.9 (0.3) | 0 | – | – | – |
| Pacmanactis sp. | 5 | −23.8 (0.2) | 14.9 (0.7) | 7.1 (0.7) | 0 | – | – | – | 0 | – | – | – |
| cf Marianactis sp. | 5 | −23.7 (0.3) | 14.0 (2.4) | 7.2 (1.8) | 0 | – | – | – | 0 | – | – | – |
| Asteroidea | ||||||||||||
| Stichasteridae sp. | 1 | −20.2 | 11.3 | 12.3 | 5 | −12.2 (0.6) | 10.0 (0.9) | 12.4 (0.4) | 5 | −14.7 (1.6) | 11.8 (2.6) | 13.4 (0.6) |
| Gastropoda | ||||||||||||
| Peltospiroidea sp. | 19 | −30.1 (0.6) | 6.0 (0.6) | 5.4 (0.4) | 22 | −31.2 (0.4) | 3.7 (0.5) | 5.8 (0.6) | 15 | −30.1 (0.5) | 4.7 (1.1) | 5.9 (0.7) |
| Peltospiroidea sp (<7 mm) | 4 | −23.9 (0.7) | 7.4 (2.0) | 6.8 (0.5) | 5 | −29.6 (2.7) | 4.2 (0.2) | 6.4 (1.3) | 0 | – | – | – |
| Provannidae sp. 1 | 1 | −26.5 | 8.0 | 4.2 | 0 | – | – | – | 0 | – | – | – |
| P–rovannidae sp. 2 | 0 | – | – | – | 0 | – | – | – | 4 | −21.8 (1.4) | 5.9 (0.8) | 4.0 (0.5) |
| Lepetodrilus sp. | 5 | −24.9 (0.8) | 6.4 (0.5) | 6.8 (0.3) | 4 | −25.2 (0.7) | 3.4 (0.3) | 8.5 (0.4) | 4 | −22.4 (0.8) | 3.6 (0.3) | 7.0 (0.3) |
| Cladorhizidae | ||||||||||||
| Cladorhiza sp. | 5 | −26.1 (0.4) | 14.7 (1.4) | 8.7 (0.4) | 0 | – | – | – | 0 | – | – | – |
| Potential food sources | ||||||||||||
| Particulate suspended material | 3 | −23.2 (5.4) | 10.0 (1.1) | −0.1 (4.9) | 0 | – | – | – | 0 | – | – | – |
| Rock scrapings | 0 | – | – | – | 1 | −23.2 | 0.8 | 2.4 | 1 | −31.1 | – | 1.9 |
| K–iwa n. sp episymbiont | 5 | −18.9 (5.3) | 7.5 (0.3) | 3.3 (1.5) | 0 | – | – | – | 5 | −9.9 (0.3) | 6.6 (0.2) | 5.2 (0.8) |
Standard deviations are in parentheses and - indicates no data.
Figure 3. δ13C and δ34S values of macroconsumers collected from the East Scotia Ridge, Southern Ocean.
The values represent means (± standard deviations) for hydrothermal vent and non-vent macroconsumers from the three sample sites: (a) E2, (b) E9N and (c) E9S. δ34S values between the triple and double dashed lines represent potential areas of isotopic mixing between chemosynthetic and photosynthetic food sources.
Intra- and Inter-site Differences in Community Trophodynamics
Eleven, ten and seven species were collected at E2, E9N and E9S respectively for stable isotope analysis (Table 3). The ranges of mean δ13C values of the vent fauna differed amongst the three sites (Fligner-Killeen test, DF = 2, χ2 = 6.46, p<0.05). E2 had the narrowest δ13C range (−29.9‰ to −19.0‰), whereas at E9N and E9S δ13C ranged from −31.4‰ to −9.9‰ and −30.0‰ to −10.5‰, respectively (Fig. 2). Across the three sites Peltospiroidea sp. had the lowest values while Kiwa sp. had the highest δ13C values (Fig. 2, Table 3), and Lepetodrilus sp., Vulcanolepas sp., Pacmanactis sp. and Colossendeis spp. all had intermediate δ13C values (Fig. 2, Table 3). However, there was no overall difference in mean δ13C values among sites for the combined data across species (Welch’s ANOVA, DF = 2.00, F = 0.59, p = 0.56). The range and mean δ34S values (Fig. 3, Table 3) did not differ among sites (Fligner-Killeen test, DF = 2, χ2 = 0.84, p = 0.65; ANOVA, DF = 2, 26, F = 1.94, p = 0.16), however Kiwa sp. had the lowest δ34S at E2 and E9S while Lepetodrilus sp. had the lowest δ34S values at E9N (Fig. 3, Table 3). The highest vent fauna δ34S values were in Pacmanactis sp. (E2), Vulcanolepas sp. (E9N) and Sericosura spp. (E9S) (Fig. 3, Table 3). Neither the range nor the mean δ15N values differed among sites (Fligner-Killeen test, DF = 2, χ2 = 0.40, p = 0.83; ANOVA, DF = 2, 26, F = 1.19, p = 0.31). The provannid gastropods at E2 and E9S had the lowest δ15N values while Peltospiroidea sp. had the lowest values at E9N (Fig. 2, Table 3). The stichasterid sp. consistently had the highest δ15N values relative to the other vent fauna at each site (Fig. 2, Table 3).
Spatial Differences in Macroconsumer Trophodynamics
Vulcanolepas sp. exhibited spatial differences in δ13C, δ15N and δ34S but there was no consistent pattern in isotopic differences among sites (Table 4). Male and female Kiwa sp. at E2 did not differ in δ13C but males were lower in δ15N and δ34S than females (Table 5). Male Kiwa sp. showed spatial differences in each stable isotope (Table 4). δ13C of the males showed a greater range (Fligner-Killeen test, DF = 2, χ2 = 10.91, p<0.01) and lower values at E2 than E9N and E9S (Table 3 & 4). The epibionts attached to the ventral surface of male Kiwa sp. also exhibited a greater spread of δ13C values at E2 than E9S (F-test, DF = 4, 3, F = 244.46, p<0.01) as well as lower δ13C but higher δ15N values at E2 than E9S (Table 3 & 5). Sericosura spp. δ13C and δ34S values varied amongst sites but δ15N values scarcely did (Table 4). δ13C and δ34S values were lowest at E9N but highest at E2 for δ13C and E9S for δ34S (Table 3). Peltospiroidea sp. showed spatial differences in δ13C and δ34S but not in δ15N (Table 4). δ34S values differed among all sites but E9N δ13C values were lower than those at E9S and E2 (Table 4). Stichasteridae sp. revealed differences between all sites for δ13C and δ15N but for δ34S only between E2 and E9N (Table 5).
Table 4. Results of ANOVA and post-hoc Tukey honest significant differences tests for the differences in stable isotope values of vent fauna among the three sites on the East Scotia Ridge.
| Species | δ13C | δ 34S | δ15N | |||||||||
| DF | F | p | Post-hoc | DF | F | p | Post-hoc | F | DF | p | Post-hoc | |
| Vulcanolepas sp. | 2, 63 | 403.18 | <0.01 | E9N<E9S<E2 | 2, 63 | 176.16 | <0.01 | E9S<E2 = E9S | 2, 63 | 138.26 | <0.01 | E2 = E9S<E9N |
| Kiwa sp. male | 2, 31.36 | 147.29 | <0.01 | E2< E9S = E9N* | 2, 66 | 19.52 | <0.01 | E2 = E9S<E9N | 2, 66 | 52.64 | <0.01 | E2< E9N = E9S |
| Sericosura spp. | 2, 15 | 215.00 | <0.01 | E9N<E9S<E2 | 2, 15 | 100.61 | <0.01 | E9N<E2< E9S | 2, 15 | 3.39 | 0.06 | NA |
| Peltospiroidea sp. | 2, 52 | 29.50 | <0.01 | E9N<E9S = E2 | 2, 52 | 49.26 | <0.01 | E9N<E9S<E2 | 2, 52 | 2.90 | 0.06 | NA |
| Lepetodrilus sp. | 2, 10 | 17.41 | <0.01 | E2 = E9N<E9S | 2, 10 | 31.99 | <0.01 | E9N = E9S<E2 | 2, 10 | 32.10 | <0.01 | E2 = E9S<E9N |
Welch’s ANOVA with post hoc analysis by t-test with Bonferroni correction (p = 0.05/3 = 0.017).
Table 5. Results of t-tests for between-sites differences in stable isotope values of vent fauna at the East Scotia Ridge.
| Species | Comparison | δ13C | δ 34S | δ 15N | ||||||
| DF | t | p | DF | t | p | DF | t | p | ||
| Kiwa sp. | E2 female v male | 36 | −0.50 | 0.62 | 36 | 2.23 | <0.05 | 36 | 5.13 | <0.01 |
| Kiwa sp. Epibionts | E2 v E9S | 4.05 | −3.81 | <0.05* | 6.93 | 4.57 | <0.01* | 2 | na | <0.05Ψ |
| Stichasteridae sp. | E2 v E9N | 4 | 28.92 | <0.01 | 4 | −3.29 | <0.05 | 4 | 5.14 | <0.01 |
| E2 v E9S | 4 | 7.28 | <0.01 | 4 | 0.385 | 0.59 | 4 | 5.94 | <0.01 | |
| E9N v E9S | 8 | 3.64 | <0.01 | 8 | −1.76 | 0.11 | 8 | −3.49 | <0.05 | |
| Colossendeis cf elphantis | E2 v E9N | 2 | 2.45 | 0.13 | 2 | −6.93 | <0.05 | 2 | 1.71 | 0.23 |
Welch’s t-test,
Wilcoxon test.
Discussion
This study described the trophic structure of a new vent biogeographical province recently discovered on the ESR in the Southern Ocean [21]. In addressing this aim, the study shared the challenges of preceding work in characterising energy sources, separating isotopic overlap and mixing of energy sources, and following energy sources into subsequent predator-prey relationships. However, the tri-isotope approach and integration of both vent chemistry and microbiology, here, provided a more holistic understanding of vent trophic ecology at within- and among-vent field scales.
Intra-site Trophic Interactions and Energy Sources
Scarcity of Δ13C estimates between inorganic carbon and cellular biomass for primary producers at hydrothermal vents [27], [30] makes interpretation of the origin of organic carbon fixed within the hydrothermal vent system and assimilated by macroconsumers tentative for species not within a symbiotic or known predator-prey relationship. Diffuse flow δ13CDIC of approximately 1‰ at the ESR vent fields suggests ESR vent macroconsumers with δ13C values <−22‰ are assimilating carbon fixed via the CBB cycle because the net fractionation associated with fixing inorganic into organic carbon for RuBisCO form I ranges from −22‰ to −30‰ [26], [27]. Peltospiroidea sp. housed an endosymbiotic gamma-Proteobacteria (K. Zwirglmaier unpublished data) and is within the δ13C range expected for carbon fixed via RuBisCO form I at all three locations. Molluscs containing a single strain of endosymbiotic gamma-Proteobacteria living in other biogeographical vent provinces include some species of bathymodiolid mussels, vesicomyid clams and Ifremeria gastropods, all of which have δ13C values between −37‰ and −27‰ [39], [40]. Other species of ESR vent macroconsumers, which had δ13C values <−22‰ included Vulcanolepas sp. (E2 and E9S), Sericusora spp., E2 anemones and Lepetodrilus sp. These species consume free-living bacteria [14], [23] so organic carbon fixed via other carbon fixation pathways cannot be ruled out as part of their assimilated diet.
Vent macroconsumers inhabiting the hottest areas of the hydrothermal vent tolerable to metazoan life, including rimicarid shrimps, polychaetes Alvinella spp. and Riftia pachyptila and some alvinoconchid gastropods, tend to assimilate rTCA-fixed carbon from their diet [11], [41], [42] and have δ13C values >−16‰ [14], [25], [43], [44]. As δ13CDIC is approximately 1‰ at the ESR sites, vent macroconsumers utilising carbon fixed via the rTCA cycle would have had δ13C values >−13‰; assuming a −2‰ to −14‰ net fractionation between the inorganic substrate and organic product catalysed by the enzymes involved in the rTCA cycle [29], [30]. Kiwa sp. living at E9N and E9S, along with its epibionts, had δ13C values that were >−12‰ and are found in areas close to discharging vent fluids [22]. This potentially indicates the epibionts living on Kiwa sp. ventral setae were fixing carbon via the rTCA cycle. Kiwa sp. was also 15N-enriched by between 3.8‰ and 4.2‰ relative to its epibionts, suggesting the epibionts were an important food source. A similar episymbiotic relationship between the ESR kiwid is therefore hypothesised to that of Kiwa puravida, for which lipid, stable isotope and behavioural analyses indicate the harvesting of epibiont bacteria [45]. Stichasteridae sp. (∼−13‰) and cf Actinostola sp. (∼−14‰) also appeared to be assimilating carbon indicative of the rTCA cycle at E9N and E9S.
Several vent macroconsumers fell within the range of δ13C values indicative of mixed carbon sources. Those within the δ13C −22‰ to −15‰ range may consume free-living bacteria or are predators or scavengers that utilise a number of trophic pathways. At the ESR hydrothermal vents, Lepetodrilus sp., Provannidae sp. 2, Vulcanolepas sp., Kiwa sp., Stichasteridae sp. and Colossendeis cf. elephantis fell into this range at one or more sites. Related species of Lepetodrilus sp., Provannidae sp. 2 and Vulcanolepas sp. are all thought to consume free-living bacteria at other vents sites [14], [23]. Such feeding can result in consuming heterogeneous bacterial communities, which have multiple pathways for carbon fixation and elemental cycling [9], [46], [47]. The biological cycling of carbon is very complex at hydrothermal vents because of the multiple single carbon substrates for carbon fixation (e.g. CO2, CH4, CO), spatial variability in the δ13C value of the substrate and various microbial primary producers associated with different carbon fixation pathways [7], [11], [48]. Furthermore, the incorporation of photosynthetic derived carbon as particulate or dissolved organic matter is possible and may provide some nutrition to vent macroconsumers [12], [13]. Therefore, complex isotopic mixes of food sources are available to these species.
The majority of ESR vent macroconsumers had δ34S values less than or equal to the 10‰ threshold, indicating chemosynthetic food sources [49]. Species exceeding the 10‰ value occurred mainly at E2 in the anemones Pacamanactis sp. and cf Marianactis sp, the sponge Cladorhiza sp., the pycnogonids C. elephantis and the stichasterid seastar along with Sericosura spp. and stichasterid seastar at E9S. All had δ34S values between 10‰ and 16‰. Mixing of epipelagic photosynthetic and hydrothermal vent chemosynthetic production sources at these sites cannot be ruled out.
Determining intra-site differences in food sources and trophic interactions using δ34S is challenging for macroconsumers with δ34S values <10‰ because the δ34S values of inorganic substrates and the net fractionation effect between inorganic substrates and products for primary producers and consumers are uncertain. At E9, δ34S appeared to increase from macroconsumers living closest to vent openings and within diffuse flow areas (i.e. Kiwa sp., Peltospiroidea sp. and Lepetodrilus sp.) to those in the periphery (i.e. anemones, stichasterid seastars and Colossendeis spp.). It is unclear why an increase in δ34S occurred from the centre of the vent to the periphery: it may be the result of changes in sulfide speciation [50] or other sulfur sources with increasing distance from the vent opening [33], differences in levels of sulfide exposure [50], incorporation of epipelagic photosynthetic primary production or a combination of the above.
Stichasterid seastars, cf Actinostola sp. and Colossendeis spp. consistently had the highest δ15N values of all the ESR vent macroconsumers, which suggested they occupied the highest trophic positions of those predators sampled. Behavioural observations [22] and δ13C values indicated that Kiwa sp. is consumed by stichasterid seastar and cf Actinostola sp. 1 but only the stichasterid seastar had δ15N values indicative of a higher trophic position than Kiwa sp. In the case of Colossendeis spp., feeding on anemones occurs at the ESR vent sites [22] and at E2 all three stable isotopes indicated a strong predator-prey link. At E9N there was a large difference in δ13C and δ34S between cf Actinostola sp. 1 and the two species of Colossendeis as well as lower δ15N in these pycnogonids compared to cf Actinostola sp. 1. This suggests that at E9N the feeding incidents between cf Actinostola sp. 1 and Colossendeis spp. are either rare or stable isotope values of Colossendeis spp. are strongly affected by isotopic mixing of different energy sources (δ13C and δ34S) and feeding over multiple trophic positions (δ15N).
It is evident from the ESR hydrothermal vent food webs that predators may have similar or lower δ15N values than their prey. Calculating trophic position assuming taxon specific nitrogen trophic discrimination factors [23] or applying the more universal value of 3.4‰ [12] was not undertaken within this study because they may have provided erroneous results. Establishing a suitable δ15N baseline is problematic because: the macroconsumer with the lowest δ15N differed among locations, is confounded by the use of different tissues (e.g. whole animals, muscle) to construct the food webs [32] and the observed high δ15N variability in potential food sources. Compound-specific amino acid stable isotope analysis may provide higher resolution information on the organic nitrogen compounds assimilated by vent macroconsumers because the isotopic values of different amino acids record trophic and basal source information [51], [52]. Thus it may circumvent some of the limitations of bulk δ15N analysis and provide a better understanding of nitrogen cycling at hydrothermal vents.
Spatial Patterns in Macroconsumer Trophodynamics
Large spatial differences in δ13C values for Kiwa sp., Stichasteridae sp. and Sericosura spp. were attributed primarily to differences in carbon fixation pathways at the base of the food web, which is in turn transferred to higher trophic positions. δ13C values of Kiwa sp. differed by ∼9‰ between E2 and E9S as did that of associated Kiwa sp. epibionts. Also, epsilon-Proteobacteria dominated the epibiont community at E9 with gamma-Proteobacteria largely absent, compared to a mix of gamma- and epsilon-Proteobacteria at E2 (K. Zwirglmaier unpublished data). All epsilon-Proteobacteria to date use the rTCA cycle to fix carbon while gamma-Proteobacteria predominantly use the CBB cycle [11]. Riftia pachyptila has similar differences in δ13C among vent sites, but this is attributed to its endosymbionts shifting between rTCA and CBB cycles [53] rather than changes in the microbial community it consumes. Alvinoconchid gastropods have δ13C values that differ by >20‰ among vent fields, which relates to whether epsilon- or gamma-Proteobacteria are the endosymbionts [54]. It is unclear why Kiwa sp. epibiont diversity is different between E2 and E9. At other hydrothermal vent locations differences in vent fluid chemical composition influences microbial communities [46], [55] and it may be similar at the ESR vent fields. The difference in carbon fixation appeared to be transferred through Kiwa sp. to the predatory stichasterid seastar. Such a predator-prey interaction may also explain the large difference in δ13C values between E2 and E9N in Sericosura spp. At E2 Sericosura spp. were collected from amongst anemones that had δ13C values indicative of a mixed carbon source but at E9 they were collected from amongst peltospiroid gastropods dependent on CBB fixed carbon, although Sericosura spp. were not observed directly feeding on either anemones or Peltospiroidea sp.
Relatively small differences in stable isotope values were observed among sites in Peltospiroidea sp., Lepetodrilus sp. and Vulcanolepas sp. To date, Peltospiroidea sp. contains a single strain of gamma-Proteobacteria endosymbiont (K. Zwirglmaier unpublished data), which means spatial differences in δ13C and δ34S are unlikely to be the result of differences in the type of endosymbiont [55]. The differences were potentially a result of site-specific variations in the δ13CDIC and inorganic δ34S values used by the endosymbionts during chemoautotrophy or physiological temperature-related effects on isotopic discrimination. Small differences among sites for the grazer Lepetodrilus sp. and suspension feeder Vulcanolepas sp. are harder to explain because of the various factors that are likely to influence their food source. δ13C values indicated these organisms consume a mixed diet of free-living microbes and particulate material. However, differences in δ13C values within sites may be related to the organism’s distribution within the vent field [56] and in turn the composition of the microbial community [46], the stable isotope values of the inorganic substrate used during chemoautotrophy [57] and temperature effects on trophic discrimination. Lepetodrilus sp. and Vulcanolepas sp. were collected from single points within each vent site and, therefore, it is not clear whether the difference in stable isotope values among sites is greater or less than that within sites.
Because of the snap-shot nature of this study, it is difficult to identify factors that caused the spatial differences in the Kiwa sp. epibiont communities that resulted in a greater range of δ13C values at the E9 sites compared to E2. Higher concentrations of dissolved sulfides in vent fluids may favour the rTCA pathway resulting in increasing numbers of organisms with δ13C values greater than −16‰ [12]. On the ESR, E9 has higher hydrogen sulfide and lower chloride concentrations than E2 meaning that there are greater concentrations of available gases for microbial primary production due to phase separation [6], [21]. Higher concentrations of reduced compounds and gases may be one of the drivers of the differences in trophic structure at the ESR vents. However, hydrothermal vent communities also undergo changes in community composition with age [58] and fluctuating hydrothermal activity [59], which will have an effect on trophic structure. As data presented here were obtained concurrently with the discovery of the new biogeographical province it is not possible to determine whether the communities at E2 and E9 represent different successional stages, are a product of varying chemistry or a mix of such processes.
Conclusion
Trophic structure differed substantially between the E2 and E9 vents fields, and only slightly between E9N and E9S. δ13CDIC of the end-member fluid and diffuse flow samples were similar among the sites but large differences in the δ13C values of some vent macroconsumers indicated spatial variations in the way microbes were fixing carbon at the base of the food chain. δ13C values >−13‰ at the E9N and E9S suggest that the relative contribution to the macroconsumer food web of carbon fixed via the rTCA cycle is likely to be greater than at E2. The greater range of δ34S values at E2 and E9S indicated a potentially greater influence of epipelagic photosynthetic primary production than at E9N. The greater contribution of rTCA fixed carbon at the E9 vent field may ultimately be related to differences in vent fluid, but more work is required to link vent fluid chemistry with microbial primary production and the related trophic structure at hydrothermal vents.
Acknowledgments
We thank the officers, crew, ROV team & scientists onboard JC42 for assistance in sample collection and sorting. We also thank Veerle Huvenne for producing the bathymetric map.
Funding Statement
The research was funded by the Natural Environment Research Council (NERC) through ChEsSO consortium grant NE/DO1249X/1 and studentships NE/F010664/1 (WDKR) and NE/H524922 (JAH). Sample analysis was funded via NERC Life Sciences Mass Spectrometry Facilities grant LSMSFBRIS043_04/10_R_09/10. The funders had no role in the study design, data collection and anlaysis, decision to publish, or preparation of the manuscript.
References
- 1. Kelley DS, Karson JA, Blackman DK, Fruh-Green GL, Butterfield DA, et al. (2001) An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30°N Nature. 412: 145–149. [DOI] [PubMed] [Google Scholar]
- 2. Staudigel H, Hart SR, Pile A, Bailey SE, Baker ET, et al. (2006) Vailulu'u seamount, Samoa: Life and death on an active submarine volcano. Proc Natl Acad Sci USA. 103: 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunnicliffe V, Juniper SK, Sibuet M (2003) Reducing environments of the deep-sea floor. In: Tyler PA, editor. Ecosystems of the Deep Ocean. Amsterdam: Elsevier Science. 81–110.
- 4. Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB (1981) Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones - possible chemoautotrophic symbionts. Science 213: 340–342. [DOI] [PubMed] [Google Scholar]
- 5. German CR, Ramirez-Llodra E, Baker MC, Tyler PA (2011) Deep-water chemosynthetic ecosystem research during the Census of Marine Life decade and beyond: A proposed deep-ocean road map. PLoS ONE 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.German CR, Von Damm KL (2003) Hydrothermal Processes. In: Elderfield H, editor. The ocean and marine geochemistry. Oxford: Elsevier. 181–222.
- 7.Karl DM (1995) Ecology of free-living, hydrothermal vent microbial communities. In: Karl DM, editor. The microbiology of deep-sea hydrothermal vents. Boca Raton: CRC Press Inc. 35–124.
- 8. McCollom TM, Shock EL (1997) Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Acta 61: 4375–4391. [DOI] [PubMed] [Google Scholar]
- 9. Campbell BJ, Cary SC (2004) Abundance of reverse tricarboxylic acid cycle genes in free-living microorganisms at deep-sea hydrothermal vents. Appl Environ Microbiol 70: 6282–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desbruyeres D, Alaysedanet AM, Ohta S, Antoine E, Barbier G, et al. (1994) Deep-sea hydrothermal communities in the southwestern Pacific back-arc basins (the North Fiji and Lau Basins)- composition, microdistribution and food web. Mar Geol 116: 227–242. [Google Scholar]
- 11.Hugler M, Sievert SM (2011) Beyond the Calvin Cycle: Autotrophic carbon fixation in the ocean. Ann Rev Mar Sci. Palo Alto: Annual Reviews. 261–289. [DOI] [PubMed]
- 12. De Busserolles F, Sarrazin J, Gauthier O, Gelinas Y, Fabri MC, et al. (2009) Are spatial variations in the diets of hydrothermal fauna linked to local environmental conditions? Deep Sea Res Part II Top Stud Oceanogr 56: 1649–1664. [Google Scholar]
- 13. Riou V, Colaco A, Bouillon S, Khripounoff A, Dando PR, et al. (2010) Mixotrophy in the deep sea: a dual endosymbiotic hydrothermal mytilid assimilates dissolved and particulate organic matter Mar Ecol Prog Ser. 405: 187–201. [Google Scholar]
- 14. Colaco A, Dehairs F, Desbruyeres D (2002) Nutritional relations of deep-sea hydrothermal fields at the Mid-Atlantic Ridge: a stable isotope approach. Deep Sea Res Part I Oceanogr Res Pap 49: 395–412. [Google Scholar]
- 15. Rau GH, Hedges JI (1979) Carbon-13 depletion in a hydrothermal vent mussel - suggestion of a chemosynthetic food source. Science 203: 648–649. [DOI] [PubMed] [Google Scholar]
- 16. Van Dover CL, Fry B (1994) Microorganisms as food resources at deep-sea hydrothermal vents. Limnol Oceanogr 39: 51–57. [Google Scholar]
- 17. Levin LA, Mendoza GF, Konotchick T, Lee R (2009) Macrobenthos community structure and trophic relationships within active and inactive Pacific hydrothermal sediments. Deep Sea Res Part II Top Stud Oceanogr 56: 1632–1648. [Google Scholar]
- 18. Limen H, Juniper SK (2006) Habitat controls on vent food webs at Eifuku Volcano, Mariana Arc. Cah Biol Mar 47: 449–455. [Google Scholar]
- 19. Van Dover CL (2002) Trophic relationships among invertebrates at the Kairei hydrothermal vent field (Central Indian Ridge). Mar Biol 141: 761–772. [Google Scholar]
- 20. German CR, Livermore RA, Baker ET, Bruguier NI, Connelly DP, et al. (2000) Hydrothermal plumes above the East Scotia Ridge: an isolated high-latitude back-arc spreading centre. Earth Planet Sci Lett 184: 241–250. [Google Scholar]
- 21. Rogers AD, Tyler PA, Connelly DP, Copley JT, James R, et al. (2012) The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol 10: e1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marsh L, Copley JT, Huvenne VAI, Linse K, Reid WDK, et al. (2012) Microdistribution of faunal assemblages at deep-sea hydrothermal vents in the Southern Ocean. PLoS ONE 7: e48348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergquist DC, Eckner JT, Urcuyo IA, Cordes EE, Hourdez S, et al. (2007) Using stable isotopes and quantitative community characteristics to determine a local hydrothermal vent food web. Mar Ecol Prog Ser 330: 49–65. [Google Scholar]
- 24. Van Dover CL, Fry B (1989) Stable isotopic compositions of hydrothermal vent organisms. Mar Biol 102: 257–263. [Google Scholar]
- 25. Fisher CR, Childress JJ, Macko SA, Brooks JM (1994) Nutritional interactions in Galapagos Rift hydrothermal vent communities - inferences from stable carbon and nitrogen isotope analyses. Mar Ecol Prog Ser 103: 45–55. [Google Scholar]
- 26. Guy RD, Fogel ML, Berry JA (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiology 101: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robinson JJ, Scott KM, Swanson ST, O'Leary MH, Horken K, et al. (2003) Kinetic isotope effect and characterization of form II RuBisCO from the chemoautotrophic endosymbionts of the hydrothermal vent tubeworm Riftia pachyptila . Limnol Oceanogr 48: 48–54. [Google Scholar]
- 28. Roeske CA, O'Leary MH (1984) Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23: 6275–6284. [DOI] [PubMed] [Google Scholar]
- 29. House CH, Schopf JW, Stetter KO (2003) Carbon isotopic fractionation by Archaeans and other thermophilic prokaryotes. Org Geochem 34: 345–356. [Google Scholar]
- 30. Suzuki Y, Sasaki T, Suzuki M, Nogi Y, Miwa T, et al. (2005) Novel chemoautotrophic endosymbiosis between a member of the Epsilonproteobacteria and the hydrothermal-vent gastropod Alviniconcha aff. hessleri (Gastropoda: Provannidae) from the Indian Ocean. Appl Environ Microbiol 71: 5440–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wirsen CO, Sievert SM, Cavanaugh CM, Molyneaux SJ, Ahmad A, et al. (2002) Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp that produces filamentous sulfur. Appl Environ Microbiol 68: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michener RH, Kaufman L (2007) Stable isotope ratios as tracers in marine food webs: an update. In: Michener RH, Lajtha K, editors. Stable isotopes in ecology and environmental science. 2nd ed. Singapore: Blackwell Publishing. 238–282.
- 33. Herzig PM, Hannington MD, Arribas A Jr (1998) Sulfur isotopic composition of hydrothermal precipitates from the Lau back-arc: implications for magmatic contributions to seafloor hydrothermal systems. Mineralium Deposita 33: 226–237. [Google Scholar]
- 34. Erickson KL, Macko SA, Van Dover CL (2009) Evidence for a chemoautotrophically based food web at inactive hydrothermal vents (Manus Basin). Deep Sea Res Part II Top Stud Oceanogr 56: 1577–1585. [Google Scholar]
- 35. Reid WDK, Wigham BD, McGill RAR, Polunin NVC (2012) Elucidating trophic pathways in benthic deep-sea assemblages of the Mid-Atlantic Ridge north and south of the Charlie-Gibbs Fracture Zone. Mar Ecol Prog Ser 463: 89–103. [Google Scholar]
- 36. Layman CA, Arrington DA, Montana CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88: 42–48. [DOI] [PubMed] [Google Scholar]
- 37.Quinn GP, Keough MJ (2002) Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press. 537 p.
- 38.R Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, URL http://www.R-project.org/.
- 39. Brooks JM, Kennicutt MC, Fisher CR, Macko SA, Cole K, et al. (1987) Deep-sea hydrocarbon seep communities - evidence for energy and nutritional carbon sources. Science 238: 1138–1142. [DOI] [PubMed] [Google Scholar]
- 40. Childress JJ, Fisher CR (1992) The biology of hydrothermal vent animals- physiology, biochemistry and autotrophic symbioses. Oceanogr Mar Biol 30: 337–441. [Google Scholar]
- 41. Campbell BJ, Engel AS, Porter ML, Takai K (2006) The versatile epsilon-Proteobacteria: key players in sulphidic habitats. Nat Rev Microbiol 4: 458–468. [DOI] [PubMed] [Google Scholar]
- 42. Campbell BJ, Stein JL, Cary SC (2003) Evidence of chemolithoautotrophy in the bacterial community associated with Alvinella pompejana, a hydrothermal vent polychaete. Appl Environ Microbiol 69: 5070–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levesque C, Juniper SK, Marcus J (2003) Food resource partitioning and competition among alvinellid polychaetes of Juan de Fuca Ridge hydrothermal vents. Mar Ecol Prog Ser 246: 173–182. [Google Scholar]
- 44. Suzuki Y, Kojima S, Sasaki T, Suzuki M, Utsumi T, et al. (2006) Host-symbiont relationships in hydrothermal vent gastropods of the genus Alviniconcha from the Southwest Pacific. Appl Environ Microbiol 72: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thurber AR, Jones WJ, Schnabel K (2011) Dancing for food in the deep sea: bacterial farming by a new species of yeti crab. PLoS ONE 6: e26243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flores GE, Campbell JH, Kirshtein JD, Meneghin J, Podar M, et al. (2011) Microbial community structure of hydrothermal deposits from geochemically different vent fields along the Mid-Atlantic Ridge. Environ Microbiol 13: 2158–2171. [DOI] [PubMed] [Google Scholar]
- 47. Takai K, Nunoura T, Horikoshi K, Shibuya T, Nakamura K, et al. (2009) Variability in microbial communities in black smoker chimneys at the NW Caldera vent field, Brothers Volcano, Kermadec Arc. Geomicrobiol J 26: 552–569. [Google Scholar]
- 48. Nakagawa S, Takai K (2008) Deep-sea vent chemoautotrophs: diversity, biochemistry and ecological significance. FEMS Microbiol Ecol 65: 1–14. [DOI] [PubMed] [Google Scholar]
- 49. Vetter RD, Fry B (1998) Sulfur contents and sulfur-isotope compositions of thiotrophic symbioses in bivalve molluscs and vestimentiferan worms. Mar Biol 132: 453–460. [Google Scholar]
- 50. Luther GW, Rozan TF, Taillefert M, Nuzzio DB, Di Meo C, et al. (2001) Chemical speciation drives hydrothermal vent ecology. Nature 410: 813–816. [DOI] [PubMed] [Google Scholar]
- 51. Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, et al. (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Methods 7: 740–750. [Google Scholar]
- 52. McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83: 2173–2180. [Google Scholar]
- 53. Markert S, Arndt C, Felbeck H, Becher D, Sievert SM, et al. (2007) Physiological proteomics of the uncultured endosymbiont of Riftia pachyptila . Science 315: 247–250. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki Y, Sasaki T, Suzuki M, Tsuchida S, Nealson KH, et al. (2005) Molecular phylogenetic and isotopic evidence of two lineages of chemoautotrophic endosymbionts distinct at the subdivision level harbored in one host-animal type: The genus Alviniconcha (Gastropoda : Provannidae). FEMS Microbiol Lett 249: 105–112. [DOI] [PubMed] [Google Scholar]
- 55. Trask JL, Van Dover CL (1999) Site-specific and ontogenetic variations in nutrition of mussels (Bathymodiolus sp.) from the Lucky Strike hydrothermal vent field, Mid-Atlantic Ridge. Limnol Oceanogr 44: 334–343. [Google Scholar]
- 56. Levesque C, Juniper SK, Limen H (2006) Spatial organization of food webs along habitat gradients at deep-sea hydrothermal vents on Axial Volcano, Northeast Pacific. Deep Sea Res Part I Oceanogr Res Pap 53: 726–739. [Google Scholar]
- 57. Levesque C, Limen H, Juniper SK (2005) Origin, composition and nutritional quality of particulate matter at deep-sea hydrothermal vents on Axial Volcano, NE Pacific. Mar Ecol Prog Ser 289: 43–52. [Google Scholar]
- 58. Shank TM, Fornari DJ, Von Damm KL, Lilley MD, Haymon RM, et al. (1998) Temporal and spatial patterns of biological community development at nascent deep-sea hydrothermal vents (9 degrees 50′ N, East Pacific Rise). Deep Sea Res Part II Top Stud Oceanogr 45: 465–515. [Google Scholar]
- 59. Cuvelier D, Sarrazin J, Colaco A, Copley JT, Glover AG, et al. (2011) Community dynamics over 14 years at the Eiffel Tower hydrothermal edifice on the Mid-Atlantic Ridge. Limnol Oceanogr 56: 1624–1640. [Google Scholar]