Abstract
Species differences in neurochemical expression and activity in the brain may play an important role in species-specific patterns of social behavior. In the present study, we used immunoreactive (ir) labeling to compare the regional density of cells containing oxytocin (OT), vasopressin (AVP), tyrosine hydroxylase (TH), or estrogen receptor alpha (ERα) staining in the brains of social Mongolian gerbils (Meriones unguiculatus) and solitary Chinese striped hamsters (Cricetulus barabensis). Multiple region- and neurochemical-specific species differences were found. In the anterior hypothalamus (AH), Mongolian gerbils had higher densities of AVP-ir and ERα-ir cells than Chinese striped hamsters. In the lateral hypothalamus (LH), Mongolian gerbils also had higher densities of AVP-ir and TH-ir cells, but a lower density of OT-ir cells, than Chinese striped hamsters. Furthermore, in the anterior nucleus of the medial preoptic area (MPOAa), Mongolian gerbils had higher densities of OT-ir and AVP-ir cells than Chinese striped hamsters, and an opposite pattern was found in the posterior nucleus of the MPOA (MPOAp). Some sex differences were also observed. Females of both species had higher densities of TH-ir cells in the MPOAa and of OT-ir cells in the intermediate nucleus of the MPOA (MPOAi) than males. Given the role of these neurochemicals in social behaviors, our data provide additional evidence to support the notion that species-specific patterns of neurochemical expression in the brain may be involved in species differences in social behaviors associated with different life strategies.
Introduction
Animals show remarkable differences in their life strategies and social behaviors. Social species, for example, usually display high levels of prosocial behavior towards conspecifics, social affiliation with mates and biparental care of their offspring [1], [2]. In contrast, solitary species generally display low levels of prosocial behavior and social affiliation, but high levels of aggression to defend their territory [1], [3]. Such species differences in life strategy and social behavior may not only reflect their adaption to the environment, but also indicate their potential differences in the central mechanisms that are involved in the regulation of social behavior.
Indeed, several neurochemicals have been implicated in social behaviors associated with different life strategies. For example, the receptor distribution and activity of the neuropeptides oxytocin (OT) and vasopressin (AVP) differ in the brains of social and nonsocial rodent species, and such differences are thought to be involved in the regulation of species-specific social behaviors, such as affiliation, pair bonding, male parental care and territory marking [4]–[7]. Similarly, release patterns of the neurotransmitter dopamine, during mating and social interaction, differ between social and nonsocial rodent species [7], and dopamine has been implicated in pair bonding behavior in the socially monogamous rodent species [8]–[10]. The distribution patterns of estrogen receptor alpha (ERα) in the brain also differ between social and nonsocial rodent species [11], [12], and ERα has been implicated in social behaviors such as social affiliation, aggression and maternal care [13]–[17]. Interestingly, ERα may regulate social behaviors by interacting with other neurotransmitter systems [18], [19]. For instance, ERα may affect AVP expression in certain brain areas [20], [21] and alters AVP-mediated behaviors, such as aggression and affiliation [22], [23].
Many comparative studies have focused on species that share a close phylogenetic relationship and that are even in the same genus [1], [6], [24]–[28]. We have expanded our efforts to examine species from different genera, as these data will help us to better understand how evolution shapes the brain and behavior. In a most recent study, we compared social Brandt's voles (Lasiopodomys brandtii) with solitary Greater long-tailed hamsters (Tscherskia triton), and found species differences in central OT and AVP immunoreactive (ir) staining in brain areas important for social behaviors [2]. These data support the notion that central OT and AVP may underlie species differences in social behaviors [1], [2], [25], [26], [29]. In the present study, we extended our efforts to additional rodent species, particularly Mongolian gerbils (Meriones unguiculatus) and Chinese striped hamsters (Cricetulus barabensis), to further test the hypothesis that differences in neurochemical systems in the brain are related to species differences in life strategies and behaviors. In addition to central OT and AVP systems, we included TH (a dopamine-related marker) and ERα in our investigation, as both have been implicated in social behaviors [7], [14]–[16].
Mongolian gerbils inhabit typical steppes in Inner Mongolia and in the south-east of the Bakal area in Russia and Mongolia [30]–[32]. These animals are diurnal and highly social. They live in large family groups that range from 2 to 17 individuals and usually consist of a breeding male and female, as well as the siblings and offspring of the breeding pair [33], [34]. Extensive social interactions have been observed among individuals [33], [35]. Females and males form monogamous pairs in nature [33], [34], [36] and both parents play an active role in nest building and caring for offspring [37]. Furthermore, female Mongolian gerbils display higher levels of parental care and food hoarding behavior compared to male conspecifics whereas males show higher levels of territorial and aggressive behavior than females [34], [36], [38]–[40]. These gerbils become sexually mature around 5 months of age, and their life span is about 1 year in the wild and 2.5 years in the laboratory [33], [35]. In contrast, Chinese striped hamsters are primarily found in the farmland or grassland of northern China [41]. They are nocturnal and solitary, and display high levels of aggressive behavior towards conspecifics [41], [42]. In Chinese striped hamsters, females raise pups alone, and they also display higher levels of aggressive behavior compared to males [42]. Chinese striped hamsters reach sexual maturity around 3 months of age, and their life span is about 10 months [41].
In the present study, we compared OT, AVP, TH, and ERα immunoreactivity between these two species in selected brain areas known to be important in social behaviors. Such species differences in neurochemical expression in the brain may be ultimately involved in the regulation of species-specific social behaviors.
Methods
Ethics statement
The project was officially approved by the Institute of Zoology (IOZ), Chinese Academy of Sciences. People involved in caring and handling experimental animals were trained, and procedures related to animal care and ethics were approved by the examination of Animal Ethics Committee of IOZ (Permit Number: IOZ13002).
Subjects
Subjects were adult male and female Mongolian gerbils (Meriones unguiculatus) and Chinese striped hamsters (Cricetulus barabensis). Mongolian gerbils were the offspring of a laboratory breeding colony that was maintained in the IOZ at the Chinese Academy of Sciences in Beijing, China. Chinese striped hamsters were captured in croplands nearby Qufu, Shandong Province in the winter of 2009. These animals were captured in private farms. Because Chinese striped hamster is a pest species, our capture got owner's permission and support. This capture does not harm other endangered species or rare species which are present in the farmland. We conducted trapping using steel-wire live traps (12 L×12 W×25 H cm). Fresh peanuts were used as bait, small pieces of cabbage were provided as a water supply and local dry leaves were provided as nest material. An iron sheet was attached on the upper side of the trap as shelter to protect from predation and sunshine. Pregnant and lactating females were released immediately on site. Captured animals were carefully transferred to the laboratory using the live trap. These animals were housed in the lab environment for about two weeks before they were used in the experiment. All subjects were housed in plastic cages (27 L×16 W×13 H cm for Mongolian gerbils and 25 L×14 W×14 H cm for Chinese striped hamsters) that contained wood shavings. Food and water were provided ad libitum. As Mongolian gerbils are social animals, they were housed in same-sex groups, consisting of two to four individuals, under a 16L:8D photoperiod (lights on 0500). Chinese striped hamsters are solitary animals and thus they were housed singly under a reversed 16L:8D photoperiod (lights on 1700). Room temperature was maintained at 20±2°C.
Tissue preparation
Eight male and eight female Mongolian gerbils, and seven male and seven female Chinese striped hamsters were deeply anesthetized with sodium pentobarbital (3 mg/100 g body weight, Sigma-Aldrich, St. Louis, MO, USA) and perfused through the ascending aorta with 0.1 M phosphate buffered solution (PBS, pH7.2) followed by 4% paraformaldehyde in PBS. Brains were quickly removed, post-fixed in 4% paraformaldehyde for 12 h and then stored in 30% sucrose in PBS. Coronal brain sections (40 µm thickness) were cut on a cryostat. Four alternate sets of brain sections at 240 µm intervals were processed for OT, AVP, TH and ERα immunocytochemistry, respectively. An additional set of brain sections was processed for Nissl staining.
Immunocytochemistry for OT, AVP, TH and ERα
Floating brain sections were processed for OT, AVP, or TH immunocytochemistry using an established method [28]. Briefly, brain sections were pre-treated with 0.5% NaBH4, followed by 0.05% H2O2 in 0.05 M Tris–NaCl (pH7.6), and then blocked in 10% normal goat serum (NGS) in Tris–NaCl with 0.5% Triton X-100 (Tris–Triton). Sections were incubated in rabbit anti-OT serum (1∶20,000), guinea pig anti-AVP serum (1∶40,000),or rabbit anti-TH serum (1∶15,000) (from Bachem California, Inc., Torrance, CA, USA), respectively, in Tris–Triton with 2% NGS for 36 h at 4°C, followed by an additional 2 h at room temperature. Thereafter, sections were incubated with a biotinylated goat-anti-rabbit, goat-anti-guinea pig or goat-anti-rabbit secondary antibody for OT, AVP and TH (1∶300; all from Vector Laboratories Inc., Burlingtone, CA, USA), respectively, in Tris–Triton for 2 h; ABC complex (Vector Laboratories Inc., Burlingtone, CA, USA) in Tris–NaCl for 90 min; and stained by 0.05% 3-3′-diaminobenzidine (Sigma-Aldrich) in Tris–NaCl with 0.009% H2O2. Sections were then mounted, air-dried and cover slipped.
The ERα immunocytochemistry was also conducted using an established method [43], [44]. Briefly, brain sections were pre-treated with 10 mM citrate buffer for 10 min at 90°C, followed by 0.5% NaBH4 for 5 min and then 0.5% H2O2 in 0.1 M PBS for 30 min. Thereafter, sections were treated in PBS with 0.6% Triton X-100 (PBT) for 20 min, blocked in 10% NGS in PBT for 30 min and incubated in rabbit ERα polyclonal antibody (1∶8000, Upstate, Millipore, Billerica, MA, USA) in PBT with 2% NGS for 36 h at 4°C and an additional 1 h at room temperature. Sections were then incubated with biotinylated goat-anti-rabbit secondary antibody (1∶300, Vector Laboratories Inc., Burlingtone, CA, USA) in PBT for 2 h, ABC complex in PBS for 90 min and stained by nickel-DAB. Sections were mounted, air-dried and cover slipped.
Brain sections for each neurochemical marker were processed concurrently to reduce variability in the staining. To control for antibody specificities, additional brain sections were incubated either in the absence of the primary antibody or with the primary antibody that was pretreated with 50 µM of OT, AVP, dopamine and estrogen, respectively. In these situations, specific staining was eliminated or substantially reduced.
Data quantification and analysis
All slides were coded to conceal group identity. Slides were inspected under a Nikon microscope to identify forebrain regions quantified. OT-ir and AVP-ir cells were counted in the anterior hypothalamus (AH), lateral hypothalamic area (LH), medial preoptic area (MPOA) and paraventricular nucleus of the hypothalamus (PVN). TH-ir cells were counted in the AH, LH, MPOA, PVN, ventral tegmental area (VTA) and substantianigra pars compacta (SNc). ERα-ir cells were counted in the AH, MPOA, PVN, lateral septum (LS), bed nucleus of the striateminalis (BST), ventromedial hypothalamus (VMH), arcuate nucleus of the hypothalamus (ARC) and medial (MeA) and anterior cortical (CoA) nuclei of the amygdala. These brain areas were chosen based on previous studies from other rodent species indicating the existence of these neurochemicals and their potential roles in social behaviors.
Brain sections were matched between animals and 2–3 sections per brain area per animal were examined. Cells stained for each neurochemical marker within each brain area were quantified bilaterally. Further, a set of Nissl stained brain sections from each species was used to identify and measure the brain areas, which were then used to convert cell counts into cell density per area. Data were analyzed by a two-way analysis of variance (ANOVA) with species and sex as between-subject variables. Significant interactions were further evaluated by a Student-Newman-Keuls (SNK) post-hoc test. The criterion for significance was set at p<0.05.
Results
OT-ir staining
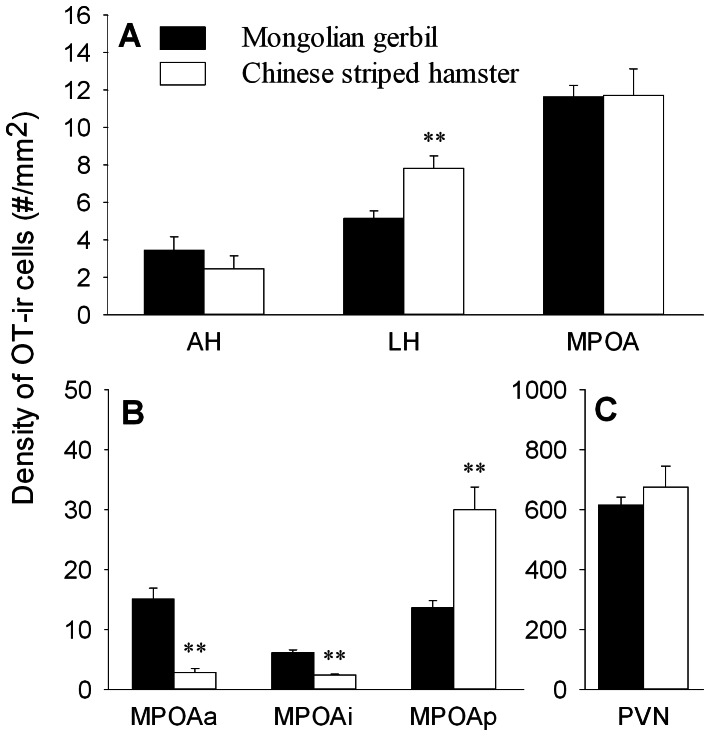
In both species, OT-ir stained cells were present either in dense clusters or scattered throughout many forebrain areas. Very intense staining of OT-ir cells was found in the PVN, while moderate clusters of OT-ir cells were present throughout the rostral–caudal extent of the MPOA. Scattered OT-ir cells were found in many brain regions including the AH and LH. Quantification of OT-ir cells in the above-mentioned brain areas (Table 1) indicates that Mongolian gerbils had a lower density of OT-ir cells in the LH than Chinese striped hamsters (F(1,26) = 11.98, p<0.01), whereas no species differences were found in the AH, MPOA or PVN (Fig. 1). Although the two species showed similar densities of OT-ir cells in the MPOA, some differences were found in the subnuclei of the MPOA. Mongolian gerbils had a higher density of OT-ir cells in the anterior (MPOAa; F(1,26) = 33.49, p<0.01) and intermediate (MPOAi; F(1,26) = 75.64, p<0.01) nuclei of the MPOA than Chinese striped hamsters, whereas an opposite pattern was found in the posterior nucleus of the MPOA (MPOAp; F(1,26) = 17.74, p<0.01) (Fig. 1B, Table 1). A sex difference was found in the OT-ir cell density in the MPOAi, in which females had a higher density of OT-ir cells than males (F(1,26) = 7.50, p<0.05) (Table 1). No sex or species-sex interaction was found in any other brain areas examined.
Table 1. Density of oxytocin immunoreactive cells (mean±SEM/mm2) in the brain of male and female Mongolian gerbils and Chinese striped hamsters.
| Brain area | Mongolian gerbil | Chinese hamster | Two-way ANOVA | ||||
| Male | Female | Male | Female | species | sex | sp X sex | |
| AH | 2.4±0.7 | 4.5±1.3 | 2.1±0.6 | 2.8±1.3 | ns | ns | ns |
| LH | 5.7±0.6 | 4.6±1.0 | 7.8±1.0 | 7.8±1.0 | ** | ns | ns |
| MPOA | 11.4±1.0 | 11.9±0.8 | 11.2±1.6 | 12.2±2.4 | ns | ns | ns |
| MPOAa | 15.4±2.6 | 14.8±2.7 | 2.1±0.9 | 3.6±1.1 | ** | ns | ns |
| MPOAi | 5.1±0.5 | 7.2±0.6 | 2.2±0.2 | 2.6±0.2 | ** | * | ns |
| MPOAp | 13.6±1.6 | 13.8±1.6 | 29.3±4.7 | 30.7±6.4 | ** | ns | ns |
| PVN | 646.2±32.9 | 583.0±62.1 | 696.7±129.6 | 654.7±62.1 | ns | ns | ns |
SEM: standard error of the mean;
p<0.05;
p<0.01;
ns, not significantly different.
Figure 1. Species differences in the density of OT-ir cells in brain areas.
(A) Species differences in the density of OT-ir cells in the anterior hypothalamus (AH), lateral hypothalamus (LH) and medial preoptic area (MPOA). (B) The two species also differed in the density of OT-ir cells in the subnuclei of the MPOA, including the anterior (MPOAa), intermediate (MPOAi) and posterior (MPOAp) part of the MPOA. (C) Species difference in the density of OT-ir cells in the paraventricular nucleus of the hypothalamus (PVN). ** p<0.01.
AVP-ir staining
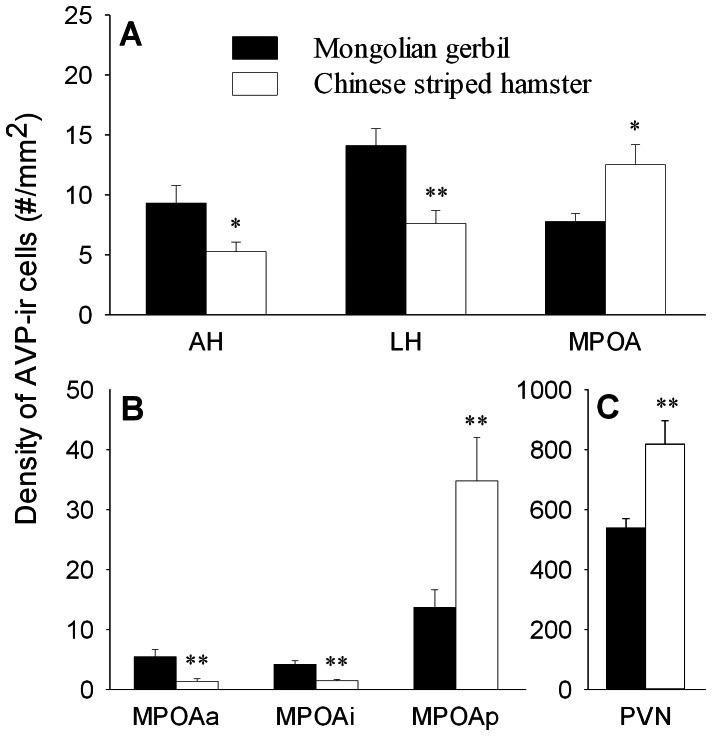
A dense cluster of AVP-ir cells was found in the PVN, moderate clusters of AVP-ir cells were found in the MPOA, mostly in the MPOAp, and scattered AVP-ir cells were observed in the AH and LH. Species differences were found in the density of AVP-ir cells in selected brain areas. Mongolian gerbils had a higher density of AVP-ir cells in the AH (F(1,26) = 6.43,p<0.05) and LH (F(1,26) = 12.50,p<0.01), but a lower density of AVP-ir cells in the MPOA (F(1,26) = 7.29,p<0.05) and PVN (F(1,26) = 11.59, p<0.01), than Chinese striped hamsters (Table 2, Fig. 2A & C). Within the MPOA, Mongolian gerbils had a higher density of AVP-ir cells in the MPOAa (F(1,26) = 16.87, p<0.01) and MPOAi (F(1,26) = 31.45, p<0.01) than Chinese striped hamsters, while an opposite pattern was found in the MPOAp (F(1,26) = 16.34, p<0.01) (Fig. 2B, Table 2). No sex or species-sex interaction was found in the density of AVP-ir cells in any brain areas examined.
Table 2. Density of vasopressin immunoreactive cells (mean±SEM/mm2) in the brain of male and female Mongolian gerbils and Chinese striped hamsters.
| Brain area | Mongolian gerbil | Chinese hamster | Two-way ANOVA | ||||
| Male | Female | Male | Female | species | sex | sp X sex | |
| AH | 6.4±1.2 | 12.3±2.3 | 5.5±1.3 | 5.0±1.0 | * | ns | ns |
| LH | 15.4±1.6 | 12.9±2.4 | 7.0±1.8 | 8.2±1.3 | ** | ns | ns |
| MPOA | 7.7±1.0 | 7.9±1.0 | 11.4±2.0 | 13.7±2.76 | * | ns | ns |
| MPOAa | 4.8±1.0 | 6.1±1.5 | 0.9±0.3 | 1.8±0.5 | ** | ns | ns |
| MPOAi | 4.0±0.6 | 4.4±0.6 | 1.0±0.2 | 1.9±0.2 | ** | ns | ns |
| MPOAp | 14.3±3.0 | 13.1±2.9 | 32.1±6.0 | 37.4±8.2 | ** | ns | ns |
| PVN | 530.4±45.6 | 546.6±48.2 | 809.6±143.2 | 828.9±71.1 | ** | ns | ns |
SEM: standard error of the mean;
p<0.05;
p<0.01;
ns, not significantly different.
Figure 2. Species differences in the density of AVP-ir cells in brain areas.
(A) Species differences in the density of AVP-ir cells in the anterior hypothalamus (AH), lateral hypothalamus (LH) and medial preoptic area (MPOA). (B) The two species also differed in the density of AVP-ir cells in the subnuclei of the MPOA, including the anterior (MPOAa), intermediate (MPOAi) and posterior (MPOAp) part of the MPOA. (C) A species difference was also found in the paraventricular nucleus of the hypothalamus (PVN). * p<0.05, ** p<0.01.
TH-ir staining
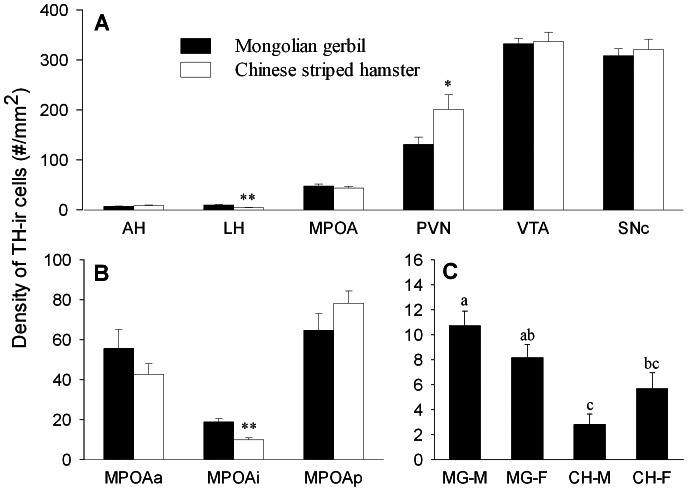
TH-ir cells were found in many brain areas in both species. For example, dense clusters of TH-ir cells were found in the PVN, VTA and SNc, a moderate cluster of TH-ir cells was found in the MPOA, and scattered TH-ir cells were found in the AH and LH. Species differences were observed (Table 3). Mongolian gerbils had a higher density of TH-ir cells in LH (F(1,26) = 19.07,p<0.01) and MPOAi (F(1,26) = 18.52, p<0.01), but a lower density of TH-ir cells in the PVN (F(1,26) = 5.21, p<0.05), than Chinese striped hamsters (Fig. 3A & B). Sex differences were found in the MPOA (F(1,26) = 9.52, p<0.01), particularly in the MPOAa (F(1,26) = 11.06, p<0.01) in which females had a higher density of TH-ir cells than males (Table 3). A species-sex interaction was also found in the LH (F(1,26) = 5.28, p<0.05) — male Mongolian gerbils had a higher density of TH-ir cells in the LH than male and female Chinese hamsters (Fig. 3C).
Table 3. Density of TH immunoreactive cells (mean±SEM/mm2) in the brain of male and female Mongolian gerbils and Chinese striped hamsters.
| Brain area | Mongolian gerbil | Chinese hamster | Two-way ANOVA | ||||
| Male | Female | Male | Female | species | sex | sp X sex | |
| AH | 7.75±1.38 | 6.90±0.86 | 7.81±1.44 | 8.80±2.54 | ns | ns | ns |
| LH | 10.73±1.15 | 8.17±1.06 | 2.82±0.83 | 5.67±1.29 | ** | ns | * |
| MPOA | 38.38±3.14 | 56.47±6.03 | 37.71±4.71 | 49.52±4.96 | ns | ** | ns |
| MPOAa | 34.01±6.05 | 77.34±14.76 | 32.50±7.08 | 52.98±5.99 | ns | ** | ns |
| MPOAi | 15.72±2.58 | 21.93±2.34 | 9.63±1.53 | 10.18±1.17 | ** | ns | ns |
| MPOAp | 65.41±12.11 | 70.15±12.32 | 71.01±8.42 | 85.39±8.75 | ns | ns | ns |
| PVN | 129.02±18.12 | 133.15±24.60 | 223.12±44.55 | 180.04±37.44 | * | ns | ns |
| VTA | 323.99±16.82 | 341.02±15.94 | 342.10±30.30 | 332.94±22.67 | ns | ns | ns |
| SNc | 295.41±15.26 | 321.44±23.17 | 328.64±34.89 | 314.16±24.38 | ns | ns | ns |
SEM: standard error of the mean;
p<0.05;
p<0.01;
ns, not significantly different.
Figure 3. Species differences in the density of TH-ir cells in brain areas.
(A) Species differences in the density of TH-ir cells in the anterior hypothalamus (AH), lateral hypothalamus (LH), medial preoptic area (MPOA), paraventricular nucleus of the hypothalamus (PVN), ventral tegmental area (VTA) and substantianigra pars compacta (SNc). (B) Species differences in the density of TH-ir cells in the anterior (MPOAa), intermediate (MPOAi) and posterior (MPOAp) subnuclei of the MPOA. (C) Student-Newman-Keuls (SNK) post hoc test indicated differences in the lateral hypothalamus (LH) between males and females of the two species (MG: Monglian gerbil; CH: Chinese striped hamster; M: male; F: female). Bars with different alphabetic letters differed significantly from each other. * p<0.05, ** p<0.01.
ERα-ir staining
In both species, specific patterns of ERα-ir stained cells were found in many forebrain areas. Very intense staining of ERα-ir cells was found in the ARC and MPOA, while moderate clusters of ERα-ir cells were found in the VMH, BST, MeA, CoA and PVN. Scattered ERα-ir cells were found in several brain regions including the AH and LS (Table 4).
Table 4. Density of ERα immunoreactive cells (mean±SEM/mm2) in the brain of male and female Mongolian gerbils and Chinese striped hamsters.
| Brain area | Mongolian gerbil | Chinese hamster | Two-way ANOVA | ||||
| Male | Female | Male | Female | species | sex | sp X sex | |
| AH | 78.24±4.81 | 80.19±5.49 | 9.85±1.16 | 17.62±3.05 | ** | ns | ns |
| LS | 25.35±3.16 | 20.22±2.97 | 4.79±1.18 | 4.07±1.05 | ** | ns | ns |
| BST | 290.05±21.78 | 271.70±17.18 | 162.24±12.94 | 179.06±19.01 | ** | ns | ns |
| MPOA | 932.49±58.36 | 880.42±50.32 | 1027.7±102.61 | 914.92±81.05 | ns | ns | ns |
| MPOAa | 716.09±152.85 | 1070.2±174.88 | 1098.1±233.5 | 759.6±144.57 | ns | ns | ns |
| MPOAi | 520.38±34.16 | 546.73±29.54 | 399.92±37.52 | 451.62±29.46 | ** | ns | ns |
| MPOAp | 1561.0±178.67 | 1024.3±167.01 | 1585.2±158.34 | 1533.5±267.6 | ns | ns | ns |
| VMH | 449.13±19.40 | 493.65±32.49 | 275.42±23.46 | 330.78±39.44 | ** | ns | ns |
| ARC | 2026.5±158.30 | 1990.4±170.37 | 1279.1±186.40 | 1217.7±117.67 | ** | ns | ns |
| MeA | 492.34±81.14 | 475.19±65.65 | 376.94±32.64 | 419.24±21.65 | ns | ns | ns |
| CoA | 308.55±47.58 | 362.93±42.79 | 230.47±14.11 | 305.48±10.06 | ns | ns | ns |
| PVN | 341.05±72.76 | 309.78±54.44 | 543.47±53.28 | 445.26±32.43 | ** | ns | ns |
SEM: standard error of the mean;
p<0.01;
ns, not significantly different.
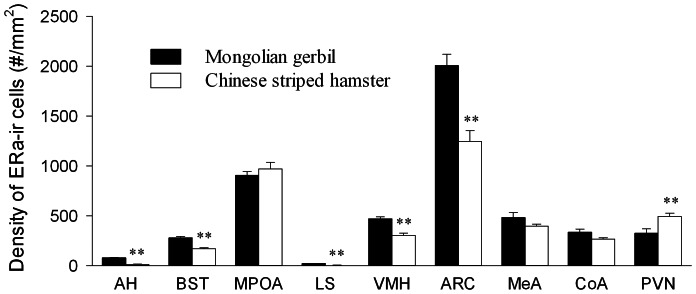
Species differences were found in the density of ERα-ir cells in some of the brain areas examined. Mongolian gerbils had a higher density of ERα-ir cells in the AH (F(1,26) = 231.58, p<0.01), BST (F(1,26) = 30.76,p<0.01), LS (F(1,26) = 53.24, p<0.01), VMH (F(1,26) = 32.64,p<0.01) and ARC (F(1,26) = 20.18,p<0.01) than Chinese striped hamsters (Fig. 4), and a similar species difference was found in the MPOAi (F(1,26) = 10.75,p<0.01) (Table 4). In the PVN, however, Chinese hamsters had a higher density of ERα-ir cells than Mongolian gerbils (F(1,26) = 8.85, p<0.01). The two species did not differ in the density of ERα-ir cells in the total MPOA, MeA and CoA. Furthermore, no sex differences or species–sex interactions were found in any brain areas examined.
Figure 4. Species differences in the density of ERα-ir cells in brain areas.
(A) Species differences in the density of ERα-ir cells in the anterior hypothalamus (AH), bed nucleus of the striaterminalis (BST), medial preoptic area (MPOA), lateral septum (LS), ventral medial hypothalamus (VMH), arcuate nucleus of the hypothalamus (ARC), medial nucleus (MeA) and anterior cortical nucleus (CoA) of the amygdala and paraventricular nucleus of the hypothalamus (PVN). ** p<0.01.
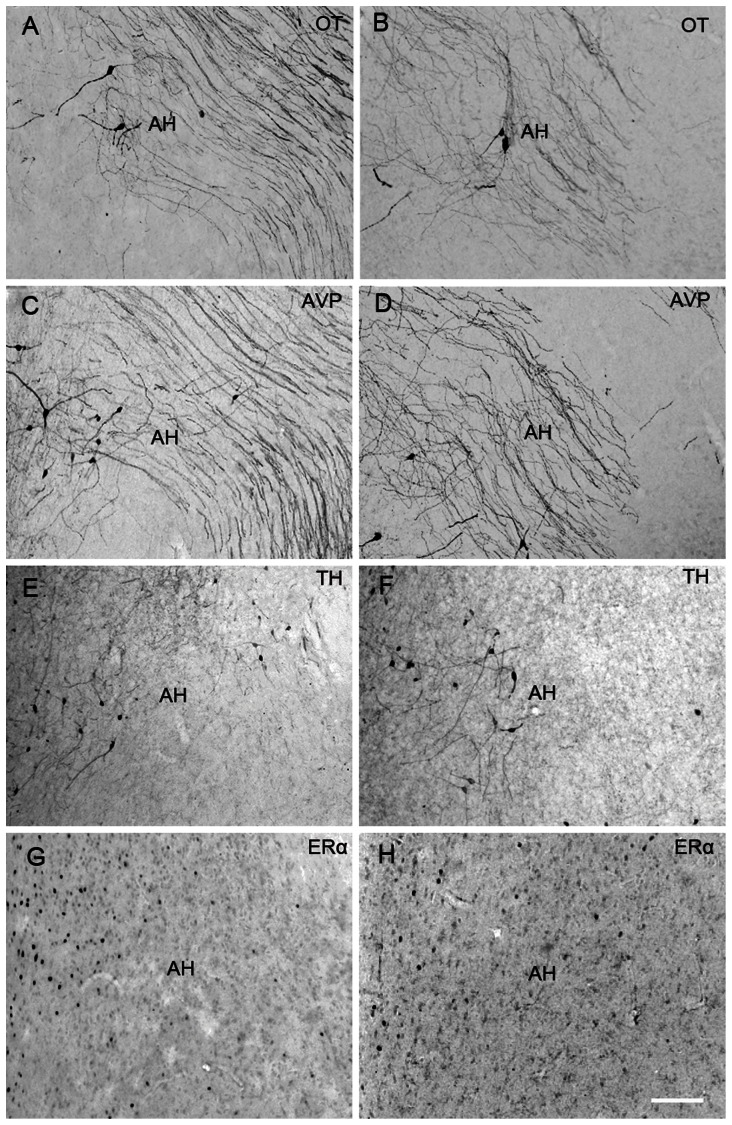
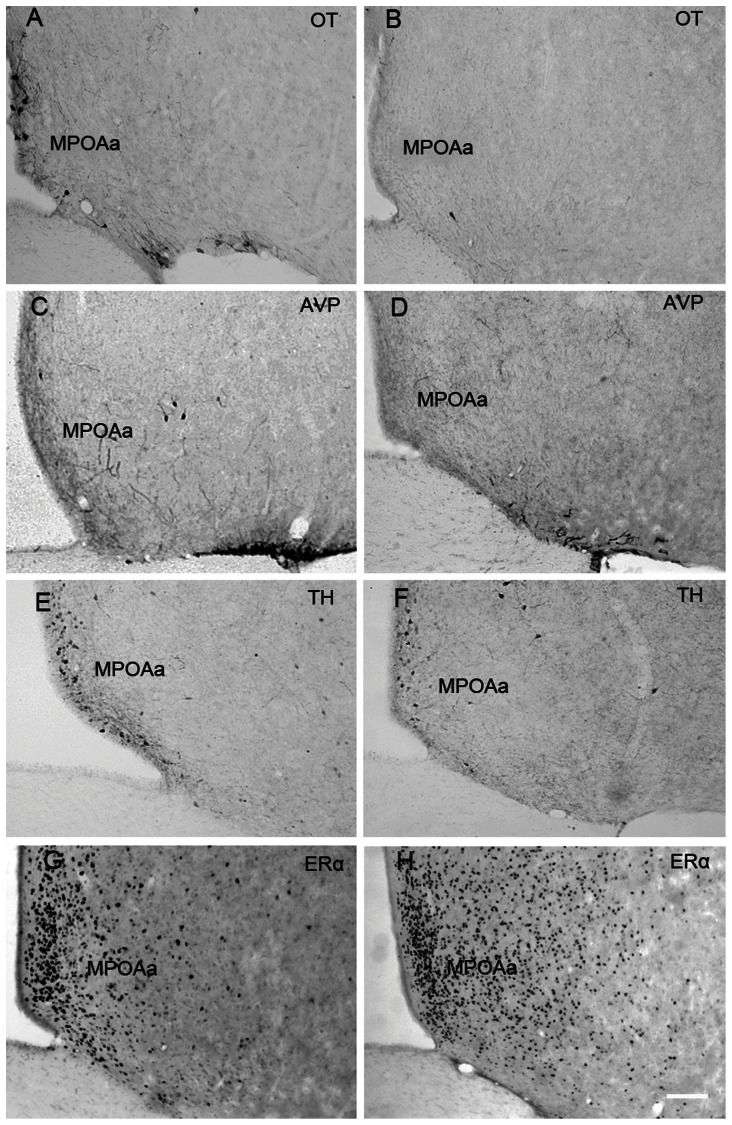
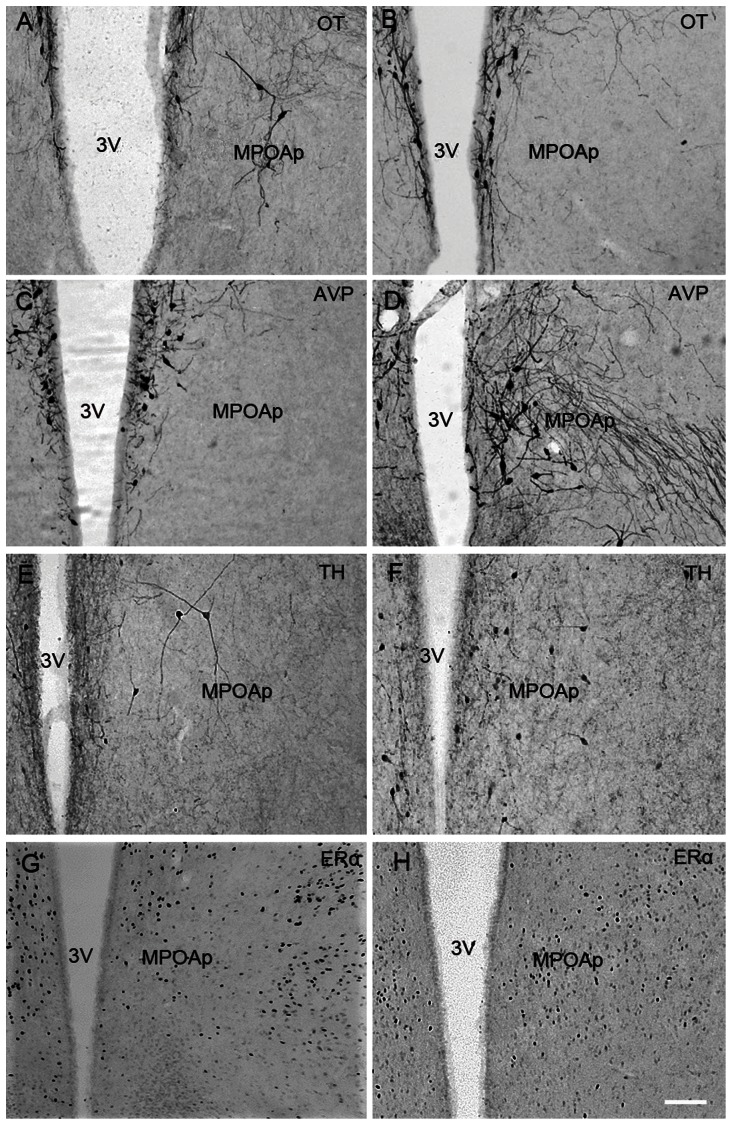
Figures 5, 6 and 7 show representative photomicrographs displaying OT-ir, AVP-ir, TH-ir and ERα-ir cells in the AH (Fig. 5), MPOAa (Fig. 6) and MPOAp (Fig. 7) in both species.
Figure 5. Photomicrographs of neuropeptides expression in the anterior hypothalamus (AH).
Photomicrographs displaying OT-ir (A & B), AVP-ir (C & D), TH-ir (E & F) and ERα-ir (G & H) stained cells in the anterior hypothalamus (AH) in the brains of Mongolian gerbils (A, C, E & G), and Chinese striped hamsters (B, D, F & H). Scale bar = 100 µm.
Figure 6. Photomicrographs of neuropeptides expression in the anterior subnucleus of the medial preoptic area (MPOAa).
Photomicrographs displaying OT-ir (A & B), AVP-ir (C & D), TH-ir (E & F) and ERα-ir (G & H) stained cells in the anterior subnucleus of the medial preoptic area (MPOAa) in the brains of Mongolian gerbils (A, C, E & G) and Chinese striped hamsters (B, D, F & H). Scale bar = 100 µm.
Figure 7. Photomicrographs of neuropeptides expression in the posterior subnucleus of the medial preoptic area (MPOAp).
Photomicrographs displaying OT-ir (A & B), AVP-ir (C & D), TH-ir (E & F) and ERα-ir (G & H) stained cells in the posterior subnucleus of the medial preoptic area (MPOAp) in the brains of Mongolian gerbils (A, C, E & G) and Chinese striped hamsters (B, D, F & H). 3V, third ventricle, Scale bar = 100 µm.
Discussion
Animals with different life strategies usually display different patterns of social behaviors. Previous studies have shown that social Mongolian gerbils display high levels of social behaviors including agonistic behavior, social affiliation and parental care [33], [35], [45]–[48]. These gerbils live in stable family groups consisting of a male and a female, suckling pups and weaning juveniles [33]. The juveniles usually display alloparental care toward their younger siblings [49]. To prepare for winter, all members in the family group participate in food hoarding [50]. In contrast, solitary Chinese striped hamsters are less social and only participate in prosocial contact with conspecifics during mating [41], [42]. In the field, single adult hamsters live alone in burrow nests [41]. A clear distinction has also been reported for their aggressive behavior; Chinese striped hamsters are territorial and display high levels of flank marking behavior and aggression towards conspecifics [41], [42], whereas Mongolian gerbils are less aggressive [33], [35]. In the present study, we compared between these two species, OT, AVP, TH and ERα immunoreactive expression in selected brain areas known to be important in social behaviors. Our data demonstrate species-specific patterns of neurochemical expression in a brain region-specific manner. These data provide further evidence to support the notion that species-specific neurochemical pathways in the brain are associated with and possibly involved in the regulation of social behaviors related to different life strategies (Table 5) [1], [5], [6].
Table 5. Neurochemical implications in social behaviors.
| Oxytocin | ||||
| Behavior | Species | Brain area | Effect | References |
| Maternal behavior | Rat | BST, MPOA, VTA | ↑ | [60], [95] |
| Naked mole-rat | NAcc, MPOA | ↑ | [61] | |
| Prairie vole | NAcc | ↑ | [96] | |
| Brandt's vole | MPOA | ↑ | [2] | |
| Greater long-tailed hamster | MPOA | ↑ | [2] | |
| Maternal aggression | Rat | PVN, CeA, BST | ↓ | [97], [98] |
| Syrian hamster | CeA | ↑ | [99] | |
| Social recognition | Rat | MPOA, LS | ↑ | [62], [63] |
| Mouse | MeA | ↑ | [100] | |
| Brandt's vole | MeA | ↑ | [2] | |
| Greater long-tailed hamster | MeA | ↑ | [2] | |
| Pair bonding | Prairie vole | NAcc | ↑ | [101], [102] |
| Sexual behavior | Rat | MPOA | ↑ | [58], [59] |
↑: increase and↓: decrease in behavior.
In the present study, species differences in neurochemical expression were found in a brain region- and neurochemical-specific manner. In the AH, for example, Mongolian gerbils had higher densities of AVP-ir and ERα-ir, but not OT-ir and TH-ir, cells than Chinese striped hamsters. In the LH, Chinese striped hamsters had a higher density of OT-ir cells, but lower densities of AVP-ir and TH-ir cells, compared to Mongolian gerbils. The AH and LH, as well as neurochemical activity within these regions, have been implicated in a variety of behavioral and physiological functions. For example, AVP and ERα in the AH are involved in flank marking and aggression [15], [51]–[53]. OT and AVP within the LH are involved in feeding and water balance [54]–[57]. It is possible that species differences in the AVP and ERα systems in the AH represent the potential involvement of these neurochemical systems in behaviors related to territory defense. On the other hand, species differences in neurochemical expression within the LH may reflect differences in the central systems regulating behaviors such as feeding and drinking that are important for maintaining one's homeostasis [54]–[56]. Although little is known about feeding and drinking behaviors in these two species, these animals live in distinct geographical regions with different ecological and environmental conditions [35], [41]. Therefore, the species differences in central neurochemical systems noted above may reflect physiological, in addition to behavioral, adaptations to the environment.
The MPOA is a brain area important for a large variety of social behaviors including mating [58], [59], maternal care [60], [61], social recognition [62], [63], territory marking [64] and aggression [65]–[67]. This is a complex brain structure consisting of several subnuclei [2], [68]–[70]. Unfortunately, we know very little about the structural and functional significance of these subnuclei within the MPOA. An interesting finding in the present study is that Mongolian gerbils had higher densities of OT-ir and AVP-ir cells in the MPOAa compared to Chinese striped hamsters, whereas an opposite pattern was found in the MPOAp for both neuropeptides. These data are generally consistent with the data from a previous study comparing social Brandt's voles with solitary Greater long-tailed hamsters [2]. One possibility is that the MPOAa and MPOAp, as well as neuropeptide activity within these regions, have opposing effects on behavioral and physiological functions associated with a social or solitary life strategy. In a previous study in rats, microinjections of an anti-androgen drug into the anteroventral MPOA decreased copulatory behavior but had no effects on sexual motivation, whereas microinjections of the same drug into the posterodorsal MPOA did not influence copulatory performance but decreased sexual motivation [71].
Our data also indicate sexually dimorphic patterns of neurochemical expression in the brain. The density of OT-ir cells in the MPOAi was significantly higher in females than in males in both species. Furthermore, the density of TH-ir cells was higher in the MPOA, particularly in the MPOAa, in females than in males in both species. Discrepancies have been found in previous studies in other rodent species focusing on the MPOA. For example, in rats, the anteroventral periventricular nucleus of the preoptic area (AVPV), which is a sub-region of MPOA, is larger in females than in males [72]. In mice, however, males have a larger MPOA than females [73]. In both rats and mice, females have more TH-expressing cells in the AVPV than males [72], [74]–[77]. However, no sex differences are found in the number of OT-ir cells in the MPOA in voles [2], [28], [61]. Furthermore, although female rats and voles have more cells labeled for ERα in the MPOA and other brain areas (e.g., the VMH, BNST and MeA) [11], [78] compared to males, we did not observe such sex differences in the two species examined in the present study. Therefore, sexually dimorphic patterns of OT-ir and TH-ir expression in the MPOA could be species-specific. Region-specific neurochemical expression is involved in behavioral functions. For example, OT in the MPOA is involved in mating behavior [58], [59], maternal care [60], [61] and social recognition [62], [63]. The functional roles of the sexually dimorphic neurochemical expression noted in the two species examined here are still unknown.
It is worth mentioning that the neurochemicals examined in the present study may interact to influence each other's expression and functions. For example, it has been well documented that estrogen can directly influence the expression and activity of central AVP and OT systems [79], [80]. Estrogen can up-regulate AVP expression in several brain areas including the LS, BST, AH and amygdala [20], [21], [79]. Estrogen can also regulate OT expression in the PVN [80], [81] and OT receptor binding in the MPOA [82]. Further, TH-ir neurons in the PVN are found to co-express AVP [83], and thus dopamine may influence local AVP expression and release [84]. Therefore, it is not surprising to see similar species differences in multiple neurochemicals in a given brain area, and in fact, synergistic effects of multiple neurotransmitters on behavior have been amply demonstrated [85], [86].
Some caveats in the present study need to be discussed. First, while Mongolian gerbils were the offspring of a laboratory breeding colony and were sexually naïve and age matched, Chinese striped hamsters were field captured and thus their ages and reproductive history were unknown. It is worth mentioning that the expression patterns in the brain of the neurochemicals under investigation have been shown to change not only during development but also as a result of reproductive experience and aging [2], [87]. Second, females of both species in the present study are spontaneous ovulators [88], [89]. The fluctuation of circulating estrogen during ovarian cycles is known to influence brain neurochemical expression, including OT [90], [91], AVP [20], [21] and TH [87], as well as to alter its own receptor expression [43], [92], [93]. Therefore, potential variations in circulating levels of estrogen among the females might have affected the observed presence or absence of sexual dimorphisms in neurochemical expression in the present study, as has been reported for typical lab rodent species [43], [94]. Finally, our subject's housing conditions reflected their species-specific life strategies. It is possible, however, that different degrees of social experience during housing may have affected the observed neurochemical staining. Although beyond the scope of the present experiment, these issues need to be considered in follow-up studies.
Acknowledgments
The authors would like to thank all the members in the Animal Ecology Group in the Institute of Zoology at the Chinese Academy of Sciences for their assistance with the experiments.
Funding Statement
This research was supported by grants from the National Natural Science Foundation of China (30930016 and 31272321), the National Basic Research Program of China (2007CB109101), the National Science and Technology Ministry (2009BAI83B01) and the Chinese Academy of Sciences (CZBZX-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bester-Meredith JK, Young LJ, Marler CA (1999) Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav 36: 25–38. [DOI] [PubMed] [Google Scholar]
- 2. Xu LX, Pan YL, Young KA, Wang ZX, Zhang ZB (2010) Oxytocin and vasopressin immunoreactive staining in the brains of Brandt's voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton). Neuroscience 169: 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madison DM, Hill JP, Gleason PE (1984) Seasonality in the nesting-behavior of Peromyscus leucopus. Am Midl Nat 112: 201–204. [Google Scholar]
- 4. Wang ZX, Hulihan TJ, Insel TR (1997) Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res 767: 321–332. [DOI] [PubMed] [Google Scholar]
- 5. Insel TR, Shapiro LE (1992) Oxytocin receptor distribution reflects social-organization in monogamous and polygamous voles. Proc Natl Acad Sci USA 89: 5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Insel TR, Wang ZX, Ferris CF (1994) Patterns of brain vasopressin receptor distribution associated with social-organization in microtine rodents. J Neurosci 14: 5381–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young KA, Liu Y, Wang ZX (2008) The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Phys C148: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang ZX (2002) A critical role for dopamine in pair bonding in male prairie voles. Horm Behav 41: 455–455. [Google Scholar]
- 9. Gingrich B, Liu Y, Cascio C, Wang ZX, Insel TR (2000) Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 114: 173–183. [DOI] [PubMed] [Google Scholar]
- 10. Wang ZX, Yu GZ, Cascio C, Liu Y, Gingrich B, et al. (1999) Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): A mechanism for pair bonding? Behav Neurosci 113: 602–611. [DOI] [PubMed] [Google Scholar]
- 11. Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le WW, et al. (2004) Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res 1016: 247–254. [DOI] [PubMed] [Google Scholar]
- 12. Cushing BS, Wynne-Edwards KE (2006) Estrogen receptor-alpha distribution in male rodents is associated with social organization. J Comp Neurol 494: 595–605. [DOI] [PubMed] [Google Scholar]
- 13. Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, et al. (1998) Roles of estrogen receptor alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 139: 5070–5081. [DOI] [PubMed] [Google Scholar]
- 14. Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E (2008) Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J Neurosci 28: 10399–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trainor BC, Greiwe KM, Nelson RJ (2006) Individual differences in estrogen receptor α in select brain nuclei are associated with individual differences in aggression. Horm Behav 50: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM (2010) Estrogen receptor-alpha in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster). Plos One 5(1): e8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lonstein JS, Greco B, De Vries G, Stern JM, Blaustein JD (2000) Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology 72: 91–101. [DOI] [PubMed] [Google Scholar]
- 18. Rissman EF (2008) Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: Beyond yin/yang. J Neuroendocrinol 20: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodo C, Kudwa AE, Rissman EF (2006) Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology 147: 415–420. [DOI] [PubMed] [Google Scholar]
- 20. Scordalakes EM, Rissman EF (2004) Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav 3: 20–26. [DOI] [PubMed] [Google Scholar]
- 21. Tetel MJ, Pfaff DW (2010) Contributions of estrogen receptor-alpha and estrogen receptor-beta to the regulation of behavior. BBA-Gen Subjects 1800: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, et al. (1997) Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci 17: 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang ZX, Young LJ, De Vries GJ, Insel TR (1998) Voles and vasopressin: A review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog Brain Res 119: 483–499. [DOI] [PubMed] [Google Scholar]
- 24. Bamshad M, Novak MA, Devries GJ (1993) Sex and species-differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus . J Neuroendocrinol 5: 247–255. [DOI] [PubMed] [Google Scholar]
- 25. Beery AK, Lacey EA, Francis DD (2008) Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J Comp Neurol 507: 1847–1859. [DOI] [PubMed] [Google Scholar]
- 26. Bester-Meredith JK, Marler CA (2001) Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus). Horm Behav 40: 51–64. [DOI] [PubMed] [Google Scholar]
- 27. Wang ZX (1995) Species-differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus). Behav Neurosci 109: 305–311. [DOI] [PubMed] [Google Scholar]
- 28. Wang ZX, Zhou L, Hulihan TJ, Insel TR (1996) Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. J Comp Neurol 366: 726–737. [DOI] [PubMed] [Google Scholar]
- 29. Bester-Meredith JK, Marler CA (2003) Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered peromyscus mice. Behav Neurosci 117: 455–463. [DOI] [PubMed] [Google Scholar]
- 30. Mallon DP (1985) The mammals of the Mongolian People's Republic. Mamm Rev 15: 71–102. [Google Scholar]
- 31.Gromov IM, Gurejew AA, Nowukov GA, Sokolov II,Strelkov PP, et al.. (1963) Fauna of the USSR. Moscow: Nauka Publ House. pp.513–528. [Google Scholar]
- 32.Luo ZX, Chen W, Gao W, Wang YX, Li CY, et al..(2000) Fauna sinica: Mammalia, vol. 6, rodentia, part iii, cricetidae. Beijing: Science Press.pp.121–128.[in Chinese]. [Google Scholar]
- 33. Agren G, Zhou Q, Zhong W (1989) Ecology and social-behavior of Mongolian gerbils, Meriones unguiculatus, at Xilinhot, Inner Mongolia, China. Anim Behav 37: 11–27. [Google Scholar]
- 34. Agren G, Zhou Q, Zhong W (1989) Territoriality, cooperation and resource priority - hoarding in the Mongolian gerbil, Meriones unguiculatus . Anim Behav 37: 28–32. [Google Scholar]
- 35.Wang MJ, Zhong WQ, Wan XR (1998) Ecology and management of Mongolian gerbils (Meriones unguiculatus). In: Zhang ZB, Wang ZW,editors. Ecology and management of rodent pests in agriculture.Beijing: Ocean Press. PP.221–236.[in Chinese]. [Google Scholar]
- 36. Agren G (1984) Pair formation in the Mongolian gerbil. Anim Behav 32: 528–535. [Google Scholar]
- 37. Elwood RW (1975) Parental and maternal behaviour in the Mongolian gerbil. Anim Behav 23: 766–772. [Google Scholar]
- 38. Nyby J, Wallace P, Owen K, Thiessen DD (1973) Influence of hormones on hoarding behavior in Mongolian gerbil (meriones-unguiculatus). Horm Behav 4: 283–288. [Google Scholar]
- 39. Prates EJ, Guerra RF (2005) Parental care and sexual interactions in Mongolian gerbils (meriones unguiculatus) during the postpartum estrus. Behav Process 70: 104–112. [DOI] [PubMed] [Google Scholar]
- 40. Bridges NJ, Starkey NJ (2004) Sex differences in Mongolian gerbils in four tests of anxiety. Physiol Behav 83: 119–127. [DOI] [PubMed] [Google Scholar]
- 41.Wang YZ, Lu HQ, Wang YS, Jiang LY (1998) Ecology and management of Chinese stripped hamsters (Cricetulus barabensis). In: Zhang ZB,Wang ZW,editors. Ecology and management of rodent pests in agriculture.Beijing: Ocean Press. PP.20–40.[in Chinese]. [Google Scholar]
- 42. Skirrow MH, Rysan M (1976) Observations on social-behavior of Chinese-hamster, Cricetulus griseus . Can J Zool 54: 361–368. [Google Scholar]
- 43. Pan YL, Xu LX, Wang ZX, Zhang ZB (2011) Expression of oestrogen receptor alpha in the brain of Brandt's voles (Lasiopodomys brandtii): Sex differences and variations during ovarian cycles. J Neuroendocrinol 23: 926–932. [DOI] [PubMed] [Google Scholar]
- 44. Fowler CD, Johnson F, Wang ZX (2005) Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol 489: 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elwood RW, Broom DM (1978) Influence of litter size and parental behavior on development of Mongolian gerbil pups. Anim Behav 26: 438–454. [Google Scholar]
- 46. Gerling S, Yahr P (1979) Effect of the male parent on pup survival in Mongolian gerbils (Meriones unguiculatus). Anim Behav 27: 310–311. [Google Scholar]
- 47. Liu W, Wang G, Wan XR, Zhong WQ (2009) Effects of supplemental food on the social organization of Mongolian gerbils during the breeding season. J Zool 278: 249–257. [Google Scholar]
- 48. Xia W, Liao C, Zhong W, Sun C, Tian Y (1982) On the population dynamics and regulation of Meriones unguiculatus in agricultural region north to Yin Mountains, Inner Mongolia. Acta Theriologica Sinica 2: 51–71 [in Chinese]. [Google Scholar]
- 49. Ostermeyer MC, Elwood RW (1984) Helpers - at the nest in the Mongolian gerbil, Meriones unguiculatus . Behaviour 91: 61–77. [Google Scholar]
- 50. Sorensen DB, Krohn T, Hansen HN, Ottesen JL, Hansen AK (2005) An ethological approach to housing requirements of golden hamsters, Mongolian gerbils and fat sand rats in the laboratory - a review. Appl Anim Behav Sci 94: 181–195. [Google Scholar]
- 51. Ferris CF, Potegal M (1988) Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav 44: 235–239. [DOI] [PubMed] [Google Scholar]
- 52.Riters LV, Panksepp J (1997) Effects of vasotocin on aggressive behavior in male japanese quail. In: Carter CS, Lederhendler II, Kirkpatrick B, editors. Integrative neurobiology of affiliation. pp. 478–480. [DOI] [PubMed] [Google Scholar]
- 53. Gobrogge KL, Liu Y, Young LJ, Wang ZX (2009) Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. P Natl Acad Sci USA 106: 19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weitzman RE, Kleeman CR (1979) Clinical physiology of water metabolism .1. Physiologic regulation of arginine vasopressin secretion and thirst. West J Med 131: 373–400. [PMC free article] [PubMed] [Google Scholar]
- 55. Dasilva RKP, Saad WA, Renzi A, Menani JV, Camargo LAD (1995) Effect of lateral hypothalamus lesions on the water and salt intake, and sodium and urine excretion induced by activation of the median preoptic nucleus in conscious rats. J Auton Nerv Syst 53: 195–204. [DOI] [PubMed] [Google Scholar]
- 56. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, et al. (1995) Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. P Natl Acad Sci USA 92: 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delgado JMR, Anand BK (1953) Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol 172: 162–168. [DOI] [PubMed] [Google Scholar]
- 58. Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA (1989) Medial preoptic area oxytocin and female sexual receptivity. Behav Neurosci 103: 655–&. [DOI] [PubMed] [Google Scholar]
- 59. Caldwell JD, Moe BD (1999) Conjugated estradiol increases female sexual receptivity in response to oxytocin infused into the medial preoptic area and medial basal hypothalamus. Horm Behav 35: 38–46. [DOI] [PubMed] [Google Scholar]
- 60. Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA (1994) Oxytocin activates the postpartum onset of rat maternal-behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci 108: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 61. Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG (2008) Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience 155: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Popik P, van Ree JM (1991) Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharm 1: 555–560. [DOI] [PubMed] [Google Scholar]
- 63. Popik P, Vos PE, Vanree JM (1992) Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav Pharmacol 3: 351–358. [PubMed] [Google Scholar]
- 64. Hennessey AC, Huhman KL, Albers HE (1994) Vasopressin and sex-differences in hamster flank marking. Physiol Behav 55: 905–911. [DOI] [PubMed] [Google Scholar]
- 65. Rosenblatt JS, Hazelwood S, Poole J (1996) Maternal behavior in male rats: Effects of medial preoptic area lesions and presence of maternal aggression. Horm Behav 30 (3) 201–215. [DOI] [PubMed] [Google Scholar]
- 66. Gammie SC, Nelson RJ (1999) Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci 19 (18) 8027–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harmon AC, Huhman KL, Moore TO, Albers HE (2002) Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol 14 (12) 963–969. [DOI] [PubMed] [Google Scholar]
- 68. Gray P, Brooks PJ (1984) Effect of lesion location within the medial preoptic-anterior hypothalamic continuum on maternal and male sexual behaviors in female rats. Behav Neurosci 98: 703–711. [DOI] [PubMed] [Google Scholar]
- 69. Giuliano F, Rampin O, Brown K, Courtois F, Benoit G, et al. (1996) Stimulation of the medial preoptic area of the hypothalamus in the rat elicits increases in intracavernous pressure. Neurosci Lett 209: 1–4. [DOI] [PubMed] [Google Scholar]
- 70. Reuss S, Hermes B, Fuchs E, Hiemke C (1999) Day- and night-time contents of monoamines and their metabolites in the medial preoptic area of the rat hypothalamus. Neurosci Lett 266: 29–32. [DOI] [PubMed] [Google Scholar]
- 71. McGinnis MY, Montana RC, Lumia AR (2002) Effects of hydroxyflutamide in the medial preoptic area or lateral septum on reproductive behaviors in male rats. Brain Res Bull 59: 227–234. [DOI] [PubMed] [Google Scholar]
- 72. Simerly RB, Swanson LW, Gorski RA (1985) The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin-release - immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res 330: 55–64. [DOI] [PubMed] [Google Scholar]
- 73. Schradin C, Anzenberger G (1999) Prolactin, the hormone of paternity. News Physiol Sci 14: 223–231. [DOI] [PubMed] [Google Scholar]
- 74. Simerly RB, Swanson LW, Handa RJ, Gorski RA (1985) Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology 40: 501–510. [DOI] [PubMed] [Google Scholar]
- 75. Simerly RB (1989) Hormonal-control of the development and regulation of tyrosine-hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Mol Brain Res 6: 297–310. [DOI] [PubMed] [Google Scholar]
- 76. Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS (1997) Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. P Natl Acad Sci USA 94: 14077–14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, et al. (2003) Overexpression of Bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci 23: 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu RY, Yuan AF, Yuan QW, Guo R, Tai FD, et al. (2011) Comparison of sociability, parental care and central estrogen receptor alpha expression between two populations of mandarin voles (Microtus mandarinus). J Comp Physiol A 197: 267–277. [DOI] [PubMed] [Google Scholar]
- 79. Plumari L, Viglietti-Panzica C, Allieri F, Honda S, Harada N, et al. (2002) Changes in the arginine-vasopressin immunoreactive systems in male mice lacking a functional aromatase gene. J Neuroendocrinol 14: 971–978. [DOI] [PubMed] [Google Scholar]
- 80. Dellovade TL, Zhu YS, Pfaff DW (1999) Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J Neuroendocrinol 11: 1–10. [DOI] [PubMed] [Google Scholar]
- 81. Akaishi T, Sakuma Y (1985) Estrogen excites oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Res 335: 302–305. [DOI] [PubMed] [Google Scholar]
- 82. Young LJ, Wang ZX, Donaldson R, Rissman EF (1998) Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport 9: 933–936. [DOI] [PubMed] [Google Scholar]
- 83. Panayotacopoulou MT, Raadsheer FC, Swaab DF (1994) Colocalization of tyrosine-hydroxylase with oxytocin or vasopressin in neurons of the human paraventricular and supraoptic nucleus. Dev Brain Res 83: 59–66. [DOI] [PubMed] [Google Scholar]
- 84. Dudas B, Semeniken KR, Merchenthaler I (2006) Morphological substrate of the catecholaminergic input of the vasopressin neuronal system in humans. J Neuroendocrinol 18: 895–901. [DOI] [PubMed] [Google Scholar]
- 85. Buccafusco JJ, Terry AV (2000) Multiple central nervous system targets for eliciting beneficial effects on memory and cognition. J Pharmacol Exp Ther 295: 438–446. [PubMed] [Google Scholar]
- 86. Bartfai T, Iverfeldt K, Fisone G, Serfozo P (1988) Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol 28: 285–310. [DOI] [PubMed] [Google Scholar]
- 87. Mohankumar PS, Thyagarajan S, Quadri SK (1997) Tyrosine hydroxylase and DOPA decarboxylase activities in the medial preoptic area and arcuate nucleus during the estrous cycle: Effects of aging. Brain Res Bull 42: 265–271. [DOI] [PubMed] [Google Scholar]
- 88. McDermott JL, Fischer J, Carter CS (1980) Long-term estrogen and progesterone and mating stimuli as regulators of female sexual receptivity in the Mongolian gerbil. Behav Neural Biol 29: 63–72. [DOI] [PubMed] [Google Scholar]
- 89. Yang YP, Li Y, Zhou YL, Li QH (2002) Study on characteristics of artificial feeding and breeding habits of striped hamster. Journal of Inner Mongolia University (Natural Science Edition) Vol. 33: 201–204 [in Chinese]. [Google Scholar]
- 90. Tabak J, Gonzalez-Iglesias AE, Toporikova N, Bertram R, Freeman ME (2010) Variations in the response of pituitary lactotrophs to oxytocin during the rat estrous cycle. Endocrinology 151: 1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kawamata M, Tonomura Y, Kimura T, Yanagisawa T, Nishimori K (2004) The differential coupling of oxytocin receptors to uterine contractions in murine estrous cycle. Biochem Biophys Res Commun 321: 695–699. [DOI] [PubMed] [Google Scholar]
- 92. Shughrue PJ, Bushnell CD, Dorsa DM (1992) Estrogen-receptor messenger-ribonucleic-acid in female rat-brain during the estrous-cycle - a comparison with ovariectomized females and intact males. Endocrinology 131: 381–388. [DOI] [PubMed] [Google Scholar]
- 93. Simerly RB, Carr AM, Zee MC, Lorang D (1996) Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 8: 45–56. [DOI] [PubMed] [Google Scholar]
- 94. Greco B, Allegretto EA, Tetel MJ, Blaustein JD (2001) Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: Effects of estradiol treatment. Endocrinology 142: 5172–5181. [DOI] [PubMed] [Google Scholar]
- 95. Francis DD, Young LJ, Meaney MJ, Insel TR (2002) Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (v1a) receptors: Gender differences. J Neuroendocrinol 14: 349–353. [DOI] [PubMed] [Google Scholar]
- 96. Olazabal DE, Young LJ (2006) Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141: 559–568. [DOI] [PubMed] [Google Scholar]
- 97. Giovenardi M, Padoin MJ, Cadore LP, Lucion AB (1998) Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav 63: 351–359. [DOI] [PubMed] [Google Scholar]
- 98. Consiglio AR, Borsoi A, Pereira GAM, Lucion AB (2005) Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav 85: 354–362. [DOI] [PubMed] [Google Scholar]
- 99. Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, et al. (1992) Oxytocin in the amygdala facilitates maternal aggression. Ann Ny Acad Sci 652: 456–457. [DOI] [PubMed] [Google Scholar]
- 100. Ferguson JN, Aldag JM, Insel TR, Young LJ (2001) Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Young LJ, Lim MM, Gingrich B, Insel TR (2001) Cellular mechanisms of social attachment. Horm Behav 40: 133–138. [DOI] [PubMed] [Google Scholar]
- 102. Liu Y, Wang ZX (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121: 537–544. [DOI] [PubMed] [Google Scholar]
- 103. Caldwell JD, Greer ER, Johnson MF, Prange AJ, Pedersen CA (1987) Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology 46: 39–47. [DOI] [PubMed] [Google Scholar]
- 104. Gobrogge KL, Liu Y, Jia X, Wang Z (2007) Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol 502: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 105. Delville W, Mansour KM, Ferris CF (1996) Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav 60: 25–29. [DOI] [PubMed] [Google Scholar]
- 106. Delville Y, De Vries GJ, Ferris CF (2000) Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evolut 55: 53–76. [DOI] [PubMed] [Google Scholar]
- 107. Caldwell HK, Albers HE (2004) Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav 46: 444–449. [DOI] [PubMed] [Google Scholar]
- 108. Engelmann M, Landgraf R (1994) Microdialysis administration of vasopressin into the septum improves social recognition in brattleboro rats. Physiol Behav 55: 145–149. [DOI] [PubMed] [Google Scholar]
- 109. Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, et al. (2003) Viral vector-mediated gene transfer of the vole v1a vasopressin receptor in the rat septum: Improved social discrimination and active social behaviour. Eur J Neurosci 18: 403–411. [DOI] [PubMed] [Google Scholar]
- 110. Liu Y, Curtis JT, Wang ZX (2001) Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (microtus ochrogaster). Behav Neurosci 115: 910–919. [DOI] [PubMed] [Google Scholar]
- 111. Keer SE, Stern JM (1999) Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav 67: 659–669. [DOI] [PubMed] [Google Scholar]
- 112. Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, et al. (2005) The effects of d1 or d2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci 119: 1588–1604. [DOI] [PubMed] [Google Scholar]
- 113. Silva MRP, Bernardi MM, Cruz-Casallas PE, Felicio LF (2003) Pimozide injections into the nucleus accumbens disrupt maternal behaviour in lactating rats. Pharmacology & Toxicology 93: 42–47. [DOI] [PubMed] [Google Scholar]
- 114. Hansen S (1994) Maternal-behavior of female rats with 6-ohda lesions in the ventral striatum - characterization of the pup retrieval deficit. Physiol Behav 55: 615–620. [DOI] [PubMed] [Google Scholar]
- 115. Lubin DA, Cannon JB, Black MC, Brown LE, Johns JM (2003) Effects of chronic cocaine on monoamine levels in discrete brain structures of lactating rat dams. Pharmacol Biochem Be 74: 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, et al. (2006) Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 9: 133–139. [DOI] [PubMed] [Google Scholar]
- 117. Northcutt KV, Wang Z, Lonstein JS (2007) Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol 500: 103–115. [DOI] [PubMed] [Google Scholar]
- 118. Aragona BJ, Liu Y, Curtis T, Stephan FK, Wang ZX (2003) A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci 23: 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pfaus JG, Phillips AG (1991) Role of dopamine in anticipatory and consummatory aspects of sexual-behavior in the male-rat. Behav Neurosci 105: 727–743. [DOI] [PubMed] [Google Scholar]
- 120. Everitt BJ (1990) Sexual motivation - a neural and behavioral-analysis of the mechanisms underlying appetitive and copulatory responses of male-rats. Neurosci Biobehav Rev 14: 217–232. [DOI] [PubMed] [Google Scholar]
- 121. Warner RK, Thompson JT, Markowski VP, Loucks JA, Bazzett TJ, et al. (1991) Microinjection of the dopamine antagonist cis-flupentixol into the mpoa impairs copulation, penile reflexes and sexual motivation in male-rats. Brain Res 540: 177–182. [DOI] [PubMed] [Google Scholar]
- 122. Pellis SM, Castaneda E, McKenna MM, Trannguyen LTL, Whishaw IQ (1993) The role of the striatum in organizing sequences of play fighting in neonatally dopamine-depleted rats. Neurosci Lett 158: 13–15. [DOI] [PubMed] [Google Scholar]
- 123. Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, et al. (2006) Maternal care associated with methylation of the estrogen receptor-alpha 1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147: 2909–2915. [DOI] [PubMed] [Google Scholar]
- 124. Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, et al. (2010) Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology 91: 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]