Abstract
Edwardsiella ictaluri is a Gram-negative facultative intracellular pathogen causing enteric septicemia of channel catfish (ESC). The disease causes considerable economic losses in the commercial catfish industry in the United States. Although antibiotics are used as feed additive, vaccination is a better alternative for prevention of the disease. Here we report the development and characterization of novel live attenuated E. ictaluri mutants. To accomplish this, several tricarboxylic acid cycle (sdhC, mdh, and frdA) and one-carbon metabolism genes (gcvP and glyA) were deleted in wild type E. ictaluri strain 93-146 by allelic exchange. Following bioluminescence tagging of the E. ictaluri ΔsdhC, Δmdh, ΔfrdA, ΔgcvP, and ΔglyA mutants, their dissemination, attenuation, and vaccine efficacy were determined in catfish fingerlings by in vivo imaging technology. Immunogenicity of each mutant was also determined in catfish fingerlings. Results indicated that all of the E. ictaluri mutants were attenuated significantly in catfish compared to the parent strain as evidenced by 2,265-fold average reduction in bioluminescence signal from all the mutants at 144 h post-infection. Catfish immunized with the E. ictaluri ΔsdhC, Δmdh, ΔfrdA, and ΔglyA mutants had 100% relative percent survival (RPS), while E. ictaluri ΔgcvP vaccinated catfish had 31.23% RPS after re-challenge with the wild type E. ictaluri.
Introduction
Channel catfish, Ictalurus punctatus, farming is the largest aquaculture industry in the United States, and enteric septicemia of catfish (ESC), caused by Edwardsiella ictaluri, is the most prevalent disease affecting this industry. Although Romet® 30, Terramycin®, and Aquaflor® are approved antibiotics to treat infections in commercial catfish by oral delivery in medicated feed, effectiveness is limited because fish develop anorexia at early stages of the infection. Also, antibiotic resistant E. ictaluri strains can emerge [1]. Therefore, vaccination is the preferred method for prevention of ESC.
Live attenuated vaccines can provide effective protection against certain diseases if they can express protective antigens without causing disease in the host [2]. In E. ictaluri, some candidate live attenuated vaccines that have been developed include chondroitinase [3] and auxotrophic (aroA and purA) [4], [5] mutants. However, none of these vaccine candidates are in commercial production. The commercial vaccine Aquavac-ESC (RE-33) was developed by selecting for rifampin resistance [6]. However, antibiotic resistance is not a desired trait for a vaccine. In addition, the genetic basis for attenuation in RE-33 is undefined [7], although it is known that RE-33 expresses shortened LPS O side chains [8]. Despite the availability of Aquavac-ESC, ESC is still the most prevalent disease in the catfish industry [9], [10].
E. ictaluri is considered a facultative intracellular pathogen, and it is capable of surviving inside channel catfish neutrophils and macrophages [11], [12]. Although E. ictaluri is effectively phagocytosed by catfish neutrophils, it is only killed by neutrophils to a limited extent [11], [13]. A recent study by Karsi et al. [14] showed that genes encoding tricarboxylic acid (TCA) cycle enzymes, glycine cleavage system, a sigmaE regulator, the SoxS oxidative response system, and a plasmid-encoded type III secretion system (TTSS) effector are important for survival in neutrophils [14]. The same study discovered that some neutrophil-susceptible E. ictaluri strains were highly attenuated and demonstrated very good potential as live attenuated vaccines. In particular, strains with insertion mutations in genes encoding TCA cycle enzymes succinate dehydrogenase (sdhC) (EiAKMut5) and malate dehydrogenase (mdh) (EiAKMut12) generated better protection than the available commercial vaccine when juvenile catfish were vaccinated by immersion [14]. Similarly, E. ictaluri glycine dehydrogenase (gcvP) mutants (EiAKMut02 and EiAKMut08) were also completely attenuated and had better vaccine efficacy than the commercial vaccine [14]. Glycine dehydrogenase is part of the glycine cleavage system pathway, which is part of one-carbon (C1) metabolism. Therefore, the objective of this research was to introduce in-frame deletions in E. ictaluri sdhC, mdh, and frdA genes (encoding enzymes in the TCA cycle) and gcvP and glyA genes (encoding C1 metabolism proteins) to determine their roles in E. ictaluri virulence.
Materials and Methods
Ethics statement
All fish experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Mississippi State University.
Bacterial strains, plasmids, and growth conditions
Bacterial strains and plasmids used in this work are listed in Table 1. E. ictaluri was grown at 30°C using brain heart infusion (BHI) broth and agar (Difco, Sparks, MD). Escherichia coli were grown at 37°C using Luria-Bertani (LB) broth and agar (Difco). E. coli CC118 λpir and SM10 λpir/S17-1 λpir were used for cloning gene deletions into suicide plasmid pMEG-375 and transferring recombinant pMEG-375 or pAKgfplux1 into E. ictaluri. Ampicillin was used at 100 µg/ml to maintain pMEG-375 and pAKgfplux1. Colistin was used at 12.5 µg/ml for counter selection against E. coli SM10 λpir following conjugation. E. ictaluri strains were cultivated for 18 h (stationary phase) for all fish challenges.
Table 1. Bacterial strains and plasmids.
| Strain | Relevant Characteristics | References |
| Edwardsiella ictaluri | ||
| 93-146 | Wild type; pEI1+; pEI2+; Colr | [32] |
| EiΔfrdA | 93-146 derivative; pEI1+; pEI2+; Colr; ΔfrdA | This study |
| EiΔgcvp | 93-146 derivative; pEI1+; pEI2+; Colr; ΔgcvP | This study |
| EiΔglyA | 93-146 derivative; pEI1+; pEI2+; Colr; ΔglyA | This study |
| EiΔsdhC | 93-146 derivative; pEI1+; pEI2+; Colr; ΔsdhC | This study |
| EiΔmdh | 93-146 derivative; pEI1+; pEI2+; Colr; Δmdh | This study |
| Escherichia coli | ||
| CC118 λpir | Δ(ara-leu); araD; ΔlacX74; galE; galK; phoA20; thi-1; rpsE; rpoB; argE(Am); recAl; λpirR6K | [33] |
| SM10 λpir | thi; thr; leu; tonA; lacY; supE; recA; ::RP4-2-Tc::Mu; Kmr; λpirR6K | [34] |
| S17-1 λpir | RP4-2 (Km::Tn7, Tc::Mu-1), ΔuidA3::pir+, recA1, endA1, thi-1,hsdR17, creC510 | [35] |
| Plasmids | ||
| pMEG-375 | 8142 bp, Ampr, Cmr, lacZ, R6K ori, mob incP, sacR sacB | [36] |
| pEiΔfrdA | 10242 bp, ΔfrdA, pMEG-375 | This study |
| pEiΔgcvp | 12231 bp, ΔgcvP, pMEG-375 | This study |
| pEiΔglyA | 14276 bp, ΔglyA, pMEG-375 | This study |
| pEiΔsdhC | 16295 bp, ΔsdhC, pMEG-375 | This study |
| pEiΔmdh | 18350 bp, Δmdh, pMEG-375 | This study |
Construction and bioluminescence tagging of in-frame deletion mutants
The method of overlap extension PCR [15] was used to generate in-frame deletions of E. ictaluri sdhC, mdh, frdA, gcvP, and glyA. Four primers were designed for each gene including forward (lflp), internal-reverse (lfrp), internal forward (rflp), and reverse primers (rfrp) (Table 2). Restriction sites were included in forward and reverse primers. Genomic DNA was isolated from E. ictaluri using a Wizard Genomic DNA Kit (Promega, Madison, WI) and used as template in PCR. Upper fragments were amplified by forward and internal-reverse primer sets while reverse and internal-forward primer sets were used to amplify lower fragments. The resulting upper and lower PCR products were gel extracted using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), mixed in a 1∶1 ratio, and then re-amplified using the forward and reverse primers. The resulting in-frame deleted fragment was purified by using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). The purified PCR product was digested with appropriate restriction enzymes (Promega) (Table 1) and cleaned using a Wizard SV Gel and PCR Clean-Up Kit (Promega).
Table 2. Primers with restriction enzyme used for the construction of the E. ictaluri mutants.
| Genes | Primer ID | Primer Sequence ((5′→3′)a | REb |
| frdA | EifrdAlflp | AAGAGCTCTCGTCCACTTCATTCATCAGAC | SacI |
| EifrdAlfrp | GTGGAAGTGGAAATCGAAAGA | ||
| EifrdArflp | TCTTTCGATTTCCACTTCCACGAAGCTCAGGAAGCCAAGAAG | ||
| EifrdArfrp | AATCTAGAGCAGGGAGATGATATTGAGGAC | XbaI | |
| EifrdA01S | CCTCAACTGAAGATTGCCTTA | ||
| gcvP | EigcvPlflp | AATCTAGACCTTTGGCGTGGAGATATGC | XbaI |
| EigcvPlfrp | AGCATCACTGTTTTCAAGCTG | ||
| EigcvPrflp | CAGCTTGAAAACAGTGATGCTGTAAAGCGCCTGGACGATGT | ||
| EigcvPrfrp | AAGAGCTCCGGACAGAGACATACCACCAA | SacI | |
| Eigcvp01S | GGCCTTTTGGTATGATTTGC | ||
| glyA | EiglyAlflp | AAGAGCTCGGGCATGGGTCAGTGAATAC | SacI |
| EiglyAlfrp | CCACAGCTCGGTATCGTAATC | ||
| EiglyArflp | GATTACGATACCGAGCTGTGGGTGAACGTCTTCCGGTCTATG | ||
| EiglyArfrp | AACCCGGGGCCTAGACGATGTCTCCTTGA | SmaI | |
| EiglyA01S | GGGCCAGATTTACTCAAAACC | ||
| sdhC | EisdhClflp | AAGAGCTCCAGCCTCCTTTGGTACTGCTA | SacI |
| EisdhClfrp | GCAAATCCAGATTGACAGGTCT | ||
| EisdhCrflp | AGACCTGTCAATCTGGATTTGCGGGTATGGTAAGCAACGCATC | ||
| EisdhCrfrp | AACCCGGGCCCCATCATGTAGTGACAGGT | SmaI | |
| EisdhC01S | CTCAGTCTCGTGGGATTTGC | ||
| mdh | Eimdhlflp | AAGAGCTCGGCTTTATAATGGCGTGTGG | SacI |
| Eimdhlfrp | AGGCAGCTGAGTCTTAAGCAG | ||
| Eimdhrflp | CTGCTTAAGACTCAGCTGCCTCTGGGCGAAGACTTTATCAAT | ||
| Eimdhrfrp | AACCCGGGGGAGCAGGCCCTACAAGACT | SmaI | |
| Eimdh01S | CAGCTCGCAATCTGAGTGTT |
RE: restriction enzyme sequence added to the 5′ end of the primer sequence.
Bold letters at the 5′ end of the primer sequence represent RE site. AA nucleotides were added to the end of each primer containing a RE site to increase the efficiency of enzyme cut. Underlined bases in internal primer (rflp) indicate reverse complemented internal primer (lfrp) sequence.
The suicide plasmid pMEG-375 was purified from an overnight E. coli culture by a QIAprep Spin Miniprep Kit (Qiagen) and cut with restriction enzymes respective to the inserts, producing compatible ends. The purified PCR product with in-frame deletion was ligated into pMEG-375 vector using T4 DNA Ligase (Promega) at 4°C overnight, generating pEiΔsdhC, pEiΔmdh, pEiΔfrdA, pEiΔgcvP, and pEiΔglyA (Table 1). Insert in each plasmid was confirmed by restriction enzyme digestion as well as sequencing.
The suicide plasmids with in-frame deleted genes were transferred into E. coli SM10 λpir/S17-1 λpir by electroporation and mobilized into E. ictaluri 93-146 by conjugation [16]. The recipient bacteria were spread on BHI plates containing colistin (12.5 ug/ml) and ampicillin (100 ug/ml) to select E. ictaluri with integrated vector by single crossover through allelic exchange. Ampicillin resistant colonies were propagated on BHI plates to allow for the second crossover allelic exchange, followed by streaking on BHI plates with 5% sucrose, 0.35% mannitol, and colistin to select for loss of pMEG-375 with sacB gene. Potential mutant colonies were tested for ampicillin sensitivity to ensure loss of the plasmid. Deleted regions were amplified from the resulting ampicillin sensitive colonies and confirmed by sequencing. After confirmation, EiΔsdhC, EiΔmdh, EiΔfrdA, EiΔgcvP, and EiΔglyA mutants were labeled with bioluminescence using pAKgfplux1 as described in Karsi and Lawrence [16].
Mutant virulence and ability to protect against E. ictaluri infection
Experimental infections were conducted in 40-L challenge tanks supplied with flow-through dechlorinated municipal water. Water temperature was maintained at 25°C (±2) throughout the experiments. Twenty-eight specific pathogen free (SPF) catfish fingerlings (14.2±0.35 cm, 25.45±1.82 g) were randomly allocated into seven groups (4 fish/group). Five treatments were injected with E. ictaluri mutants, one group was injected with wild type E. ictaluri strain 93-146, and the last group served as negative control (phosphate-buffered saline (PBS). Fish were anesthetized in water containing 100 mg/L MS222 and injected with approximately 1×104 colony forming units (CFU) in 100 µl PBS.
Bioluminescent imaging (BLI) was conducted using an IVIS 100 Imaging System to measure number of photons emitted by bioluminescent bacteria in fish [17]. Briefly, catfish were anesthetized in water containing 100 mg/L MS222 and transferred immediately to the photon collection chamber for image capture. Total photon emissions from the whole fish body were collected at an exposure time of one min. Following BLI imaging, fish were returned to well-aerated water for recovery. BLI was conducted at 2, 4, 8, and 24 h post-infection, and subsequent daily intervals until 168 h. Bioluminescence was quantified from the fish images using Living Image Software v 2.5 (Caliper Corporation., Hopkinton, Massachusetts), and mean photon counts for each treatment were used in statistical analysis.
To determine the ability of mutants to protect against E. ictaluri infection, the juvenile catfish vaccinated with mutants (virulence challenge) were immersion challenged [14] with 4.8×107 bioluminescent wild type E. ictaluri at 4 weeks post-vaccination. Photon emissions from fish were collected at 2, 4, 8, 24, 48, 72, and 96 h post-infection using an IVIS 100 as described above, and statistical analysis was performed on the mean photon counts.
Mutant ability to protect against ESC induced mortalities
Approximately 420 eight-month-old SPF channel catfish fingerlings (17.61±0.63 cm, 47.47±5.31 g) were stocked into 21 tanks at a rate of 20 fish/tank. Each treatment had three replicate tanks. Treatments consisted of EiΔsdhC, EiΔmdh, EiΔfrdA, EiΔglyA and EiΔgcvP (vaccination), wild type E. ictaluri (positive control), and BHI (sham control). Channel catfish were vaccinated by immersion in water containing approximately 4.3×107 CFU/ml of water for 1 h, followed by gradual removal of bacteria. Mortalities were recorded for 21 days following vaccination. At 21 days post-vaccination, both vaccinated and non-vaccinated treatments were immersion exposed to wild type parent E. ictaluri 93-146 (approximately 3.06×107 CFU/ml), and fish mortalities were recorded daily for 14 days. Relative percent survival (RPS) was calculated according to the following formula: RPS = [1−(% mortality of vaccinated fish/% mortality of non-vaccinated fish)]×100 [18].
Statistical analysis
Photon counts were transformed by taking the base 10 logarithm to improve normality. One-way ANOVA was conducted using SPSS V19 (IBM Corp., Armonk, NY) to compare mean photon counts at each time point (p<0.05). Pairwise comparison of the means was done using Tukey procedure. Data was then retransformed for interpretation.
Results
Construction of the E. ictaluri in-frame deletion mutants
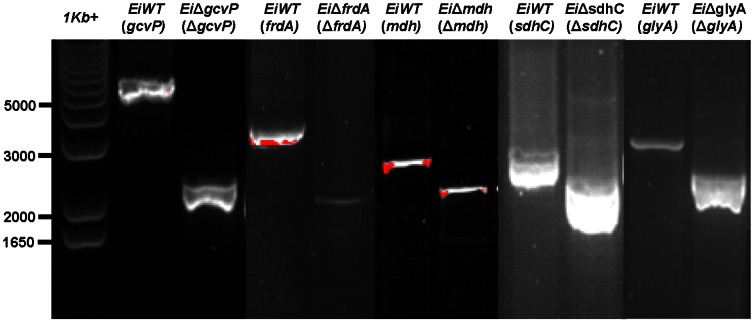
Five in-frame mutants (EiΔsdhC, EiΔmdh, EiΔfrdA, EiΔgcvP, and EiΔglyA) were obtained successfully (Fig. 1) by deleting on average over 90% of each gene (Table 3).
Figure 1. Genotypic confirmation of the E. ictaluri gcvP, frdA, mdh, sdhC, and glyA mutants.
Genomic DNAs was amplified from the E. ictaluri wild type and mutants using the two outside primers (lflp and rfrp) and separated on 1% agarose gel.
Table 3. Properties of the E. ictaluri TCA cycle and C1 metabolism genes and percentage of gene deleted.
| Gene | Locus | Product | ORF (bp/aa) | Remaining (bp/aa) | % Deletion |
| sdhC | NT01EI_2872 | Succinate dehydrogenase, cytochrome b556 subunit, putative | 390/129 | 57/18 | 86.05 |
| mdh | NT01EI_0446 | Malate dehydrogenase, NAD-dependent, putative | 939/312 | 99/32 | 89.74 |
| frdA | NT01EI_0392 | Fumarate reductase, flavoprotein subunit, putative | 1800/899 | 126/41 | 95.44 |
| gcvP | NT01EI_3351 | Glycine dehydrogenase, putative | 2884/960 | 114/37 | 96.15 |
| glyA | NT01EI_3190 | Serine hydroxymethyltransferase, putative | 1254/417 | 75/24 | 94.24 |
Mutant virulence and ability to protect against E. ictaluri infection
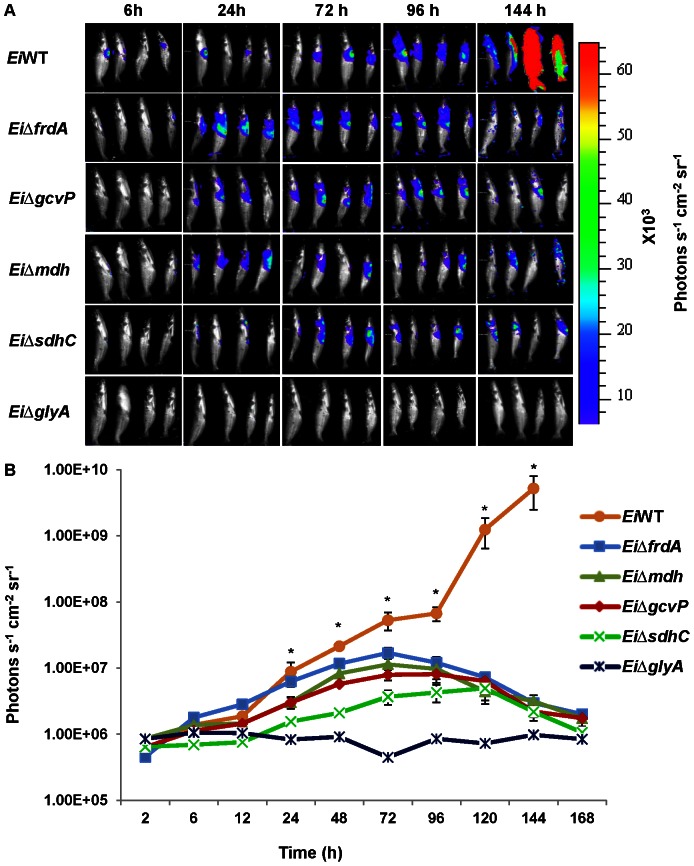
BLI results revealed that bioluminescence (quantified as photon counts) from the catfish infected with EiΔsdhC, EiΔmdh, EiΔfrdA, EiΔgcvP, and EiΔglyA mutants were low at 2, 6, and 12 h post-infection. However, bioluminescence for mutants EiΔmdh, EiΔfrdA, and EiΔgcvP increased from 24 h to 72 h and then decreased thereafter. Bioluminescence for EiΔsdhC followed the same pattern as the other mutants, except the signal peaked at 120 h. However, in mutant EiΔglyA, very low bioluminescence was detected at all time points. In fish infected with wild type E. ictaluri, bioluminescence increased until all fish died (Fig. 2). Average photon counts in the fish infected with 93-146 at 72 h post-infection were approximately 7-fold higher than the average of all fish infected with mutant strains, and it was 2,265-fold higher at 144 h. At this time point, fish infected with wild type E. ictaluri strain died, while bioluminescence from fish infected with mutant strains was in decline (Fig. 2). Photon counts were 118- and 5,329-fold higher in wild type E. ictaluri compared to EiΔglyA at 72 h and 144 h post-infection, respectively (Fig. 2). In the wild type infected treatment, two fish died at 144 h post-infection, and the remaining two fish died at168 h. Mean photon counts between all mutants (except EiΔfrdA) and wild type E. ictaluri were significantly different (p<0.5) at 24 h and thereafter. Mean photon counts for wild type E. ictaluri were significantly higher than EiΔfrdA at 48 h and thereafter.
Figure 2. Bioluminescent imaging of vaccination/attenuation in live catfish after intraperitoneal injection.
A, BLI imaging of catfish. B, Total photon emissions from each fish. Each data point represents the mean photon emissions from four fish. Two of the four channel catfish injected with wild type died at 144 h post-infection. The remaining two died at 168 h post-infection. Star indicates significant difference between wild type E. ictaluri and other mutants, except for EiΔfrdA at 24 h.
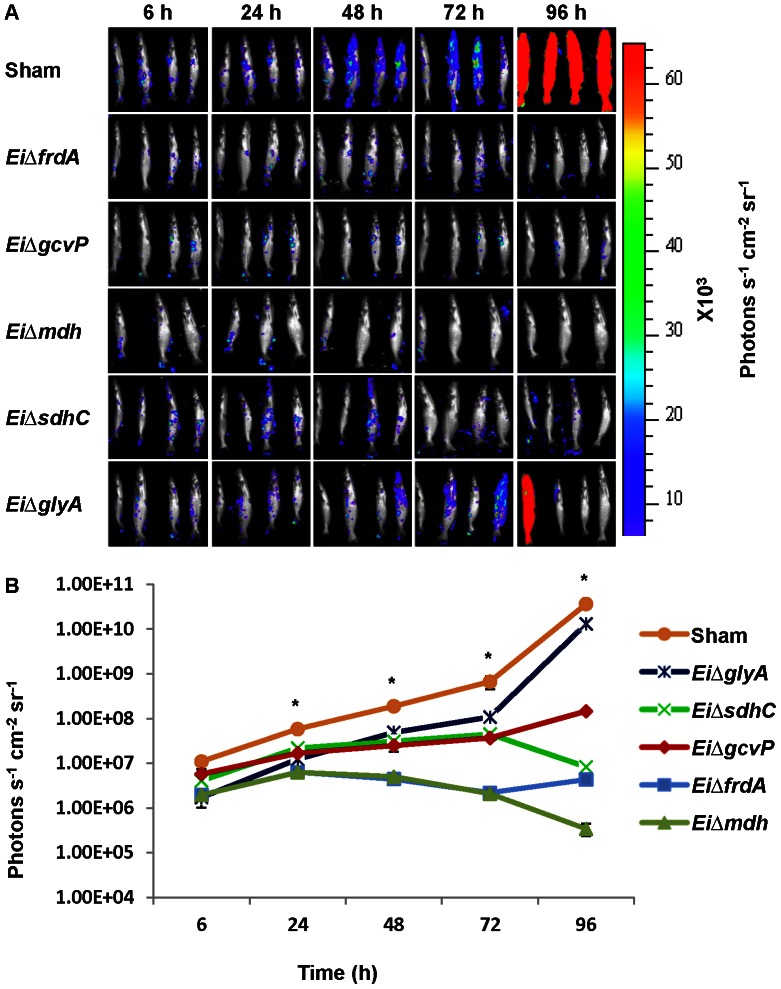
When mutant challenged fish were immersion exposed to wild type E. ictaluri at 4 weeks post-vaccination, photon counts were significantly lower (p<0.5) at each time point for the vaccinated fish compared to the sham-vaccinated control (Fig. 3). Average photon counts in sham-vaccinated fish at 6 h post-infection were 4-fold higher than the average of all five mutant-vaccinated fish treatments, which increased to 14-fold at 96 h. At this time, bioluminescence in EiΔsdhC and EiΔmdh vaccinated fish was declining, while bioluminescence in EiΔfrdA, EiΔgcvP, and EiΔglyA vaccinated fish was increasing (Fig. 3).At 96 h post-infection, all fish in the sham vaccinated group died. In summary, BLI demonstrated that all mutants are significantly attenuated compared to wild type E. ictaluri, and all mutants except EiΔglyA provided significant protection against E. ictaluri infection.
Figure 3. Bioluminescence imaging of juvenile catfish after immersion exposure to wild type E. ictaluri.
Fish were challenged with E. ictaluri mutants as described in the virulence trial, and at 4 weeks post-vaccination they were challenged with bioluminescent wild type E. ictaluri. A, BLI imaging of catfish. B, Total photon emissions from each fish. Each data point represents the mean photon emissions from four fish. Star indicates significant difference between the E. ictaluri mutants and wild type.
Mutant ability to protect against ESC induced mortalities
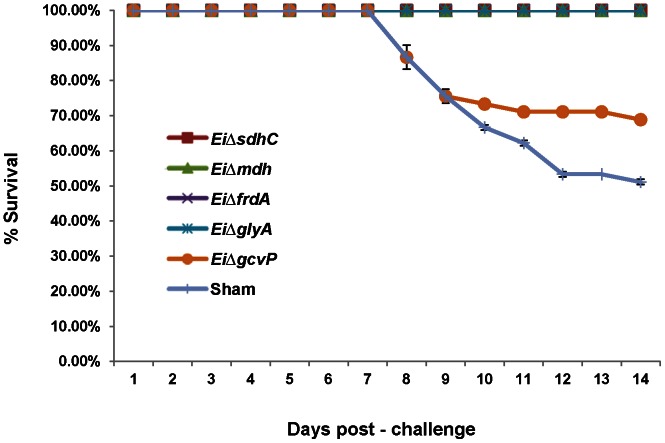
Vaccination of channel catfish with EiΔsdhC, EiΔmdh, EiΔfrdA, and EiΔglyA provided complete protection (100% survival) against wild type E. ictaluri 93-146 while the EiΔgcvP mutant showed lower efficacy (68.89% survival) (Fig. 4). Survival in EiΔsdhC, EiΔmdh, EiΔfrdA, and EiΔglyA vaccinated groups was 1.96-fold higher than that of the non-vaccinated group when re-challenged with wild type E. ictaluri (100% vs 51.11%).
Figure 4. Percent survival of immersion vaccinated catfish.
Catfish fingerlings were immersion vaccinated with the EiΔmdh, EiΔsdhC, EiΔfrdA, EiΔglyA, and EiΔgcvP mutants and challenged with wild type E. ictaluri strain 93-146. Data points represent the mean percent survival of 20 fish per tank for each treatment.
Discussion
The primary objective of this study was to construct live attenuated E. ictaluri strains based on mutations in genes encoding enzymes in the TCA cycle (mdh, schC, and frdA) and enzymes involved in C1 metabolism (gcvP and glyA). Additional aims included assessing the mutant strains' virulence in catfish and ability to protect against wild type E. ictaluri infection. We constructed in-frame deletion mutants to avoid polar effects of the mutations and to avoid insertion of antibiotic resistance genes, which is undesirable in vaccine strains. Splicing overlap extension combined with allelic exchange is an effective method for gene deletion in E. ictaluri and has been reported previously [19], [20].
We utilized bioluminescence imaging to assess virulence of mutants, which allows better quantification compared to percent mortalities. It also enables sensitive detection of subclinical infection and mutants' abilities to invade and establish infection. Mutant strains EiΔsdhC, EiΔmdh, EiΔfrdA, and EiΔgcvP were clearly able to establish infection because bioluminescence was detected after 12 h post-infection. However, channel catfish injected with the mutant strains started clearing the bacteria after 72 h post-infection. Thus, our results showed that although EiΔsdhC, EiΔmdh, EiΔfrdA, and EiΔgcvP do not cause mortalities, they are able to invade and establish infection before being cleared. Because of mutants' abilities to survive and replicate in fish up to 72 h post-infection, we expected them to generate an immune response and protection against wild type E. ictaluri. On the other hand, the EiΔglyA mutant did not replicate well in the host, and we anticipated much less systemic protection from this mutant. By contrast, wild type E. ictaluri increased in quantity until mortality occurred. Our current study corroborated an earlier study showing that 1×109 photons−1 cm−2 steradian−1 seems to be a critical threshold for bacterial tissue concentrations where mortality is imminent [21].
Ultimately, prevention of mortalities is used as a common measure of vaccine efficacy. Thus, we used percent survival to evaluate efficacy of our candidate vaccines in catfish fingerlings using immersion exposure, which is a practical route of vaccination of catfish fry in catfish production systems. Results for mutant strains EiΔsdhC, EiΔmdh and EiΔgcvP were similar to our previous study that evaluated vaccine efficacy of E. ictaluri sdhC, mdh, and gcvP transposon insertion mutants [21]. In our previous study, sdhC and mdh insertion mutants gave 100% protection against E. ictaluri infection, and a gcvP insertion mutant gave 89.15% survival in catfish fingerlings. Our current results with deletion mutants show that attenuation is not due to polar effects of the insertion mutations. The deletion mutants have an additional advantage in that they do not carry antibiotic resistance genes. The current study is the first to report vaccine efficacy of E. ictaluri EiΔglyA and EiΔfrdA mutants; both provided significant protection against mortalities by immersion vaccination.
We also evaluated vaccine efficacy of our candidate mutant strains using a more sensitive measure than percent survival; namely, we evaluated the ability of the mutant strains to prevent invasion of virulent E. ictaluri as monitored using BLI. Vaccination in this trial was by injection, which is not a practical route of vaccination for commercial catfish production, but it does allow accurate vaccine dose delivery. Protection results by injection vaccination were very similar to results obtained by immersion vaccination, except that EiΔglyA vaccination provided better protection by immersion vaccination than injection (Fig. 4). It is possible that immersion vaccination using EiΔglyA may activate mucosal immunity better, preventing wild type E. ictaluri septicemia. We saw the opposite trend when fish were vaccinated with the EiΔgcvP mutant, which protects fish better when vaccination is applied by injection rather than immersion.
Succinate dehydrogenase (SDH) is part of the aerobic respiratory chain in the TCA cycle, oxidizing succinate to fumarate while reducing ubiquinone to ubiquinol [22]. It is closely related to fumarate reductase, which catalyzes the reverse reaction. Succinate dehydrogenase and fumarate reductase can replace each other [22], [23]. Although SdhC has similar function, hydrophobicity, and protein size to the membrane-binding subunit fumarate reductase (FrdC), sdhC and frdC do not share significant sequence identity [24]. The organic acids formate and succinate have a protective effect in stationary phase cells against killing effects of antimicrobial peptide BPI, which appears to disrupt the bacterial respiratory chain [25]. Maintenance of protective levels of formate and succinate requires the activity of formate dehydrogenase and succinate dehydrogenase, respectively.
In E. coli and Salmonella, succinate dehydrogenase is known to contribute to pathogenicity. Recently, it was shown that a full TCA cycle is required for Salmonella enterica virulence, and a sdhDCA mutant is attenuated in an oral mouse infection model [26], which is similar to our finding. In Helicobacter pylori, fumarate reductase was found to be essential for colonization of mouse gastric mucosa [27]. In Salmonella enterica, deletion of sdhCDA caused partial attenuation, and complete attenuation was achieved when both sdhCDA and frdABCD were deleted [28]. Our results indicated that deletion of only the E. ictaluri sdhC gene and deletion of only frdA resulted in full attenuation in catfish fingerlings. However, our previous results showed that catfish fry are more sensitive to E. ictaluri than catfish fingerlings (unpublished data), so further testing in catfish fry is warranted. Regardless, the data show that succinate dehydrogenase and fumarate reductase play an important role in pathogenesis. The other mutant that was tested in this study was mdh, which encodes malate dehydrogenase. Our results show that mdh is also important in E. ictaluri virulence, which was consistent with findings in Salmonella using the mouse oral challenge model, where a mdh mutant was found to be highly attenuated [26].
The glycine cleavage system is a loosely associated four subunit enzyme complex that catalyzes the reversible oxidation of glycine to form 5, and 10-methylenetetrahydrofolate, which serves as a one carbon donor. It is one of two sources of C1 units; serine hydroxymethyltransferase is another source, and it is considered a more important source. Expression of the glycine cleavage enzyme system is induced by glycine [29], [30], and gcv mutants are unable to use glycine as a C1 source and excrete glycine [31]. We have previously shown that E. ictaluri gcvP is required for virulence [14]. This is the first report that glyA is required for E. ictaluri, virulence, and to our knowledge, this is the first report that serine hydroxymethyltransferase is associated with virulence in any bacterial species.
Although BLI for real-time monitoring of E. ictaluri infection in live fish was shown by our group [17], this is the first time we report the use of BLI to quantify the degree of E. ictaluri attenuation in channel catfish. It appears that BLI could be used for vaccine evaluation by using a relatively low number of fish (four fish in this work). Also, use of BLI provides a more sensitive measure of vaccine protection than percent mortalities.
In summary, our results showed that the EiΔsdhC, EiΔmdh, EiΔfrdA, EiΔgcvP, and EiΔglyA mutants were significantly attenuated and provided protection against ESC under controlled laboratory conditions. Thus, EiΔsdhC, EiΔmdh, EiΔfrdA, and EiΔgcvP mutants have potential for use as live attenuated vaccines for catfish fingerlings. The E. ictaluri ΔglyA mutant was found to be incapable of persisting in catfish when injected, which might be the reason for lower protection than when it is used in immersion vaccination. Based on these results, testing of these vaccine candidates in catfish fry is warranted.
Acknowledgments
We thank Michelle Banes for technical assistance. We also thank Dr. Scott Willard and Dr. Peter Ryan for use of the IVIS Imaging System in the Laboratory for Organismal and Cellular Imaging at the Department of Animal and Diary Sciences. Further, we are grateful for the SPF catfish provided by the Laboratory Animal Resources and Care (LARAC) at the College of Veterinary Medicine.
Funding Statement
The research reported herein was supported by United States Department of Agriculture grant #2009-65119-05671 to MLL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dung TH, Haesebrouck F, Nguyen AH, Sorgeloos P, Baele M, et al. (2008) Antimicrobial susceptibility pattern of Edwardsiella ictaluri isolates from natural outbreaks of bacillary necrosis of Pangasianodon hypophthalmus in Vietnam. Microbial Drug Resistance 14: 311–316. [DOI] [PubMed] [Google Scholar]
- 2. Lan MZ, Peng X, Xiang MY, Xia ZY, Bo W, et al. (2007) Construction and characterization of a live, attenuated esrB mutant of Edwardsiella tarda and its potential as a vaccine against the haemorrhagic septicaemia in turbot, Scophthamus maximus (L.). Fish & Shellfish Immunology 23: 521–530. [DOI] [PubMed] [Google Scholar]
- 3. Cooper RK, Shotts EB, Nolan LK (1996) Use of a mini-transposon to study chondroitinase activity associated with Edwardsiella ictaluri . Journal of Aquatic Animal Health 8: 319–324. [Google Scholar]
- 4. Lawrence ML, Cooper RK, Thune RL (1997) Attenuation, persistence, and vaccine potential of an Edwardsiella ictaluri purA mutant. Infect Immun 65: 4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thune RL, Fernandez DH, Battista JR (1999) An aroA mutant of Edwardsiella ictaluri is safe and efficacious as a live, attenuated vaccine. Journal of Aquatic Animal Health 11: 358–372. [Google Scholar]
- 6. Klesius PH, Shoemaker CA (1999) Development and use of modified live Edwardsiella ictaluri vaccine against enteric septicemia of catfish. Adv Vet Med 41: 523–537. [DOI] [PubMed] [Google Scholar]
- 7. Klesius PH, Shoemaker CA (1997) Heterologous isolates challenge of channel catfish, Ictalurus punctatus, immune to Edwardsiella ictaluri . Aquaculture 157: 147–155. [Google Scholar]
- 8. Arias CR, Shoemaker CA, Evans JJ, Klesius PH (2003) A comparative study of Edwardsiella ictaluri parent (EILO) and E. ictaluri rifampicin-mutant (RE-33) isolates using lipopolysaccharides, outer membrane proteins, fatty acids, Biolog, API 20E and genomic analyses. Journal of Fish Disease 26: 415–421. [DOI] [PubMed] [Google Scholar]
- 9.USDA (2003) Highlights of NAHMS catfish 2003: part II. USDA:APHIS:VS:CEAH. Fort Collins, Colorado.
- 10.Annonymous (2006) Case summary report aquatic diagnostic laboratory. Mississippi State University College of Veterinary Medicine.
- 11. Ainsworth AJ, Dexiang C (1990) Differences in the phagocytosis of four bacteria by channel catfish neutrophils. Developmental and Comparative Immunology 14: 201–209. [DOI] [PubMed] [Google Scholar]
- 12. Booth NJ, Elkamel AA, Thune RL (2006) Intracellular replication of Edwardsiella ictaluri in channel catfish macrophages. Journal of Aquatic Animal Health 18: 101–108. [Google Scholar]
- 13. Waterstrat PR, Ainsworth AJ, Capley G (1988) Use of a discontinuous Percoll gradient technique for the separation of channel catfish, Ictalurus punctatus (Rafinesque), peripheral blood leucocytes. Journal of Fish Disease 289–294. [Google Scholar]
- 14. Karsi A, Gulsoy N, Corb E, Dumpala PR, Lawrence ML (2009) A high-throughput bioluminescence mutant screening strategy for identification of bacterial virulence genes. Applied Environmental Microbiology 75: 2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horton RM, Cai ZL, Ho SN, Pease LR (1990) Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8: 528–535. [PubMed] [Google Scholar]
- 16. Karsi A, Lawrence ML (2007) Broad host range fluorescence and bioluminescence expression vectors for gram-negative bacteria. Plasmid 57: 286–295. [DOI] [PubMed] [Google Scholar]
- 17. Karsi A, Menanteau-Ledouble S, Lawrence ML (2006) Development of bioluminescent Edwardsiella ictaluri for noninvasive disease monitoring. FEMS Microbiology Letters 260: 216–223. [DOI] [PubMed] [Google Scholar]
- 18. Amend DF (1981) Potency testing of fish vaccines. Developments in Biological Standardization 49: 447–454. [Google Scholar]
- 19. Abdelhamed H, Lu J, Shaheen A, Abbass A, Lawrence M, et al. (2013) Construction and evaluation of an Edwardsiella ictaluri fhuC mutant. Veterinary Microbiology 162: 858–865. [DOI] [PubMed] [Google Scholar]
- 20. Santander J, Xin W, Yang Z, Curtiss R (2010) The aspartate-semialdehyde dehydrogenase of Edwardsiella ictaluri and its use as balanced-lethal system in fish vaccinology. PLoS One 5: e15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karsi A, Menanteau-Ledouble S, Lawrence ML (2006) Development of bioluminescent Edwardsiella ictaluri for noninvasive disease monitoring. FEMS Microbiol Lett 260: 216–223. [DOI] [PubMed] [Google Scholar]
- 22. Maklashina E, Berthold DA, Cecchini G (1998) Anaerobic expression of Escherichia coli succinate dehydrogenase: Functional replacement of fumarate reductase in the respiratory chain during anaerobic growth. Journal of Bacteriology 22: 5989–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guest JR (1981) Partial replacement of succinate dehydrogenase function by phage- and plasmid-specified fumarate reductase in Escherichia coli . Journal of General Microbiology 122: 171–179. [DOI] [PubMed] [Google Scholar]
- 24. Wood WI, Capon DJ, Simonsen CC, Eaton DL, Gitschier J, et al. (1984) Expression of active human factor VIII from recombinant DNA. Nature (London) 312: 330–337. [DOI] [PubMed] [Google Scholar]
- 25. Barker HC, Kinsella N, Jaspe A, Friedrich T, O'Connor CD (2000) Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Molecular Mircobiology 35: 1518–1529. [DOI] [PubMed] [Google Scholar]
- 26. Tchawa Yimga M, Leatham MP, Allen JH, Laux DC, Conway T, et al. (2006) Role of gluconeogenesis and the tricarboxylic acid cycle in the virulence of Salmonella enterica serovar typhimurium in BALB/c mice. Infection and Immunity 74: 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ge Z, Feng Y, Dangler CA, Xu S, Taylor NS, et al. (2000) Fumarate reductase is essential for Helicobacter pylori colonization of the mouse stomach. Microbial Pathogenesis 29: 279–287. [DOI] [PubMed] [Google Scholar]
- 28. Mercado-Lubo R, Gauger EJ, Leatham MP, Conway T, Cohen PS (2008) A Salmonella enterica serovar typhimurium succinate dehydrogenase/fumarate reductase double mutant is avirulent and immunogenic in BALB/c mice. Infection and Immunity 76: 1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meedel TH, Pizer LI (1974) Regulation of one-carbon biosynthesis and utilization in Escherichia coli . Journal of Bacteriology 118: 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stauffer LT, Fogarty SJ, Stauffer GV (1994) Characterization of the Escherichia coli gcv operon. Gene 142: 17–22. [DOI] [PubMed] [Google Scholar]
- 31. Plamann MD, Rapp WD, Stauffer GV (1983) Escherichia coli K12 mutants defective in the glycine cleavage enzyme system. Molecular and General Genetics 192: 15–20. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence ML (1997) Development and in vivo evaluation of a purA mutant strain of the channel catfish pathogen Edwardsiella ictaluri. Veterinary Microbiology and Parasitology Ph.D.
- 33. Herrero M, de Lorenzo V, Timmis KN (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. Journal of Bacteriology 172: 6557–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller VL, Mekalanos JJ (1998) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR . Journal of Bacteriology 170: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metcalf WW, Jiang W, Wanner BL (1994) Use of the rep technique for allele replacement to construct new Eschericia coli hosts for maintenance of R6K gamma origin plasmids at different copy numbers. Gene 138: 1–7. [DOI] [PubMed] [Google Scholar]
- 36. Dozois CM, Daigle F, Curtiss RI (2003) Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proceedings of the National Academy of Sciences of the United States of America 100: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]