Abstract
Background
It is not known whether the addition of chemotherapy to radiation therapy improves outcomes in primary vaginal cancer. Here, we review clinical outcomes in patients with primary vaginal cancer treated with radiation therapy (RT) or concurrent chemoradiation therapy (CRT).
Methods
Seventy-one patients with primary vaginal cancer treated with definitive RT with or without concurrent chemotherapy at a single institution were identified and their records reviewed. A total of 51 patients were treated with RT alone; 20 patients were treated with CRT. Recurrences were analyzed. Overall survival (OS) and disease-free survival (DFS) rates were estimated using the Kaplan-Meier method. Cox regression analysis was performed.
Results
The median age at diagnosis was 61 years (range, 18–92 years) and the median follow-up time among survivors was 3.0 years. Kaplan-Meier estimates for OS and DFS differed significantly between the RT and CRT groups (3-yr OS = 56% vs. 79%, log-rank p = 0.037; 3-yr DFS = 43% vs. 73%, log-rank p = 0.011). Twenty-three patients (45%) in the RT group had a relapse at any site compared to 3 (15%) in the CRT group (p = 0.027). With regard to the sites of first relapse, 10 patients (14%) had local only, 4 (6%) had local and regional, 9 (13%) had regional only, 1 (1%) had regional and distant, and 2 (3%) had distant only relapse. On univariate analysis, the use of concurrent chemotherapy, FIGO stage, tumor size, and date of diagnosis were significant predictors of DFS. On multivariate analysis, the use of concurrent chemotherapy remained a significant predictor of DFS (hazard ratio 0.31 (95% CI, 0.10–0.97; p = 0.04)).
Conclusions
Vaginal cancer results in poor outcomes. Adequate radiation dose is essential to ensure curative management. Concurrent chemotherapy should be considered for vaginal cancer patients.
Introduction
Vaginal cancer is a rare disease, comprising only 1%–2% of all gynecologic malignancies. Known prognostic factors for recurrence of vaginal cancer include stage, lymph node involvement [1], [2], size of the initial lesion [3] and advanced age [4]. Location of the lesion, grade, and HPV status have conflicting evidence [5].
The standard treatment for non-metastatic vaginal cancer has historically consisted of definitive radiation therapy (RT) alone, using external-beam radiation therapy (EBRT) with or without brachytherapy [6], [7], [8]. Optimal outcomes are achieved with careful selection of treatment modalities to ensure coverage of the tumor and involved regions. Over the past two decades, the integration of computed tomography (CT) and magnetic resonance imaging (MRI) for staging has allowed more accurate assessment of nodal involvement and appropriate treatment to suspicious lymph node regions. Similarly, 3-dimensional (3D) imaging used for radiation planning has facilitated the identification and delineation of the target volume with greater accuracy [9].
Given the favorable outcome of cervical cancer patients treated with chemoradiation therapy (CRT) [10], [11], translation to vaginal cancer remains a topic of discussion. Studies published to date address the feasibility and outcomes of CRT for primary vaginal cancer, but have few patients and therefore comparisons to RT alone are limited [12], [13], [14]. In this study, we analyze and compare the clinical outcomes of patients with primary vaginal cancer treated at our institution with either definitive RT or with CRT.
Patients and Methods
Patients
Records of patients with a diagnosis of biopsy-proven primary vaginal cancer treated with definitive RT with or without concurrent chemotherapy at Brigham & Women’s Hospital/Dana-Farber Cancer Institute between 1972 and 2009 were identified and retrospectively reviewed under an Institutional Review Board (Dana-Farber Cancer Institute IRB)-approved protocol with a waiver of consent. Exclusion criteria were prior gynecological malignancy, prior pelvic radiation therapy, metastatic disease and cancer involvement of the cervix or vulva. For all patients, pretreatment patient and tumor characteristics were recorded, including date of diagnosis, stage according to the International Federation of Gynecology and Obstetrics (FIGO) 2000 classification, histology, tumor size, tumor site, tumor differentiation, presence of lymphatic vessel invasion (LVI) and lymph node involvement. For each patient, medical records were reviewed to determine whether diagnostic imaging, including pelvic CT, pelvic MRI and PET scan, had been performed to evaluate for lymph node involvement and/or distant metastases.
Treatment
Treatment characteristics for all patients were recorded, including the type of radiation therapy used (EBRT, brachytherapy, or both), EBRT technique, brachytherapy type and dose rate, treatment duration, and the use of chemotherapy. For patients treated with high-dose-rate (HDR) brachytherapy, the total radiation dose was converted to the biologically equivalent dose (BED) in 2-Gy fractions, using the linear quadratic BED equation with an α/β ratio of 10. For patients treated with CRT, the type of chemotherapy used and the number of cycles of chemotherapy were recorded. Information was also collected on whether surgery was performed and the type of surgery.
Clinical Outcomes
Data on outcomes were collected for each patient, including local, regional and distant relapse, disease-free survival (DFS) and overall survival (OS). Local relapse was defined as recurrence in the vagina or paravaginal area. Regional relapse was defined as recurrence in the pelvic or inguinal lymph nodes. Distant relapse included para-aortic recurrences and distant metastases.
Statistical Analysis
We report medians with interquartile range or means with standard deviation for numeric variables and percentages for categorical or ordinal variables. Treatment groups were compared using the exact Wilcoxon test for numeric or ordinal variables, the Fisher exact test for binary variables, and the likelihood ratio test for multicategory discrete variables. Two-sided p-values <0.05 were considered statistically significant. OS was measured from the date of the start of RT to the date of death or last follow-up. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox regression analyses were used to identify predictors of OS and DFS. Statistical analyses were performed using R, version 2.12.0.
Results
We identified 71 patients with primary vaginal cancer treated with definitive RT with or without concurrent chemotherapy at Brigham & Women’s Hospital/Dana-Farber Cancer Institute between 1972 and 2009. A total of 51 patients were treated with RT without chemotherapy and 20 were treated with CRT.
Patient and Tumor Characteristics
Detailed patient and tumor characteristics are listed in Table 1. The median age was 61 years for all patients. There were no significant differences between the RT-alone and CRT groups with regard to FIGO stage, tumor size, histology, differentiation or presence of LVI. There was a statistically significant difference in terms of the year of diagnosis; more patients treated with RT alone were diagnosed prior to 1999, and more patients treated with CRT were diagnosed after 1999. With regard to tumor site, there was a trend towards a higher proportion of patients in the CRT group having involvement of the lower 1/3 of the vagina. A significantly higher percentage of patients in the CRT group than the RT-alone group underwent diagnostic imaging; also, significantly more patients in that cohort had radiographically detected lymph node involvement.
Table 1. Patient and tumor characteristics.
| Category | Subcategory | All patients (n = 71) | RT alone (n = 51) | ChemoRT (n = 20) | p value |
| Median follow-up (range) | All patients | 2.3 years (0.3–14.6) | 2.3 years (0.5–14.6) | 2.6 years (0.3–7.76) | 0.625 |
| Survivors | 3.0 years (0.3–14.6) | 3.0 years (0.7–14.6) | 3.6 years (0.3–7.8) | 0.462 | |
| Median Age (range) | All patients | 61 (18–92) | 64 (20–92) | 60 (18–81) | 0.112 |
| Year of diagnosis | 1970–1979 | 8 (11%) | 8 (16%) | 0 (0%) | <0.001 |
| 1980–1989 | 14 (20%) | 13 (26%) | 1 (5%) | ||
| 1990–1999 | 17 (24%) | 16 (32%) | 1 (5%) | ||
| 2000–2009 | 32 (45%) | 14 (28%) | 18 (90%) | ||
| Stage (FIGO) | I | 22 (31%) | 18 (35%) | 4 (20%) | 0.395 |
| II | 30 (42%) | 19 (37%) | 11 (55%) | ||
| III | 12 (17%) | 8 (16%) | 4 (20%) | ||
| IVA | 7 (10%) | 6 (12%) | 1 (5%) | ||
| Prior hysterectomy | No | 33 (47%) | 21 (41%) | 12 (60%) | 0.191 |
| Yes | 38 (54%) | 30 (59%) | 8 (40%) | ||
| Diagnostic imaging | No | 30 (42%) | 29 (57%) | 1 (5%) | <0.001 |
| Yes | 41 (58%) | 22 (43%) | 19 (95%) | ||
| CT | 21 (30%) | 16 (31%) | 5 (25%) | ||
| MRI | 9 (13%) | 2 (4%) | 7 (35%) | ||
| PET | 1 (1%) | 1 (2%) | 0 (0%) | ||
| CT and MRI | 4 (6%) | 1 (2%) | 3 (15%) | ||
| MRI and PET | 5 (7%) | 1 (2%) | 4 (20%) | ||
| CT and PET | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Tumor size | ≤4 cm | 41 (58%) | 27 (53%) | 14 (70%) | 0.202 |
| >4 cm | 24 (34%) | 18 (35%) | 6 (30%) | ||
| Unknown | 6 (9%) | 6 (12%) | 0 (0%) | ||
| Tumor site | Apex | 15 (21%) | 12 (24%) | 3 (15%) | 0.055 |
| Upper 2/3 | 39 (55%) | 31 (61%) | 8 (40%) | ||
| Lower 1/3 | 10 (14%) | 4 (8%) | 6 (30%) | ||
| Entire | 6 (9%) | 4 (8%) | 2 (10%) | ||
| Unknown | 1 (1%) | 0 (0%) | 1 (5%) | ||
| Histology | Squamous cell | 54 (76%) | 36 (71%) | 18 (90%) | 0.384 |
| Adenocarcinoma | 7 (10%) | 7 (14%) | 0 (0%) | ||
| Clear cell | 5 (7%) | 4 (8%) | 1 (5%) | ||
| Epidermoid | 2 (3%) | 2 (4%) | 0 (0%) | ||
| Unknown | 2 (3%) | 1 (2%) | 1 (5%) | ||
| Other | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Differentiation | Well | 10 (14%) | 9 (18%) | 1 (5%) | 0.116 |
| Moderately | 18 (25%) | 15 (29%) | 3 (15%) | ||
| Poorly | 25 (35%) | 14 (28%) | 11 (55%) | ||
| Unknown | 18 (25%) | 13 (26%) | 5 (25%) | ||
| LVI | No | 10 (14%) | 7 (14%) | 3 (15%) | 0.923 |
| Yes | 5 (7%) | 3 (6%) | 2 (10%) | ||
| Unknown | 56 (79%) | 41 (80%) | 15 (75%) | ||
| Lymph node involvement | None | 61 (86%) | 48 (94%) | 13 (65%) | 0.004 |
| Pelvic | 4 (6%) | 2 (4%) | 2 (10%) | ||
| Inguinal | 2 (3%) | 0 (0%) | 2 (10%) | ||
| Pelvic and inguinal | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Para-aortic | 3 (4%) | 0 (0%) | 3 (15%) |
Key: CRT, concurrent chemoradiation; FIGO, Federation Internationale de Gynecologie et Obstetrique; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; LVI, lymphatic vessel invasion.
Treatment Details
Treatment characteristics for all patients are listed in Table 2. In the RT-alone group, most patients were treated with EBRT and brachytherapy, although some patients received EBRT alone or brachytherapy alone. In the CRT group, all patients were treated with both EBRT and brachytherapy. Twenty-eight percent of all patients underwent surgery as part of their initial management prior to RT or CRT, including 35% of patients in the RT-only group and 10% of those in the CRT group. Most of these patients underwent either a vaginectomy (16%) or a wide local excision of tumor (10%).
Table 2. Treatment characteristics.
| Category | Subcategory | All patients (n = 71) | RT alone (n = 51) | ChemoRT (n = 20) | p value |
| Radiation therapy given | Brachytherapy alone | 9 (13%) | 9 (18%) | 0 (0%) | 0.006 |
| EBRT alone | 10 (14%) | 10 (20%) | 0 (0%) | ||
| EBRT+brachytherapy | 52 (73%) | 32 (63%) | 20 (100%) | ||
| RT dose to Vagina (EQD2) | All RT groups | 68.2±15.4 Gy | 65.1±16.8 Gy | 76.0±6.5 Gy | 0.99 |
| Brachy alone group | 54.2±22.2 Gy | 54.2±22.2 Gy | – | – | |
| EBRT alone group | 48.4±7.1 Gy | 48.4±7.1 Gy | – | – | |
| EBRT+brachy group | 74.4±9.3 Gy | 73.5±10.7 Gy | 76.0±6.5 Gy | 0.90 | |
| EBRT fields | 4-field | 25 (35%) | 18 (35%) | 7 (35%) | 0.654 |
| AP/PA | 32 (45%) | 21 (41%) | 11 (55%) | ||
| Opposed laterals | 1 (1%) | 1 (2%) | 0 (0%) | ||
| 3-field | 1 (1%) | 0 (0%) | 1 (5%) | ||
| En face | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Unknown | 2 (3%) | 1 (2%) | 1 (5%) | ||
| Brachytherapy type | Cylinder | 26 (37%) | 21 (41%) | 5 (25%) | 0.235 |
| Interstitial | 26 (37%) | 16 (31%) | 10 (50%) | ||
| Tandem and ovoid | 4 (6%) | 1 (2%) | 3 (15%) | ||
| Cylinder and tandem | 2 (3%) | 1 (2%) | 1 (5%) | ||
| Interstitial and tandem | 2 (3%) | 1 (2%) | 1 (5%) | ||
| Sutures | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Brachytherapy dose rate | HDR | 23 (32%) | 12 (24%) | 11 (55%) | 0.090 |
| LDR | 38 (54%) | 29 (57%) | 9 (45%) | ||
| Concurrent Chemotherapy | Yes | 20 (28%) | – | – | – |
| Cisplatin | 17 (24%) | – | 17 (85%) | ||
| Carboplatin | 2 (3%) | – | 2 (10%) | ||
| 5-FU | 1 (1%) | – | 1 (5%) | ||
| Unknown | 0 (1%) | – | 0 (0%) | ||
| Number of cycles chemo | ChemoRT group | 5 (1–8) | – | 5 (1–8) | – |
| Surgery | Yes | 20 (28%) | 18 (35%) | 2 (10%) | 0.040 |
| Wide excision | 7 (10%) | 6 (12%) | 1 (5%) | ||
| Vaginectomy | 11 (16%) | 10 (20%) | 1 (5%) | ||
| Pelvic exenteration | 2 (3%) | 2 (4%) | 0 (0%) |
Key: CRT, concurrent chemoradiation; EBRT, external-beam radiation therapy; EQD2, Equivalent dose in 2Gy fractions; AP/PA, anterioposterior/posterioanterior; HDR, high-dose-rate; LDR, low-dose-rate.
The mean total radiation dose received for all patients, calculated as the BED in 2-Gy fractions, was 68.2±15.4 Gy. The difference in radiation dose received between patients in the RT-alone group and those treated with CRT was not statistically significant (Table 2). The subgroup of patients treated with both EBRT and brachytherapy received a higher total dose of radiation (BED, 74.4±9.3 Gy) than those treated with EBRT alone; however, this also did not significantly differ between the RT-alone and CRT groups. The technique and field arrangements of EBRT are described in Table 2. There were no significant differences in technique between the RT-alone and CRT groups. Most patients received EBRT with a 4-field approach or anterioposterior/posterioanterior fields. With regard to brachytherapy technique, there was no significant difference in the types of brachytherapy applicators used between the two groups. However there was a trend towards a higher percentage of patients in the CRT group receiving HDR brachytherapy as opposed to low-dose-rate (LDR) brachytherapy.
Among the CRT patients, 85% received weekly cisplatin concurrently with RT at a dose of 40 mg/m2; the remainder received either carboplatin or 5-FU during RT. The average number of cycles of concurrent chemotherapy completed was five. One patient received adjuvant doxorubicin and paclitaxel after completion of CRT with concurrent cisplatin. Another patient received neoadjuvant cisplatin and 5-FU prior to undergoing CRT with concurrent 5-FU. One patient who was included in the RT-only group received carboplatin and cyclophosphamide prior to her radiation.
Outcome
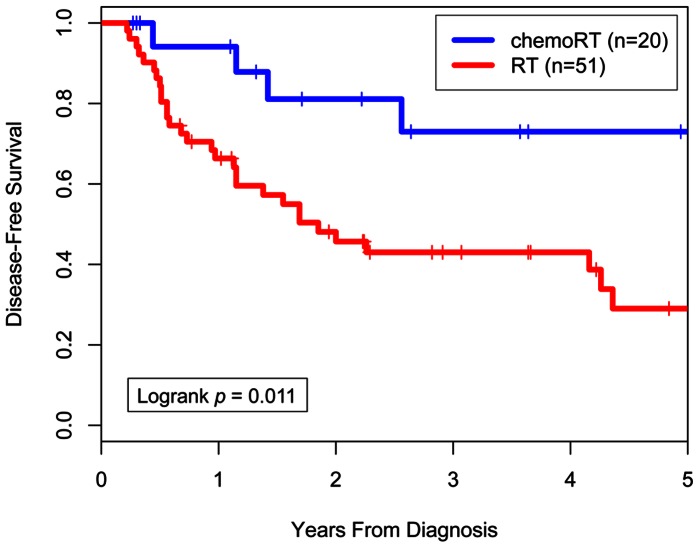
The median follow-up time among survivors was 3.0 years for all patients. Median follow-up time was not significantly different between the RT-alone and CRT groups (Table 1). Kaplan-Meier actuarial OS rates at 3 years were 56% for the RT-alone group and 79% for the CRT group (log-rank p = 0.037; Figure 1). Median OS time was 4.3 years for the RT-alone group and had not been reached for the CRT group. DFS was also significantly different between the two groups, with actuarial 3-year DFS rates of 43% for the RT-alone group and 73% for the CRT group (log-rank p = 0.011; Figure 2). Median DFS time was 1.9 years for the RT-alone group and had not been reached for the CRT group.
Figure 1. Overall survival by treatment (chemoradiation therapy or radiation therapy alone).
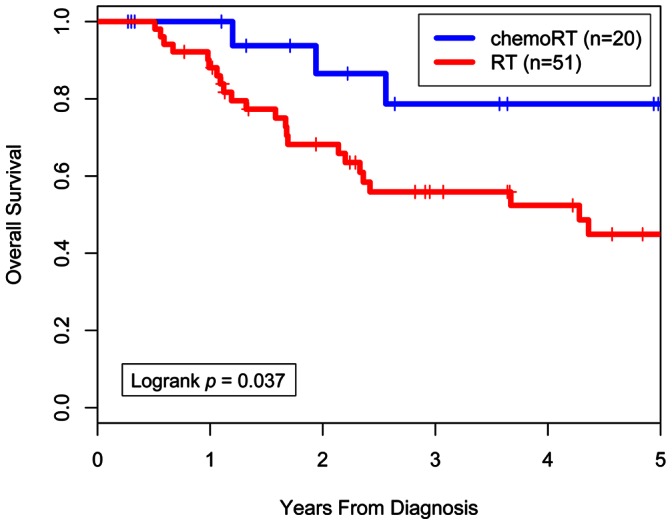
Figure 2. Disease-free survival by treatment (chemoradiation therapy or radiation therapy alone).
A subgroup analysis was performed for the 52 patients who received both EBRT and brachytherapy; 32 of these were in the RT-alone group and 20 in the CRT group. Within this subgroup, actuarial 3-year DFS rates remained significantly different between the RT-alone and CRT groups (48% vs. 73%; log-rank p = 0.027) and actuarial 3-year OS trended towards a difference favoring the CRT group (62% vs. 79%; log-rank p = 0.113).
Patterns of Tumor Relapse
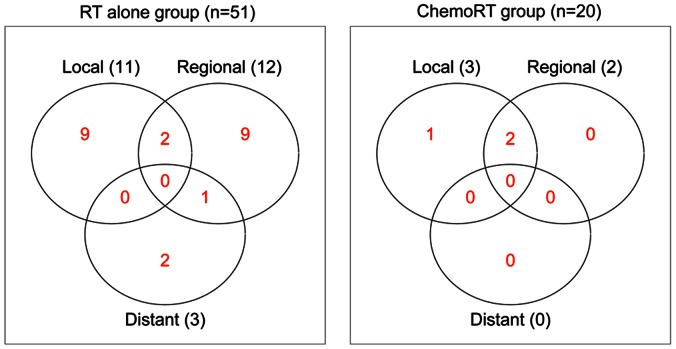
A total of 26 patients (37%) had a relapse of vaginal cancer after treatment. With regard to the sites of first relapse, 10 patients (14%) had local only, 4 (6%) had local and regional, 9 (13%) had regional only, 1 (1%) had regional and distant, and 2 (3%) had distant only relapse. The percentage of patients with relapse was significantly higher in the RT-alone group (n = 23, 45%) than in the CRT group (n = 3, 15%; p = 0.027). The distribution of the first sites of vaginal-cancer relapse is depicted in graphical form in Figure 3.
Figure 3. Sites of first relapse by treatment group (radiation therapy alone compared to chemoradiation therapy).
Cox Proportional Hazards Analyses
Results from univariate Cox proportional hazards analyses for DFS and OS are presented in Table 3. On univariate analysis for predictors of DFS, year of diagnosis, use of concurrent chemotherapy, FIGO stage, and tumor size >4 cm were significant variables. Variables that were not significant included lymph node involvement, tumor differentiation, age at diagnosis, use of surgery, prior hysterectomy, treatment duration, and use of 3D diagnostic imaging. Significant univariate predictors of OS included year of diagnosis, use of concurrent chemotherapy, radiation dose (EQD2), use of both EBRT and brachytherapy, and any use of brachytherapy. Radiation dose was significant both as a continuous variable and when dichotomized by the median dose of 70 Gy. Lymph node involvement, tumor differentiation, age at diagnosis, use of surgery, prior hysterectomy, treatment duration, use of 3D diagnostic imaging, FIGO stage, and tumor size were not significant univariate predictors of OS.
Table 3. Cox proportional hazards analyses.
| Univariate | Disease-Free Survival | Overall Survival | ||
| Variable | Crude HR (95% CI) | P value (Wald) | Crude HR (95% CI) | P value (Wald) |
| Date of diagnosis (year) | 0.96 (0.93–0.99) | 0.011 | 0.96 (0.93–0.99) | 0.030 |
| Chemotherapy | 0.28 (0.10–0.80) | 0.017 | 0.30 (0.09–0.99) | 0.049 |
| FIGO stage | 1.53 (1.08–2.16) | 0.017 | 1.38 (0.95–2.00) | 0.096 |
| Tumor size >4 cm | 2.12 (1.04–4.35) | 0.040 | 1.84 (0.83–4.06) | 0.131 |
| Treatment duration (days) | 0.99 (0.97–1.001) | 0.072 | 0.98 (0.97–1.001) | 0.062 |
| 3D Diagnostic imaging | 0.54 (0.28–1.07) | 0.077 | 0.67 (0.31–1.42) | 0.295 |
| Brachytherapy (any) | 0.50 (0.22–1.10) | 0.084 | 0.42 (0.18–0.97) | 0.041 |
| RT dose to vagina (>70 Gy EQD2) | 0.55 (0.27–1.09) | 0.085 | 0.34 (0.15–0.77) | 0.009 |
| Age at diagnosis | 1.02 (0.99–1.04) | 0.096 | 1.02 (0.999–1.04) | 0.066 |
| Prior hysterectomy | 1.80 (0.89–3.62) | 0.100 | 1.71 (0.81–3.60) | 0.156 |
| Tumor differentiation | 0.71 (0.45–1.11) | 0.130 | 0.65 (0.39–1.11) | 0.107 |
| EBRT and brachytherapy | 0.59 (0.30–1.18) | 0.138 | 0.44 (0.20–0.94) | 0.034 |
| Lymph node involvement | 0.90 (0.35–2.32) | 0.822 | 0.86 (0.30–2.49) | 0.781 |
| Surgery | 0.99 (0.49–2.00) | 0.980 | 0.93 (0.42–2.07) | 0.866 |
Key: HR, hazard ratio; FIGO, Federation Internationale de Gynecologie et Obstetrique; EQD2, Equivalent dose in 2Gy fractions; EBRT, external-beam radiation therapy.
Given the total of 26 relapse events, the number of variables that could be included in the multivariate model was limited. We therefore tested several clinically relevant variables in combination, realizing that the final multivariate model would be weakened by the small number of events. We included year of diagnosis in the models to attempt to control for changes over time. On including tumor size in a model for DFS that included the use of concurrent chemotherapy, FIGO stage, and year of diagnosis, tumor size was no longer significant, and the HR for chemotherapy remained significant at 0.31 (95% CI 0.10–0.98; p = 0.05). To assess the impact of 3D diagnostic imaging, this variable was evaluated in the multivariate model for DFS. With this exploratory analysis, the HR for the use of concurrent chemotherapy remained significant at 0.31 (95% CI 0.10–0.97; p = 0.04). However, the use of diagnostic imaging itself was not significant on univariate analysis (HR 0.54; 95% CI, 0.28–1.07; p = 0.08) and was therefore not included in the final model. Similarly, total RT dose to the vagina (≥70 Gy EQD2) was not a significant predictor for DFS on MVA. Therefore, our final multivariate model for DFS included the use of chemotherapy, FIGO stage, and year of diagnosis, with which the DFS hazard ratio for the use of concurrent chemotherapy remained significant at 0.31 (95% CI, 0.10–0.97; p = 0.04).
Discussion
This retrospective single-institution study demonstrates a potential benefit in overall survival and disease-free survival with the addition of concurrent chemotherapy in the treatment of primary vaginal cancer. In addition to the use of concurrent chemotherapy, other significant covariates in univariate models for disease-free and overall survival included radiation dose, FIGO stage, tumor size and year of diagnosis. Multivariate analysis was limited given the small number of events. Whether concurrent chemotherapy contributes to a long-term overall survival benefit in patients who receive sufficient radiation in the modern era must be tested in a larger study.
Prospective randomized controlled trials have demonstrated a survival benefit with the addition of concurrent chemotherapy to RT in the treatment of cervical cancer [10], [11]. Other studies have demonstrated the efficacy of CRT in the treatment of vulvar cancer [15], [16], [17], [18]. A number of retrospective studies have examined the tolerability of and outcomes after chemoradiation therapy in patients with primary vaginal cancer, but none have had a comparative radiation-only group. Dalrymple and colleagues reported on a retrospective series of 14 patients with squamous carcinoma of the vagina treated with CRT consisting of 5-FU, cisplatin or mitomycin-C; 9 patients were alive and cancer-free 74–168 months after treatment [12]. Samant and colleagues reported a similar retrospective series of 12 patients with primary vaginal cancer treated with CRT, with a more homogeneous treatment regimen consisting of concurrent weekly cisplatin [13]. The 5-year overall, progression-free, and locoregional-progression-free survival rates were 66%, 75%, and 92%, respectively, in that series. Nashiro and colleagues reported on a smaller retrospective series of 6 patients treated with cisplatin-based CRT, of whom 4 remained free of disease at 18, 23, 33 and 55 months, respectively. [14] Our current study, with 51 RT-only and 20 CRT patients, represents the largest series of chemoradiation compared to radiation only for patients with primary vaginal cancer treated at the same institution.
The management of vaginal cancer has changed in several ways over the course of the study. Patients treated before 2002 received LDR brachytherapy, whereas those treated from 2002 forward received HDR brachytherapy. Prospective randomized trials in cervical cancer have shown no difference in outcome between LDR and HDR [19]. In addition, the advent of 3-D imaging since the 1980s has enabled more accurate staging of vaginal cancer and improved delineation of the tumor. In order to assess the impact of 3-D diagnostic imaging, this variable was assessed as a potential confounder. In a multivariate model, diagnostic imaging was not significant, whereas CRT remained significant in the final model, indicating the independent predictive role of the use of CRT. The introduction of 3-D imaging has also allowed the implementation of image-guided adaptive brachytherapy approaches over the past decade [2]. Since 2002, our institution has used MRI guidance followed by MR- and CT-based treatment planning for vaginal brachytherapy treatment [20], [21]. In the current study, the prescription dose was 74.4+/−9.3 Gy EQD2 for patients treated with EBRT and brachytherapy. The question of whether patients treated with an adequate dose of radiation, using image-based conformal brachytherapy planning, benefit from concurrent chemotherapy must be assessed in larger clinical trials. Chemotherapy does cause rapid regression of the tumor, resulting in treatment of a small radiation volume. This smaller tumor volume may reduce the amount of rectal tissue exposed to radiation, and the D2cc (dose to 2cc of rectum) predicts for the risk of future rectal morbidity [22].
A previous study using RT alone for vaginal cancer demonstrated excellent 5-year DFS rates of 82% for tumors <4 cm and 60% for tumors >4 cm [6]. It also demonstrated stage-dependent locoregional relapse rates of 68% for Stages I–II and 83% for Stages III–IVA [6]. Our study showed a 3-year DFS rate of 73% for all patients receiving CRT.
Limitations of our study include its retrospective nature, its scope as a single institutional experience, and its inclusion of patients who were treated over a long period. The year of diagnosis was therefore included in the analysis to account for shifts in technology and treatments over time, some of which are known confounders and others that may be unknown. Therefore, appropriate adjustment for year of diagnosis in the multivariate modeling was valuable.
Our data suggest that chemoradiation therapy may be considered in the treatment of patients with primary vaginal cancer. Adequate radiation dosing with the combination of external beam and brachytherapy is critical to the curative management of patients. Though accrual may require international collaboration, prospective trials may be warranted to more thoroughly assess the benefit of adding concurrent chemotherapy to radiation therapy in these patients.
Acknowledgments
Presented in part at the 52nd Annual Meeting of the American Society for Therapeutic Radiology and Oncology, San Diego, CA, November, 2010.
We thank Cynthia Tanaka and Paula Bu for assistance with data management.
Funding Statement
This research was supported by a donation to Dr Viswanathan by the St. Laurent Family Fund. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Creasman WT, Phillips JL, Menck HR (1998) The National Cancer Data Base report on cancer of the vagina. Cancer 83: 1033–1040. [PubMed] [Google Scholar]
- 2. Beriwal S, Demanes DJ, Erickson B, Jones E, De Los Santos JF, et al. (2012) American Brachytherapy Society consensus guidelines for interstitial brachytherapy for vaginal cancer. Brachytherapy 11: 68–75. [DOI] [PubMed] [Google Scholar]
- 3. Shah CA, Goff BA, Lowe K, Peters WA 3rd, Li CI (2009) Factors affecting risk of mortality in women with vaginal cancer. Obstet Gynecol 113: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hellman K, Silfversward C, Nilsson B, Hellstrom AC, Frankendal B, et al. (2004) Primary carcinoma of the vagina: factors influencing the age at diagnosis. The Radiumhemmet series 1956–96. Int J Gynecol Cancer 14: 491–501. [DOI] [PubMed] [Google Scholar]
- 5.Cardenes H, Perez C (2007) Vaginal Cancer. In: Perez C BL, editor. Principles and Practice of Radiation Oncology. Philadelphia: Lippincott, Williams and Wilkins. 1657–1681.
- 6. Perez CA, Camel HM, Galakatos AE, Grigsby PW, Kuske RR, et al. (1988) Definitive irradiation in carcinoma of the vagina: long-term evaluation of results. Int J Radiat Oncol Biol Phys 15: 1283–1290. [DOI] [PubMed] [Google Scholar]
- 7. Frank SJ, Jhingran A, Levenback C, Eifel PJ (2005) Definitive radiation therapy for squamous cell carcinoma of the vagina. Int J Radiat Oncol Biol Phys 62: 138–147. [DOI] [PubMed] [Google Scholar]
- 8. Tran PT, Su Z, Lee P, Lavori P, Husain A, et al. (2007) Prognostic factors for outcomes and complications for primary squamous cell carcinoma of the vagina treated with radiation. Gynecol Oncol 105: 641–649. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan A, Kirisits C, Erickson B, Potter R (2011) Gynecologic radiation therapy: Novel approaches to image-guidance and management. Heidelberg: Springer-Verlag.
- 10. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, et al. (1999) Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 340: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 11. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, et al. (1999) Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340: 1144–1153. [DOI] [PubMed] [Google Scholar]
- 12. Dalrymple JL, Russell AH, Lee SW, Scudder SA, Leiserowitz GS, et al. (2004) Chemoradiation for primary invasive squamous carcinoma of the vagina. Int J Gynecol Cancer 14: 110–117. [DOI] [PubMed] [Google Scholar]
- 13. Samant R, Lau B, E C, Le T, Tam T (2007) Primary vaginal cancer treated with concurrent chemoradiation using Cis-platinum. Int J Radiat Oncol Biol Phys 69: 746–750. [DOI] [PubMed] [Google Scholar]
- 14. Nashiro T, Yagi C, Hirakawa M, Inamine M, Nagai Y, et al. (2008) Concurrent chemoradiation for locally advanced squamous cell carcinoma of the vagina: case series and literature review. Int J Clin Oncol 13: 335–339. [DOI] [PubMed] [Google Scholar]
- 15. Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, et al. (1998) Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 42: 79–85. [DOI] [PubMed] [Google Scholar]
- 16. Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, et al. (2000) Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 48: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 17. Landrum LM, Skaggs V, Gould N, Walker JL, McMeekin DS (2008) Comparison of outcome measures in patients with advanced squamous cell carcinoma of the vulva treated with surgery or primary chemoradiation. Gynecol Oncol 108: 584–590. [DOI] [PubMed] [Google Scholar]
- 18. Mak RH, Halasz LM, Tanaka CK, Ancukiewicz M, Schultz DJ, et al. (2011) Outcomes after radiation therapy with concurrent weekly platinum-based chemotherapy or every-3–4-week 5-fluorouracil-containing regimens for squamous cell carcinoma of the vulva. Gynecol Oncol 120: 101–107. [DOI] [PubMed] [Google Scholar]
- 19. Stewart AJ, Viswanathan AN (2006) Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer 107: 908–915. [DOI] [PubMed] [Google Scholar]
- 20. Viswanathan AN, Cormack R, Holloway CL, Tanaka C, O'Farrell D, et al. (2006) Magnetic resonance-guided interstitial therapy for vaginal recurrence of endometrial cancer. Int J Radiat Oncol Biol Phys 66: 91–99. [DOI] [PubMed] [Google Scholar]
- 21. Kapur T, Egger J, Damato A, Schmidt EJ, Viswanathan AN (2012) 3-T MR-guided brachytherapy for gynecologic malignancies. Magn Reson Imaging 30: 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee LJ, Viswanathan AN (2012) Predictors of toxicity after image-guided high-dose-rate interstitial brachytherapy for gynecologic cancer. Int J Radiat Oncol Biol Phys 84: 1192–1197. [DOI] [PubMed] [Google Scholar]