Abstract
Objectives
The objectives of this study were to determine if ABCB1 polymorphisms are associated with interindividual variability in sitagliptin pharmacokinetics, and if atorvastatin alters the pharmacokinetic disposition of sitagliptin in healthy volunteers.
Methods
In this open-label, randomized, two-phase crossover study, healthy volunteers were prospectively stratified according to ABCB1 1236/2677/3435 diplotype (n=9, CGC/CGC; n=10, CGC/TTT; and n=10, TTT/TTT). In one phase, participants received a single 100 mg dose of sitagliptin. In the other phase, participants received 40 mg of atorvastatin for five days, with a single 100 mg dose of sitagliptin administered on day 5. A 24 hour pharmacokinetic study followed each sitagliptin dose, and the study phases were separated by a 14-day washout period.
Results
Sitagliptin pharmacokinetic parameters did not differ significantly between ABCB1 CGC/CGC, CGC/TTT, and TTT/TTT diplotype groups during the monotherapy phase. Atorvastatin administration did not significantly affect sitagliptin pharmacokinetics, with GMRs (90% CIs) for sitagliptin Cmax, AUC0-∞, CLR, and fe of 0.93 (0.86, 1.01), 0.96 (0.91, 1.01), 1.02 (0.93, 1.12), and 0.98 (0.90, 1.06), respectively.
Conclusions
ABCB1 CGC/CGC, CGC/TTT, and TTT/TTT diplotypes did not influence sitagliptin pharmacokinetics in healthy volunteers. Furthermore, atorvastatin had no effect on the pharmacokinetics of sitagliptin in the setting of ABCB1 CGC/CGC, CGC/TTT, and TTT/TTT diplotypes.
Keywords: sitagliptin, atorvastatin, ABCB1, P-glycoprotein, pharmacogenetic, pharmacokinetic
Introduction
Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is used in the treatment of type 2 diabetes [1-3]. Sitagliptin undergoes little hepatic metabolism, and about 80% of the drug is excreted unchanged in the urine, mainly through active tubular secretion [2,4,5]. In vitro and clinical data suggest that sitagliptin is a substrate for the efflux transporter, P-glycoprotein (P-gp) [6,7]. P-gp is expressed on the brush border membrane of intestinal enterocytes and the luminal side of renal proximal tubular cells [8]. It can be hypothesized that interindividual variability in P-gp-mediated transport of sitagliptin, as a result of genetic polymorphisms or drug-drug interactions, may contribute to alterations in sitagliptin pharmacokinetics in humans.
The most commonly studied polymorphisms in ABCB1, the gene that encodes P-gp, are c.1236C>T (rs1128503), c.2677G>T/A (rs2032582), and c.3435C>T (rs1045642), and these polymorphisms are often evaluated in haplotype form (i.e., 1236/2677/3435) [9]. In vitro, ABCB1 3435T, in combination with 1236T and/or 2677T, has been associated with changes in P-gp conformation and decreased P-gp function [10]. ABCB1 3435T has also been shown to affect mRNA stability [11]. In vivo, there are conflicting reports regarding the association between ABCB1 genetics and drug exposure for different P-gp substrates [9,12]. To our knowledge, the extent to which common ABCB1 SNPs affect sitagliptin pharmacokinetics has not been studied.
Atorvastatin, an HMG-CoA reductase inhibitor (statin), is used to lower cholesterol and reduce the risk of cardiovascular disease in patients with type 2 diabetes [13-19]. In vitro data show that atorvastatin is both a substrate and an inhibitor of P-gp [20-27]. In clinical studies, atorvastatin has been shown to alter the pharmacokinetics of the P-gp substrates, digoxin and aliskiren [21,28]. Sitagliptin and atorvastatin are frequently co-administered in patients with type 2 diabetes; however, a pharmacokinetic assessment of the effect of atorvastatin on sitagliptin disposition has not been conducted in humans.
Given the gaps in knowledge regarding the impact of ABCB1 genetics and P-gp-mediated drug-drug interactions on sitagliptin disposition, the objectives of our study were to: 1) determine if ABCB1 polymorphisms are associated with interindividual variability in sitagliptin pharmacokinetics, and 2) determine if atorvastatin alters sitagliptin pharmacokinetics in healthy volunteers.
Materials and methods
Study design
The investigation was conducted as an open-label, randomized, two-phase crossover study in healthy volunteers prospectively stratified according to ABCB1 diplotype. In one phase, participants received a single 100 mg dose of sitagliptin by mouth at 8:00 AM. In the other phase, participants received 40 mg of atorvastatin by mouth at 8:00 AM for five days, with a single 100 mg dose of sitagliptin administered by mouth at 8:00 AM on day 5. A 14-day washout period separated the two phases. After each sitagliptin dose, an intensive 24-hour pharmacokinetic study was conducted at the University of Colorado Clinical and Translational Research Center (CTRC). Participants started the pharmacokinetic studies in the fasted state and a standardized breakfast (600 calories; 55% carbohydrates, 15% protein, and 30% fat) was administered two hours after sitagliptin dosing. Warm meals were also given 6, 10, and 24 hours after sitagliptin administration. Participants abstained from caffeine and smoking during the 24-hour period. Blood samples (5 ml in EDTA) were collected pre-dose and 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, and 24 hours post-dosing in both phases. Plasma was harvested within 30 minutes and stored at−80°C until analytical processing. Urine, which was kept on ice, was collected from 0 to 12 hours and 12 to 24 hours. The volume of urine collected during each 12-hour period was recorded. A 10 ml urine aliquot was removed and stored at−80°C until analytical processing. Blood glucose was measured by finger-stick at 1, 2, 3, and 4 hours after sitagliptin administration to assess for hypoglycemia.
Study participants
Healthy men and women between 21 to 60 years of age were recruited to participate in the study, which was approved by the Colorado Multiple Institutional Review Board. All subjects provided written informed consent. Participants were prospectively screened and stratified according to ABCB1 (1236/2677/3435) diplotype as follows: Group 1, CGC/CGC (reference); Group 2, CGC/TTT; and Group 3, TTT/TTT.
For inclusion in the study, metabolic, renal, hepatic, and hematologic laboratory parameters had to be within normal limits. Participants were excluded for any of the following: current or past history of cardiovascular, hepatic, renal, endocrine, gastrointestinal, pulmonary, hematologic, immunologic, or neurologic diseases. Other pertinent exclusion criteria were: body mass index (BMI) <18 kg/m2 or >30 kg/m2; body weight <50 kg; creatinine clearance <60 ml/min based on the Cockcroft-Gault formula; lipid-lowering therapy (e.g., statins); inhibitors or inducers of P-gp or CYP3A4;, and/or medications known to affect drug absorption (e.g., cholestyramine).
Genetic analyses
For the screening genetic sample, a buccal cell sample was collected via a mouthwash method [29]. Genomic DNA was isolated using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). The ABCB1 polymorphisms, c.1236C>T (rs1128503), c.2677G>T/A (rs2032582), and c.3435C>T (rs1045642), were genotyped by PCR-pyrosequencing (PSQ 96MA, Qiagen, Valencia, CA, USA). ABCB1 haplotypes and diplotypes were computationally assigned using HelixTree Genetics Analysis Software (Golden Helix, Bozeman, MT, USA).
Drug concentration analyses
Plasma and urine concentrations of sitagliptin and plasma concentrations of atorvastatin and its metabolites (i.e., atorvastatin lactone, 4-hydroxyatorvastatin, 4-hydroxyatorvastatin lactone, 2-hydroxyatorvastatin, and 2-hydroxyatorvastatin lactone) were measured with a validated LC/MS-MS assay. The detailed bioanalytical methods are provided in the supplementary appendix. The limits of quantification (LOQs) for sitagliptin in plasma and in urine were 0.22 ng/ml and 0.11 ng/ml, respectively. The LOQs for atorvastatin and its metabolites in plasma were: atorvastatin, 0.60 ng/ml; atorvastatin lactone, 0.31 ng/ml; 4-hydroxyatorvastatin, 0.30 ng/ml; 4-hydroxyatorvastatin lactone, 0.29 ng/ml; 2-hydroxyatorvastatin, 0.60 ng/ml; and 2-hydroxyatorvastatin lactone, 0.62 ng/ml. The between-day coefficient of variation (CV) for plasma sitagliptin was 12% for low concentration quality controls, and less than 10% for medium and high concentration quality controls. For plasma atorvastatin and its metabolites, the between-day CVs for low concentration quality controls were 13% (atorvastatin) and less than 20% (atorvastatin metabolites). The CVs for medium and high concentration quality controls were less than 16% for atorvastatin and its metabolites.
Pharmacokinetic analyses
Plasma concentration-time curves of sitagliptin, atorvastatin (acid), atorvastatin lactone, and atorvastatin metabolites were generated and the maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) were taken from the curves. Pharmacokinetic parameters were estimated using noncompartmental methods with WinNonlin version 5.2.1 software (Pharsight Corporation, Mountain View, CA, USA). The terminal elimination rate constant (λz) was obtained by regression of the log-linear portion of the concentration-time curves. Sitagliptin area under the plasma concentration-time curve from 0 to infinity (AUC0-∞) and 0 to 24 hours (AUC0-24), and atorvastatin acid, atorvastatin lactone, and atorvastatin metabolites AUC over the dosing interval (AUCtau) were calculated using the linear-log trapezoidal rule. Half-lives (t1/2) were calculated as 0.693 divided by λz. For the calculation of sitagliptin renal clearance (CLR), the amount of sitagliptin collected over each 12-hour period was multiplied by the urine volume collected during that period and the two amounts were summed. The fraction of sitagliptin excreted unchanged in the urine (fe) was calculated as the total amount of sitagliptin excreted during the 24-hour period divided by dose. CLR was determined as the product of fe and CL/F.
Statistical analyses
Baseline demographics were compared between ABCB1 1236/2677/3435 diplotype groups using Fisher exact tests for categorical variables and one-way analysis of variance for continuous variables. Pharmacokinetic parameters that did not follow a normal distribution were log-transformed for statistical analysis and then back-transformed for data presentation. For the assessment of the potential interaction between sitagliptin and atorvastatin, sitagliptin pharmacokinetic parameters were presented as 90% confidence intervals (CI) surrounding the geometric mean ratios (GMR). The 90% CIs were compared to the pre-specified no effect bounds of 0.8-1.25. If the 90% CI of sitagliptin AUC0-∞ fell entirely within the pre-specified bounds, it was concluded that atorvastatin did not have a clinically significant effect on sitagliptin exposure [30]. Pharmacokinetic data were compared within each ABCB1 diplotype group using paired t tests or Wilcoxon signed rank tests (for time data). Pharmacokinetic data, including relative changes, were compared between ABCB1 diplotype groups using one-way analysis of variance, Kruskal-Wallis tests (for time data), or generalized linear model analysis (for assessment of covariates). An a priori power calculation is provided in the supplementary appendix. Statistical analyses were conducted using SPSS version 18.0 software. A P value <0.05 was considered statistically significant.
Results
We prospectively screened 145 subjects for ABCB1 1236/2677/3435 diplotypes, and 33 subjects started the study protocol. Four subjects participated in only one phase of the study—two withdrew for personal reasons and two were withdrawn for adverse events (i.e., increased liver function tests and difficulty maintaining intravenous access). Here, we present the results of 29 subjects who completed both phases of the study. The study consisted of 16 men and 13 women, mean age 33 ± 10 years, mean weight 73.0 ± 9.9 kg, and mean creatinine clearance 100.0 ± 14.5 ml/min. Table 1 shows baseline demographics by ABCB1 diplotype group [CGC/CGC (n=9), CGC/TTT (n=10), and TTT/TTT (n=10)]. With the exception of race (p=0.05), baseline variables did not differ significantly among the three diplotype groups.
Table 1.
Baseline demographics (n=29) by ABCB1 1236/2677/3435 diplotype group
| Variable |
ABCB1 CGC/CGC n=9 |
ABCB1 CGC/TTT n=10 |
ABCB1 TTT/TTT n=10 |
P value |
|---|---|---|---|---|
| Male, n | 4 | 8 | 4 | 0.17 |
|
| ||||
| Race, n | ||||
| Caucasian | 5 | 6 | 10 | 0.05 |
| African American | 3 | 1 | 0 | |
| Other | 1 | 3 | 0 | |
|
| ||||
| Age, years | 36 ± 10 | 33 ± 10 | 32 ± 10 | 0.71 |
|
| ||||
| Weight, kg | 75.9 ± 10.5 | 68.7 ± 7.5 | 74.8 ± 10.9 | 0.23 |
|
| ||||
| Creatinine Clearance, ml/min |
99.2 ± 12.9 | 95.8 ± 15.7 | 104.9 ± 14.7 | 0.38 |
|
| ||||
| Current smoker | 2 | 2 | 0 | 0.42 |
|
| ||||
| Hormonal birth control | 2 | 1 | 1 | 0.66 |
Data are expressed as number or mean ± standard deviation. Creatinine clearance was calculated by the Cockcroft-Gault equation.
During the sitagliptin monotherapy phase, there was modest variability in sitagliptin Cmax, AUC0-∞, and AUC0-24, with CV% of 37%, 33%, and 32%, respectively. In terms of ABCB1 genetics, the pharmacokinetics of sitagliptin (when administered alone) did not differ significantly between CGC/CGC, CGC/TTT, and TTT/TTT diplotype groups (Table 2). Sitagliptin CLR and fe were numerically greater in the TTT/TTT group as compared with the CGC/TTT and CGC/CGC groups; however these results did not reach statistical significance (p=0.21 and p=0.44, respectively), even when controlling for creatinine clearance (p=0.36 and p=0.64, respectively).
Table 2.
Single-dose sitagliptin pharmacokinetics in the presence and absence of atorvastatin by ABCB1 diplotype group
| Pharmacokinetic parameter | Sitagliptin Alone Arithmetic Mean ± SD |
Sitagliptin + Atorvastatin Arithmetic Mean ± SD |
Mean relative change (95% CI) |
P Value (within diplotype groups) |
|---|---|---|---|---|
| Cmax (ng/ml) | ||||
| ABCB1 CGC/CGC | 454 ± 180 | 428 ± 201 | 0.96 (0.75-1.17) | 0.43 |
| ABCB1 CGC/TTT | 438 ± 152 | 420 ± 137 | 1.0 (0.79-1.21) | 0.69 |
| ABCB1 TTT/TTT | 394 ± 160 | 370 ± 217 | 0.93 (0.76-1.11) | 0.26 |
| P value, between diplotype groups | 0.67 | 0.56 | 0.86 | |
|
| ||||
| AUC0-∞ (ng*h/ml) | ||||
| ABCB1 CGC/CGC | 3638 ± 1439 | 3501 ± 1617 | 0.96 (0.81-1.11) | 0.41 |
| ABCB1 CGC/TTT | 3502 ± 1133 | 3430 ± 1279 | 0.98 (0.88-1.07) | 0.43 |
| ABCB1 TTT/TTT | 3129 ± 795 | 3117 ± 1216 | 0.97 (0.87-1.08) | 0.45 |
| P value, between diplotype groups | 0.83 | 0.90 | 0.97 | |
|
| ||||
| AUC0-24 (ng*h/ml) | ||||
| ABCB1 CGC/CGC | 3227 ± 1216 | 3205 ± 1420 | 0.99 (0.83-1.15) | 0.66 |
| ABCB1 CGC/TTT | 3081 ± 925 | 3073 ± 1101 | 0.99 (0.89-1.09) | 0.69 |
| ABCB1 TTT/TTT | 2809 ± 820 | 2799 ±1195 | 0.98 (0.92-1.04) | 0.42 |
| P value, between diplotype groups | 0.80 | 0.82 | 0.97 | |
|
| ||||
| CLRa (ml/min) | ||||
| ABCB1 CGC/CGC | 254 ± 176 | 280 ± 124 | 1.22 (0.97-1.48) | 0.11 |
| ABCB1 CGC/TTT | 232 ± 62 | 226 ± 66 | 0.99 (0.82-1.16) | 0.67 |
| ABCB1 TTT/TTT | 359 ± 182 | 339 ± 188 | 1.0 (0.81-1.18) | 0.68 |
| P value, between diplotype groups | 0.21 | 0.22 | 0.14 | |
|
| ||||
| fea | ||||
| ABCB1 CGC/CGC | 0.50 ± 0.09 | 0.55 ± 0.08 | 1.14 (0.89-1.39) | 0.25 |
| ABCB1 CGC/TTT | 0.50 ± 0.06 | 0.47 ± 0.10 | 0.96 (0.81-1.11) | 0.44 |
| ABCB1 TTT/TTT | 0.62 ± 0.22 | 0.56 ± 0.16 | 0.96 (0.78-1.14) | 0.41 |
| P value, between diplotype groups | 0.44 | 0.36 | 0.24 | |
|
| ||||
| t1/2 (h) | ||||
| ABCB1 CGC/CGC | 7.3 ± 3.0 | 6.2 ± 1.5 | 0.94 (0.70-1.17) | 0.26 |
| ABCB1 CGC/TTT | 7.7 ± 2.5 | 7.3 ± 2.4 | 0.98 (0.78-1.17) | 0.51 |
| ABCB1 TTT/TTT | 7.3 ± 2.3 | 7.4 ± 1.9 | 1.04 (0.90-1.17) | 0.65 |
| P value, between diplotype groups | 0.82 | 0.16 | 0.49 | |
fe and CLR could not be calculated for three subjects (n=2, CGC/CGC; n=1, CGC/TTT). Data are expressed as arithmetic mean ± standard deviation or mean relative change (95% CI). Relative change=(sitagliptin + atorvastatin)/sitagliptin alone.
ABCB1 CGC/CGC, n=9; ABCB1 CGC/TTT, n=10; ABCB1 TTT/TTT, n=10.
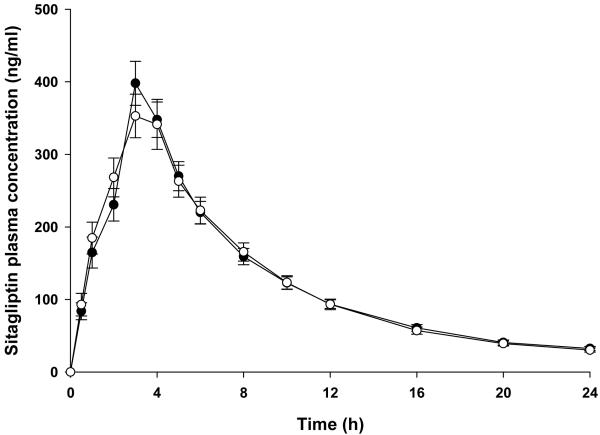
Atorvastatin administration did not significantly affect sitagliptin pharmacokinetics (Figure 1 and Table 3), with 90% CIs that fell within the pre-specified bounds of 0.8 to 1.25. Median sitagliptin Tmax was 3 hours in the absence and presence of atorvastatin. ABCB1 diplotype did not influence sitagliptin pharmacokinetics in the presence of atorvastatin (Table 2). Likewise, mean relative changes in sitagliptin pharmacokinetic parameters did not differ significantly within or between ABCB1 diplotype groups (Table 2). When the analysis was restricted to Caucasians only, there were no significant differences in sitagliptin pharmacokinetic parameters between ABCB1 diplotype groups in the monotherapy phase, in the presence of atorvastatin, or for relative changes (data not shown).
Fig.1.
Sitagliptin plasma concentrations (mean ± standard error) in the absence (closed circles) and presence (open circles) of atorvastatin in all study participants (n=29).
Table 3.
Single-dose sitagliptin pharmacokinetics in the absence and presence of atorvastatin (n=29)
| Pharmacokinetic parameter |
Sitagliptin Geometric Mean (90% CI) |
Sitagliptin + Atorvastatin Geometric Mean (90% CI) |
GMR (90% CI) |
P value |
|---|---|---|---|---|
| Cmax (ng/ml) | 399 (352, 451) | 371 (326, 424) | 0.93 (0.86, 1.01) | 0.16 |
| AUC0-∞ (ng*h/ml) | 3218 (2865, 3614) | 3082 (2696, 3522) | 0.96 (0.91, 1.01) | 0.15 |
| CLRa (ml/min) | 254 (215, 300) | 259 (225, 298) | 1.02 (0.93, 1.12) | 0.69 |
| fea | 0.52 (0.47, 0.58) | 0.51 (0.47, 0.56) | 0.98 (0.90, 1.06) | 0.68 |
| t1/2 (h)b | 7.4 ± 2.5 | 7.0 ± 2.0 | - | 0.39 |
CLR and fe could not be calculated for three subjects due to missing urine samples or incomplete urine collection
arithmetic mean ± standard deviation. GMR=geometric mean ratio; CI=confidence interval.
Atorvastatin and atorvastatin lactone pharmacokinetic parameters did not differ significantly between ABCB1 diplotype groups (Table 4). Along the same lines, the pharmacokinetics of 2-hydroxyatorvastatin, 2-hydroxyatorvastatin lactone, 4-hydroxyatorvastatin, and 4-hydroxyatorvastatin lactone were not significantly affected by ABCB1 diplotype (Table 4).
Table 4.
Atorvastatin and atorvastatin metabolite pharmacokinetics by ABCB1 diplotype group.
| ABCB1 Diplotype | Pharmacokinetic Parameters |
||
|---|---|---|---|
| Cmax (ng/ml) | AUCtau (ng*h/ml) | T1/2 (h) | |
| Atorvastatin | |||
| CGC/CGC | 8.6 ± 6.2 | 44.4 ± 28.0 | 5.7 ± 1.2 |
| CGC/TTT | 7.5 ± 7.6 | 35.4 ± 35.6 | 6.9 ± 2.0 |
| TTT/TTT | 10.6 ± 7.4 | 49.0 ± 37.1 | 6.4 ± 2.1 |
| P value, between diplotype groups | 0.43 | 0.29 | 0.55 |
|
| |||
| Atorvastatin lactone | |||
| CGC/CGC | 11.0 ± 11.9 | 87.3 ± 69.3 | 7.0 ± 2.5 |
| CGC/TTT | 7.4 ± 4.7 | 69.8 ± 54.7 | 6.7 ± 2.3 |
| TTT/TTT | 13.8 ± 11.2 | 101.0 ± 64.2 | 9.2 ± 6.0 |
| P value, between diplotype groups | 0.23 | 0.35 | 0.66 |
|
| |||
| 2-hydroxyatorvastatin | |||
| CGC/CGC | 5.5 ± 3.4 | 43.2 ± 21.6 | 6.3 ± 2.7 |
| CGC/TTT | 5.1 ± 4.1 | 45.0 ± 38.7 | 7.5 ± 3.0 |
| TTT/TTT | 4.5 ± 2.3 | 38.6 ± 19.5 | 7.0 ± 1.5 |
| P value, between diplotype groups | 0.93 | 0.93 | 0.32 |
|
| |||
| 2-hydroxyatorvastatin lactone | |||
| CGC/CGC | 4.4 ± 3.5 | 37.3 ± 22.5 | 6.8 ± 1.8 |
| CGC/TTT | 4.1 ± 3.0 | 42.6 ± 31.1 | 7.8 ± 4.2 |
| TTT/TTT | 3.9 ± 1.9 | 38.5 ± 19.3 | 9.9 ± 8.5 |
| P value, between diplotype groups | 0.98 | 0.96 | 0.92 |
|
| |||
| 4-hydroxyatorvastatin | |||
| CGC/CGC | 0.48 ± 0.61 | 4.6 ± 4.6 | 14.2 ± 4.3 |
| CGC/TTT | 0.25 ± 0.14 | 3.2 ± 1.6 | 19.3 ± 12.9 |
| TTT/TTT | 0.39 ± 0.34 | 5.1 ± 4.5 | 12.6 ± 5.2 |
| P value, between diplotype groups | 0.61 | 0.61 | 0.29 |
|
| |||
| 4-hydroxyatorvastatin lactone | |||
| CGC/CGC | 0.84 ± 0.11 | 9.1 ± 10.2 | 10.5 ± 5.5 |
| CGC/TTT | 0.7 ± 0.43 | 7.2 ± 4.0 | 12.8 ± 4.3 |
| TTT/TTT | 0.91 ± 0.64 | 10.4 ± 7.0 | 14.5 ± 8.8 |
| P value, between diplotype groups | 0.63 | 0.46 | 0.29 |
ABCB1 CGC/CGC, n=9; ABCB1 CGC/TTT, n=10; ABCB1 TTT/TTT, n=10. Data are expressed as arithmetic mean ± standard deviation.
Discussion
We sought to determine the impact of ABCB1 polymorphisms and atorvastatin on sitagliptin pharmacokinetics in healthy volunteers. Our main finding was that sitagliptin pharmacokinetics did not differ between ABCB1 diplotypes (i.e., CGC/CGC, CGC/TTT, and TTT/TTT). We also found that atorvastatin did not alter the disposition of sitagliptin.
Given in vitro data showing that ABCB1 polymorphisms have putative functional consequences [10,11,31,32], we hypothesized that the ABCB1 1236T/2677T/3435T haplotype may be associated with increased sitagliptin plasma exposure as a result of decreased renal clearance and/or increased oral bioavailability of sitagliptin. Our findings do not support this hypothesis, as sitagliptin plasma exposure did not differ significantly between ABCB1 CGC/CGC, CGC/TTT, and TTT/TTT diplotype groups. This does not imply that P-gp does not have a role in sitagliptin disposition, but rather, that ABCB1 CGC/CGC, CGC/TTT, and TTT/TTT diplotypes do not significantly influence sitagliptin pharmacokinetics in vivo. Similar findings have been observed for the P-gp substrate, digoxin, whereby ABCB1 polymorphisms have been shown to have no impact on digoxin pharmacokinetics in some clinical studies [31,33]. It is well-known that ABCB1 pharmacogenetic studies are influenced by confounding factors (e.g., race, co-medication, genotype versus haplotype); therefore the influence of ABCB1 genetics on the disposition of sitagliptin merits study in additional cohorts [31]. In vitro data also indicate that sitagliptin is a substrate for other drug transporters such as human organic anion transporter 3 (hOAT3) and organic anion transporting polypeptide 4C1 (OATP4C1) [6]. As such, the effect of OAT3 and OATP4C1 gene polymorphisms on sitagliptin disposition warrants investigation in clinical studies.
Sitagliptin is commonly co-administered with atorvastatin in clinical practice [19]. Atorvastatin has been shown to significantly increase the plasma concentrations of P-gp substrates such as digoxin, aliskiren, and verapamil, in healthy volunteers [21,28,34]. We found that sitagliptin pharmacokinetic parameters were unchanged following steady-state administration of atorvastatin. These results are consistent with the fact that atorvastatin is not a potent inhibitor of P-gp in vivo, producing plasma concentrations that are often much lower than its IC50 [35,7]. In terms of other, more potent, P-gp inhibitors, a healthy volunteer study showed a single 600 mg dose of cyclosporine modestly increased sitagliptin Cmax, but did not have a significant effect on overall sitagliptin plasma exposure [7]. Taken together, it does not appear that inhibition of P-gp alters sitagliptin disposition to a great extent in humans. Our drug interaction assessment was conducted in the setting of a diplotype enrichment design; therefore the findings are only applicable to individuals with ABCB1 CGC/CGC, CGC/TTT, or TTT/TTT diplotypes. We also studied a moderate, 40 mg dose of atorvastatin. It remains to be determined whether atorvastatin administration in the setting of other ABCB1 diplotypes, or high-dose atorvastatin (80 mg), has a clinically meaningful impact on sitagliptin concentrations. This is unlikely given the low frequency and questionable functional significance of other polymorphic ABCB1 diplotypes, the absence of any interaction with the 40 mg dose, and the wide therapeutic index of sitagliptin [2,36,37,7].
Our drug interaction findings are consistent with data showing that statins do not alter the concentrations of other DPP-4 inhibitors (e.g., alogliptin, vildagliptin, saxagliptin) to an appreciable extent [38]. For example, atorvastatin 80 mg and simvastatin 80 mg did not alter the multiple-dose pharmacokinetics of alogliptin and vildagliptin, respectively, in healthy volunteers [39,40]. Simvastatin 40 mg increased saxagliptin Cmax by 21% and AUC by 12%; however the magnitude of this interaction was determined not to be clinically significant [41]. We did not assess the impact of sitagliptin on atorvastatin pharmacokinetics because evidence suggests that sitagliptin does not inhibit or induce CYP3A4 or P-gp [3]. However, a few patient case reports have been published, which attribute the development of rhabdomyolysis to the concomitant administration of sitagliptin with various statins (e.g., lovastatin, simvastatin, and atorvastatin) [42-44]. Healthy volunteer studies do not indicate that DPP-4 inhibitors have a major effect on statin pharmacokinetics; therefore, the mechanisms underlying the observations in these case reports are unclear [38].
We found no differences in atorvastatin or atorvastatin metabolite disposition between CGC/CGC, CGC/TTT, and TTT/TTT diplotypes [45]. In contrast, a few studies have shown that ABCB1 haplotypes influence atorvastatin pharmacokinetics in healthy volunteers. Keskitalo et al. found that atorvastatin acid, 2-hydroxyatorvastatin, and 4-hydroxyatorvastatin plasma exposures were greater in Caucasian subjects with the TTT/TTT diplotype than in those with the CGC/CGC diplotype [45]. Lee et al. found that atorvastatin, atorvastatin lactone, 2-hydroxyatorvastatin, and 2-hydroxyatorvastatin lactone half-lives, but not plasma exposures, were greater in Korean subjects with two copies of the 2677T/3435T haplotype as compared with the GC/GC and GC/TT diplotypes [46]. Unlike these previous studies, we did not interrogate SLCO1B1 hepatic uptake transporter polymorphisms, which are known to influence statin pharmacokinetics [47]. Therefore, SLCO1B1 polymorphisms may have confounded our assessment of the influence of ABCB1 polymorphisms on atorvastatin disposition. Other possible reasons for the discrepancies between studies may include: different race/ethnic demographics of the study cohorts, small sample size, concomitant medications (e.g., sitagliptin), and failure to consider polymorphisms in other drug metabolizing enzyme and transporter genes (e.g., CYP3A5, ABCG2).
In conclusion, sitagliptin pharmacokinetics did not differ between ABCB1 CGC/CGC, CGC/TTT, and TTT/TTT diplotypes in healthy volunteers. Furthermore, atorvastatin had no effect on the pharmacokinetics of sitagliptin in the setting of this ABCB1 diplotype enrichment study.
Supplementary Material
Acknowledgements
We would like to thank the study volunteers for their participation, and the nursing and administrative staff at the University of Colorado Clinical and Translational Research Center for assisting with the conduct of the study. The study was funded by National Institutes of Health (NIH) grants R03 DK084089 (to CLA) and UL1 TR000154 (to University of Colorado). The research utilized the services of the Medicinal Chemistry Core facility (MFW) housed within the Department of Pharmaceutical Sciences at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. The Medicinal Chemistry Core facility receives funding via the Colorado Clinical and Translational Sciences Institute (CCTSI), which is supported in part by CTSA grant UL1TR000154 from NIH/NCRR. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
References
- 1.Herman GA, Stein PP, Thornberry NA, Wagner JA. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther. 2007;81:761–767. doi: 10.1038/sj.clpt.6100167. [DOI] [PubMed] [Google Scholar]
- 2.Herman GA, Stevens C, Van Dyck K, Bergman A, Yi B, De Smet M, Snyder K, Hilliard D, Tanen M, Tanaka W, Wang AQ, Zeng W, Musson D, Winchell G, Davies MJ, Ramael S, Gottesdiener KM, Wagner JA. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–688. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Januvia (sitagliptin) prescribing information. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc; Whitehouse Station, New Jersey, USA: Apr, 2012. http://www.merck.com/product/usa/pi_circulars/j/januvia/januvia_pi.pdf. [Google Scholar]
- 4.Vincent SH, Reed JR, Bergman AJ, Elmore CS, Zhu B, Xu S, Ebel D, Larson P, Zeng W, Chen L, Dilzer S, Lasseter K, Gottesdiener K, Wagner JA, Herman GA. Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans. Drug Metab Dispos. 2007;35:533–538. doi: 10.1124/dmd.106.013136. [DOI] [PubMed] [Google Scholar]
- 5.Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab. 2010;12:648–658. doi: 10.1111/j.1463-1326.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 6.Chu XY, Bleasby K, Yabut J, Cai X, Chan GH, Hafey MJ, Xu S, Bergman AJ, Braun MP, Dean DC, Evers R. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007;321:673–683. doi: 10.1124/jpet.106.116517. [DOI] [PubMed] [Google Scholar]
- 7.Krishna R, Bergman A, Larson P, Cote J, Lasseter K, Dilzer S, Wang A, Zeng W, Chen L, Wagner J, Herman G. Effect of a single cyclosporine dose on the single-dose pharmacokinetics of sitagliptin (MK-0431), a dipeptidyl peptidase-4 inhibitor, in healthy male subjects. J Clin Pharmacol. 2007;47:165–174. doi: 10.1177/0091270006296523. [DOI] [PubMed] [Google Scholar]
- 8.Cascorbi I. P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. Handb Exp Pharmacol. 2011:261–283. doi: 10.1007/978-3-642-14541-4_6. [DOI] [PubMed] [Google Scholar]
- 9.Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein) Pharmacogenet Genomics. 2011;21:152–161. doi: 10.1097/FPC.0b013e3283385a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704. [PubMed] [Google Scholar]
- 12.Chinn LW, Kroetz DL. ABCB1 pharmacogenetics: progress, pitfalls, and promise. Clin Pharmacol Ther. 2007;81:265–269. doi: 10.1038/sj.clpt.6100052. [DOI] [PubMed] [Google Scholar]
- 13.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 16.Sever PS, Poulter NR, Dahlof B, Wedel H, Collins R, Beevers G, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA) Diabetes Care. 2005;28:1151–1157. doi: 10.2337/diacare.28.5.1151. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, Waters D. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 18.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 19.Scheen AJ. Pharmacokinetic evaluation of atorvastatin and sitagliptin in combination for the treatment of type 2 diabetes. Expert Opin Drug Metab Toxicol. 2012;8:745–758. doi: 10.1517/17425255.2012.686603. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm Res. 2000;17:209–215. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- 21.Boyd RA, Stern RH, Stewart BH, Wu X, Reyner EL, Zegarac EA, Randinitis EJ, Whitfield L. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol. 2000;40:91–98. doi: 10.1177/00912700022008612. [DOI] [PubMed] [Google Scholar]
- 22.Wang E, Casciano CN, Clement RP, Johnson WW. HMG-CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharm Res. 2001;18:800–806. doi: 10.1023/a:1011036428972. [DOI] [PubMed] [Google Scholar]
- 23.Bogman K, Peyer AK, Torok M, Kusters E, Drewe J. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br J Pharmacol. 2001;132:1183–1192. doi: 10.1038/sj.bjp.0703920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochman JH, Pudvah N, Qiu J, Yamazaki M, Tang C, Lin JH, Prueksaritanont T. Interactions of human P-glycoprotein with simvastatin, simvastatin acid, and atorvastatin. Pharm Res. 2004;21:1686–1691. doi: 10.1023/b:pham.0000041466.84653.8c. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Mireles RJ, Campbell SD, Lin J, Mills JB, Xu JJ, Smolarek TA. Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos. 2005;33:537–546. doi: 10.1124/dmd.104.002477. [DOI] [PubMed] [Google Scholar]
- 26.Sakaeda T, Fujino H, Komoto C, Kakumoto M, Jin JS, Iwaki K, Nishiguchi K, Nakamura T, Okamura N, Okumura K. Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res. 2006;23:506–512. doi: 10.1007/s11095-005-9371-5. [DOI] [PubMed] [Google Scholar]
- 27.Holtzman CW, Wiggins BS, Spinler SA. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26:1601–1607. doi: 10.1592/phco.26.11.1601. [DOI] [PubMed] [Google Scholar]
- 28.Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, Bizot MN, Dieterich HA, Howard D, Dole WP. Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J Clin Pharmacol. 2008;48:1323–1338. doi: 10.1177/0091270008323258. [DOI] [PubMed] [Google Scholar]
- 29.Andrisin TE, Humma LM, Johnson JA. Collection of genomic DNA by the noninvasive mouthwash method for use in pharmacogenetic studies. Pharmacotherapy. 2002;22:954–960. doi: 10.1592/phco.22.12.954.33598. [DOI] [PubMed] [Google Scholar]
- 30.Guidance for Industry: Drug interaction studies —study design, data analysis, implications for dosing, and labeling recommendations. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research; [Last accessed August 13, 2012]. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/uc m292362.pdf. Draft published in February 2012. [Google Scholar]
- 31.Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 2007;7:154–179. doi: 10.1038/sj.tpj.6500413. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowbay B, Li H, David M, Cheung YB, Lee EJ. Meta-analysis of the influence of MDR1 C3435T polymorphism on digoxin pharmacokinetics and MDR1 gene expression. Br J Clin Pharmacol. 2005;60:159–171. doi: 10.1111/j.1365-2125.2005.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi DH, Shin WG, Choi JS. Drug interaction between oral atorvastatin and verapamil in healthy subjects: effects of atorvastatin on the pharmacokinetics of verapamil and norverapamil. Eur J Clin Pharmacol. 2008;64:445–449. doi: 10.1007/s00228-007-0447-5. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno N, Niwa T, Yotsumoto Y, Sugiyama Y. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- 36.Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72. doi: 10.1016/j.clinthera.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, Davies MJ, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7. doi: 10.1186/1472-6823-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheen AJ. Dipeptidylpeptidase-4 inhibitors (gliptins): focus on drug-drug interactions. Clin Pharmacokinet. 2010;49:573–588. doi: 10.2165/11532980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Karim A, Fleck P, Harris S, Munsaka M, Weiss M, Mekki Q. Assessment of drug interaction between alogliptin, a highly selective dipeptidyl peptidase-4 inhibitor, and atorvastatin in healthy subjects. Clin Pharmacol Ther. 2008;83:S14. [Google Scholar]
- 40.Ayalasomayajula SP, Dole K, He YL, Ligueros-Saylan M, Wang Y, Campestrini J, Humbert H, Sunkara G. Evaluation of the potential for steady-state pharmacokinetic interaction between vildagliptin and simvastatin in healthy subjects. Curr Med Res Opin. 2007;23:2913–2920. doi: 10.1185/030079907X233296. [DOI] [PubMed] [Google Scholar]
- 41.Patel CG, Li L, Girgis S, Kornhauser DM, Frevert EU, Boulton DW. Two-way pharmacokinetic interaction studies between saxagliptin and cytochrome P450 substrates or inhibitors: simvastatin, diltiazem extended-release, and ketoconazole. Clin Pharmacol. 2011;3:13–25. doi: 10.2147/CPAA.S15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao DP, Kohrt HE, Kugler J. Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use. Diabet Med. 2008;25:1229–1230. doi: 10.1111/j.1464-5491.2008.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiGregorio RV, Pasikhova Y. Rhabdomyolysis caused by a potential sitagliptin-lovastatin interaction. Pharmacotherapy. 2009;29:352–356. doi: 10.1592/phco.29.3.352. [DOI] [PubMed] [Google Scholar]
- 44.Bhome R, Penn H. Rhabdomyolysis precipitated by a sitagliptin-atorvastatin drug interaction. Diabet Med. 2012;29:693–694. doi: 10.1111/j.1464-5491.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- 45.Keskitalo JE, Kurkinen KJ, Neuvoneni PJ, Niemi M. ABCB1 haplotypes differentially affect the pharmacokinetics of the acid and lactone forms of simvastatin and atorvastatin. Clin Pharmacol Ther. 2008;84:457–461. doi: 10.1038/clpt.2008.25. [DOI] [PubMed] [Google Scholar]
- 46.Lee YJ, Lee MG, Lim LA, Jang SB, Chung JY. Effects of SLCO1B1 and ABCB1 genotypes on the pharmacokinetics of atorvastatin and 2-hydroxyatorvastatin in healthy Korean subjects. Int J Clin Pharmacol Ther. 2010;48:36–45. doi: 10.5414/cpp48036. [DOI] [PubMed] [Google Scholar]
- 47.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.