Abstract
Background
SET domain is responsible for the catalytic activity of histone lysine methyltransferases (HKMTs) during developmental process. Histone lysine methylation plays a crucial and diverse regulatory function in chromatin organization and genome function. Although several SET genes have been identified and characterized in plants, the understanding of OsSET gene family in rice is still very limited.
Methodology/Principal Findings
In this study, a systematic analysis was performed and revealed the presence of at least 43 SET genes in rice genome. Phylogenetic and structural analysis grouped SET proteins into five classes, and supposed that the domains out of SET domain were significant for the specific of histone lysine methylation, as well as the recognition of methylated histone lysine. Based on the global microarray, gene expression profile revealed that the transcripts of OsSET genes were accumulated differentially during vegetative and reproductive developmental stages and preferentially up or down-regulated in different tissues. Cis-elements identification, co-expression analysis and GO analysis of expression correlation of 12 OsSET genes suggested that OsSET genes might be involved in cell cycle regulation and feedback.
Conclusions/Significance
This study will facilitate further studies on OsSET family and provide useful clues for functional validation of OsSETs.
Introduction
SET domain, named after the three Drosophila proteins SUPPRESSOR OF VARIEGATION 3–9 [SU(VAR)3–9], ENHANCER OF ZESTE [E(Z)] and TRITHORAX (TRX) [1], has been known to be involved in the biochemical process of the histone lysine methyltransferases (HKMTs) [2]. It contains an approximately 130-amino acid, presenting as an evolutionarily conserved motif in chromosome proteins from yeast to mammals and higher plants [3]. It consists of two non-contiguous regions formed by N- and C-terminal ends of the primary sequence, known as SET-N and SET-C, respectively, and an insert region (SET-I) [4]. SET domain protein methyltransferases have enormous impacts on the regulation of chromatin structure and function [5], [6]. They catalyze the transfer of methyl groups from the cofactor S-adenosylmethionine (AdoMet) to specific lysine residues of protein substrates, such as the N-terminal tails of histone (H3 or H4) and the large subunit of the Rubisco holoenzyme complex [7], [8].
Baumbusch [1] first identified 39 SET domain genes in Arabidopsis thaliana and divided them into four classes based on the SET domains, cysteine-rich regions and additional conserved domains. Springer et al. identified 32 SET domain genes in Arabidopsis and 22 ones in Zea mays, and classified the SET domain proteins into five subfamilies, on the basis of phylogenetic analyses and domain organization [9]. It revealed that the duplication of SET domain proteins in plants was extensive and had occurred via multiple. Ng et al. [10] inferred that there were at least 47 SET genes in Arabidopsis, 35 members in maize and 34 ones in rice, based on the annotation in Pfam and ChromDB database, which were classified into seven groups. Pontvianne [11] reported that SET domain genes in Arabidopsis can be divided into five classes (I to V), based on their domain architectures and/or differences in enzymatic activity of SET domain-containing proteins.
Presently, a number of SET genes have been functionally identified in plants. Mutation of Arabidopsis SET domain genes resulted in phenotypic abnormalities due to the improper regulation of important developmental genes [1], [9]–[11]. Arabidopsis CURLY LEAF (CLF), a homolog of Drosophila E(Z) gene, was involved in the division and elongation of cells during leaf morphogenesis [12]–[14]. Further evidences revealed that CLF can directly mediate the repression of AGAMOUS (AG), FLOWERING LOCUS C (FLC) and FLOWERING LOCUS T (FT) via lysine 27 of histone H3 trimethylation (H3K27me3) and thus control floral organogenesis in a Polycomb repressive complex 2 (PRC2)[15]–[18]. MEDEA (MEA), another E(Z) homolog in Arabidopsis, is a self-controlled imprinting gene and functions in controlling the proliferation of central cell [19]–[23]. Su(VAR)3–9 homolog (SUVH) proteins KRYPTONITE (KYP, also known as SUVH4), SUVH5 and SUVH6 are shown to function in a locus specific manner to undergo H3K9 methylation and cytosine metyltransferase 3 (CMT3)-mediated non-CG DNA methylation [24]–[27]. In contrast to the E(Z) and SUVH proteins suppressing transcription, ATX1 functions as an activator of homeotic genes by lysine 4 of histone H3 (H3K4) methylation [28]–[30]. These results uncovered the extensive functions of SET proteins in plant development.
Rice is one of the major staple foods and an ideal model species of monocotyledons for functional genomics analysis. In previous studies, several SET proteins have been characterized in rice. OsCLF and OsiEZ1, both of which are E(Z) homologies, is expressed preferentially in young seedlings and during reproductive development [31]–[33]. SET Domain Group 714 (SDG714) and SDG728, encoding H3K9me2 histone methyltransferase, display specific functions in chromatin modification and retrotransposon repression [34], [35]. The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice [36]. Therefore, it is necessary to carry out a comprehensive functional exploration for OsSET gene family in rice.
In this study, the members of SET family in rice have been analyzed based on complete genome and protein sequences and annotations. Expression patterns and co-expression analyses were performed to discover the potential functions of OsSET gene family. Promoter cis-elements identification and the combined analysis of expression correlation suggest that most of OsSET genes may be cell cycle modulated and linked to the cell cycle progression by histone modifications. Our results will provide a useful reference for further functional analysis of members of OsSET gene family in rice.
Results and Discussion
Identification and Classification of OsSET Gene Family
In the previous report [10], 34 SET genes in rice genome were inferred, on the basis of annotation in Pfam and ChromDB database. However, based on the update Pfam and ChromDB database and MSU data, 43 SET family genes in rice were identified in our study. These 43 OsSET genes were named from OsSET1 to OsSET43 according to their positions on chromosomes. Nine genes, OsSET12, OsSET13, OsSET18, OsSET23, OsSET29, OsSET31, OsSET36, OsSET37 and OsSET39, were novel OsSET genes compared to the ones in previous study. OsSET genes vary substantially in the size of their encoded proteins and their physicochemical properties (Table S1). OsSET33 has 25 exons and 24 introns, whereas OsSET14, OsSET20 and OsSET30 have no intron. The position of the SET domain also varies within the proteins. The shortest OsSET protein is OsSET9 with 231 amino acids, while the longest one is OsSET30 with 1292 amino acids. EXPASY analysis showed a large variation in isoelectric point (pI) values (ranging from 4.4119 to 9.2625) and molecular weights (ranging from 110.892 kDa to 138.5423 kDa). Nevertheless, only 3 (OsSET4, OsSET14 and OsSET29) of the 43 OsSET genes were predicted to be stable proteins. Details on other parameters of protein sequences were shown in Table S1.
Chromosomal Localization and Gene Duplication
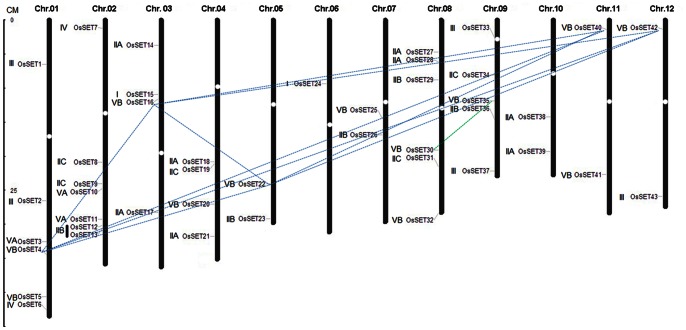
The genomic distribution of OsSET genes was determined by their chromosomal positions on rice chromosome. Totally, 43 OsSET genes were dispersed on the 12 chromosomes, presenting unevenly in all regions of the chromosomes. A brief diagrammatic representation of chromosomal distribution of OsSET genes was described ( Figure 1 , the exact position on rice chromosome pseudomolecules was given in Table S1). Seven OsSET genes are on chromosome 2, six OsSET genes are on chromosome 1 and 8, respectively; five OsSET genes are on chromosome 9; four OsSET genes are on chromosome 3 and chromosome 4; two OsSET genes are on chromosome 5, 7, 10, 11, 12, respectively; only one OsSET gene is on chromosome 6.
Figure 1. Chromosomal distribution, and tandem and segmental genome duplications of the OsSET gene family.
The scale on the left is in megabases (Mb). The ovals on the chromosomes (vertical bars) indicate the positions of centromeres; the chromosome numbers are shown on the top of each bar. The segmental duplication genes are connected by a dotted line.
During the evolution of a gene family, segmental duplication and tandem duplication play important roles in generating new members [37]. Therefore, both segmental and tandem duplication events were investigated for elucidating the potential mechanism of evolution of OsSET gene family. Analysis of the MSU RGAP rice segmental duplication database revealed that 10 pairs of OsSET genes could be assigned to MSU RGAP segmental duplication blocks. The overall similarity of the cDNA sequences of these genes ranged from 25.6% to 77.0% and all of them were found to have their counterparts on duplicated segments (Table S2). Interestingly, these duplicated segments can be clustered in 2 groups. Five OsSET genes (OsSET4, OsSET16, OsSET22, OsSET40 and OsSET42), which had high overall identities between each other, belonged to the same group. While the overall identity between OsSET30 and OsSET35 was 52.0%, and was included in another group. None of the OsSET genes seemed to be generated from tandem duplications in our analysis. These results implicated that much of the diversity of the OsSET gene family in rice was mainly due to the segmental duplication events.
Phylogenetic and Structural Analysis of OsSET Gene Family
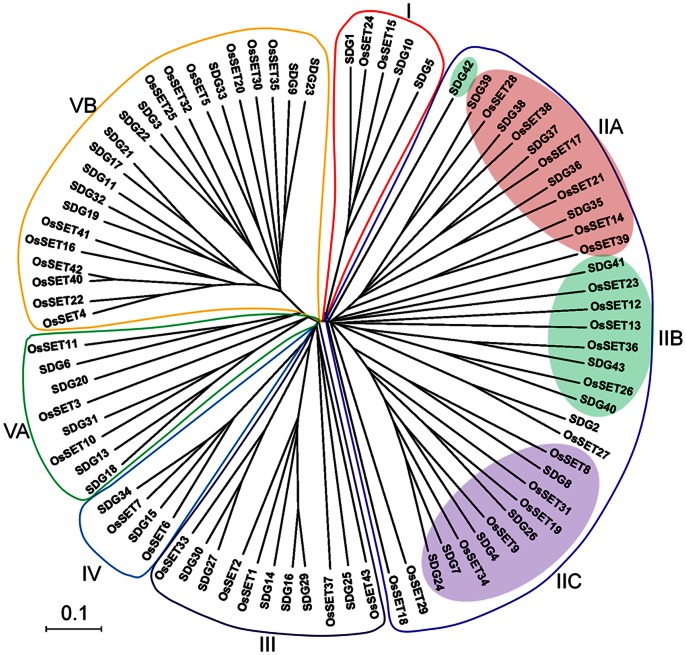
To determine the evolutionary relationships of SET family genes between rice and Arabidopsis, an unrooted phylogenetic tree was constructed from alignments of their full-length protein sequences. The latest data showed that Arabidopsis SET family genes can be divided into five classes (I to V), based on their domain architectures and/or differences in enzymatic activity of SET domain-containing proteins [11]. Coincidently, our phylogenetic analysis and their domain architectures support the classification of rice and Arabidopsis SET gene family into five classes ( Figure 2 , 3 ; Table S1).
Figure 2. Phylogenetic analysis of Arabidopsis and rice SET proteins.
Phylogenetic tree of rice and Arabidopsis SET proteins. An unrooted NJ tree of rice and Arabidopsis SET proteins is shown. The six classes are marked by different colors. Scale bar represents 0.1 amino acid substitution per site.
Figure 3. Structure of representative OsSET proteins from each subfamily.
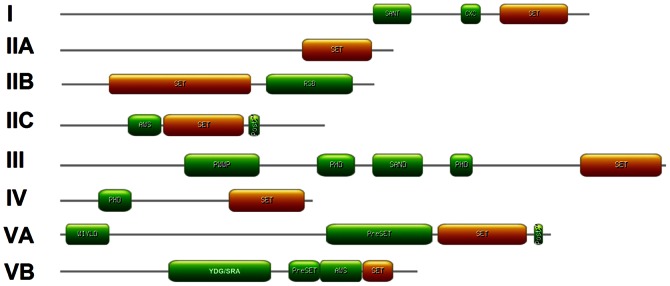
The protein structure is based on the presence of OsSET and other additional domains as identified by Pfam. Subfamily name of each corresponding protein belonged to are given on the left. The length and order of domains represent actual situation in each protein.
Class I SET proteins include 2 rice OsSET proteins and 3 Arabidopsis SET proteins. OsSET15/OsiEZ1 and OsSET24/OsCLF are the homologs of Arabidopsis SWINGER (SWN)/SDG10 and CLF/SDG1, respectively. No Arabidopsis MEDEA (MEA)/SDG5 homolog was found in rice. In addition to the C-terminal SET domain, SANT domain (Swi3, Ada2, N-Cor, and TFIIIB DNA binding domain) and cysteine rich CXC domain were found in this subfamily. This result is in agreement with previous studies [10]. Recent studies suggested that SANT domains might be a histone-tail-binding module [38], [39]. It is reported that E(Z)-like proteins are components of PRC2 complexes and function as transcriptional repressors by H3K27me3 in diverse eukaryotes [13], [16], [21], [22]. Therefore, OsSET15/OsiEZ1 and OsSET24/OsCLF may have H3K27me3 activities by these conserved domains.
Class II subfamily can be divided into three clusters of IIA, B and C based on their domains, which is also in accordant with the previous reports [9], [10]. Eight members in rice (OsSET14, OsSET17, OsSET18, OsSET21, OsSET27, OsSET28, OsSET38 and OsSET39) and five in Arabidopsis (SDG35–39) belonged to Class IIA, which only contain the SET domain. Class IIB proteins (OsSET12, OsSET13, OsSET23, OsSET26, OsSET36, SDG40, SDG41, SDG42, SDG43) have a Rubisco LSMT substrate-binding domain (RSB domain), which allows the binding of the protein to its substrate, such as the N-terminal tails of histones H3 and H4 and the large subunit of the Rubisco holoenzyme complex [7]. Class IIC has five SET proteins in rice (OsSET8, OsSET9, OsSET19, OsSET31 and OsSET34/SDG724) and five in Arabidopsis (ABSENT, SMALL, OR HOMEOTIC DISCS 1 HOMOLOG 1 (ASHH1)/SDG26, ASHH2/SDG8, ASHH3/SDG7, ASHH4/SDG24 and ASH1-related 3 (ASHR3)/SDG4 [40]–[42],). All proteins except OsSET31 in class IIC have an AWS domain (Associated With SET, a sub unit of pre-SET domain) [10]. Five of the members in class IIC have an additional cysteine-rich post-SET domain. Although some of the class II subfamily genes have been demonstrated to methylate H3K36 at the region of actively transcribed genes [43], the functions of the additional domains are still little known.
Class III HKMTs consist of four rice SET members (OsSET1, OsSET2, OsSET33 and OsSET37) and seven Arabidopsis genes. Five of the Arabidopsis genes encode homologs of Trithorax (trxG), which named as Arabidopsis Trithorax-like protein (ATX1/SDG27, ATX2/SDG30, ATX3/SDG14, ATX4/SDG16 and ATX5/SDG29), while another two genes, ATXR3/SDG2 and ATXR7/SDG25, are ATX-RELATED (ATXR) genes [44]. Class III subfamily genes have several additional highly conserved protein domains, including PWWP, FYRN/C and plant homeodomain (PHD). The PWWP domain was shown to be a DNA or methyl-lysine histone binding domain [45]–[47]. In Arabidopsis, class III proteins are able to methylate H3K4me2/3, acting as antagonistic regulators with Polycomb Group (PcG) proteins to maintain transcriptional OFF and ON states of their target genes [48], [49]. Interestingly, PHD finger is considered to be specific and highly robust binding modules for H3K4me2/3 in humans and plants, resulting the recruitment of basal transcriptional active factor(s)[50]–[53]. ATX1 has been demonstrated to interact with ASHH1/SDG26, suggesting that trxG complexes could involve different sets of histone lysine methyltransferases in Arabidopsis [40]. These results provide an efficient insight for functional identification of trxG in rice.
OsSET6, OsSET7, ATXR5/SDG15 and ATXR6/SDG34 are included in class IV, which characterized with a PHD domain and a SET domain. ATXR5 and ATXR6 are involved in DNA replication [54]. Although class IV proteins are near to class III on their evolutionary relationship, they are distinct from class III for the absence of PWWP domain. The result suggests that the PWWP domain may be crucial for the antagonistic mechanism between PcG and trxG.
Class V proteins are characterized by the presence of pre-SET and SET domains. This class can be divided into VA [SU(VAR)3–9 (SUVR)] and VB (SUVH) for the latter having an YDG/SRA domain [1]. Class VA contains three OsSET proteins (OsSET3, OsSET10 and OsSET11) and 5 SU(VAR)3–9 (SUVR) proteins (SUVR1/SDG13, SUVR2/SDG18, SUVR3/SDG20, SUVR4/SDG31 and SUVR5/SDG6). OsSET10, SDG18 and SDG31 also have an N-teminal plant-specific domain, WIYLD, which binds ubiquitin and enables conversion of H3K9me1 to H3K9me3 [55]. Subfamily VB consists of 12 OsSET proteins (OsSET5/SDG714, OsSET14, OsSET16, OsSET20, OsSET22/SDG728, OsSET25, OsSET30/SDG710, OsSET32, OsSET35/SDG727, OsSET40, OsSET41, OsSET42) and 9 SUVH proteins (SUVH1/SDG32, SUVH2/SDG3, SUVH3/SDG19, SUVH4/KYP/SDG33, SUVH5/SDG9, SUVH6/SDG23, SUVH7/SDG17, SUVH8/SDG21, SUVH9/SDG22 and SDG11). It has reported that the YDG/SRA domain can mediate epigenetic inheritance by recruiting histone deacetylase (HDAC), DNA methyltransferase (DNMT) to methyl-CpG site [56]–[58]. Similarly, the YDG/SRA domain of KYP, SUVH5 and SUVH6 binds directly to methylated DNA at both CpG and non-CpG site, thereafter, providing a binding site for CMT3 via its chromodomain to CHG methylation [59]. OsSET5/SDG714, an H3K9 methyltransferase, is also involved in DNA methylation in rice [35], [60], which implies a similar mechanism between class VB OsSET proteins and DNA methylation. SUVR5 establishes the heterochromatic state by H3K9me2 deposition in a DNA methylation–independent manner through zinc fingers [61]. However, no such DNA binding domain was identified in rice SUVR like proteins. Therefore, there must be a distinct mechanism for SUVR in rice.
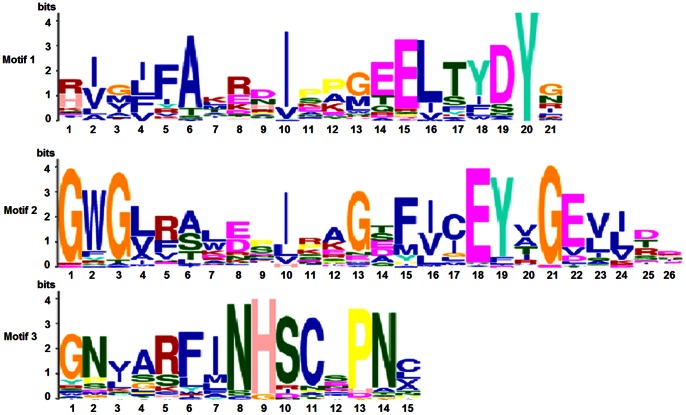
Because SET domain is essential for the catalytic activity of SET proteins, the MEME motif search tool was employed to identify the conserved motifs of SET domains from 84 SET proteins in rice and Arabidopsis. Three distinct motifs, motifs 2, 3 and 1, were located orderly at SET-N, SET-I and SET-C region of SET domain, respectively ( Figure 4 ). 55 out of 84 (65.5%) SET proteins have motifs 1, 2 and 3. 17 (20.2%) SET proteins only have motifs 1 and 3. OsSET29 and OsSET39 have motifs 2 and 3. OsSET12, OsSET13 and OsSET23 only have motif 1. OsSET3 and OsSET16 only have motif 2. The other five SET proteins (OsSET26, OsSET36, SDG40, SDG42 and SDG43) have none of the three motifs. Interestingly, 24 out of 29 (82.8%) proteins that have no more than 2 motifs are subfamily IIA or IIB members, which showed diversity in these two subfamilies.
Figure 4. Sequence LOGOs for each motif of SET domains using the MEME algorithm.
MEME motifs are displayed by stacks of letters at each position. The total height of the stack is the “information content” of that position in the motif in bits. The height of the individual letters in a stack is the probability of the letter at that position multiplied by the total information content of the stack. X- and Y-axis represents the width of motif and the bits of each letters, respectively.
Identification of Cis-elements in OsSET Gene Promoters
To understand the molecular mechanism of OsSET genes in transcriptional regulation, cis-elememts at the promoter regions were identified (Table S3). As a result, 255 cis-elements were obtained. In addition to basic TATA-box and CCAAT box, cis-elements such as MYB recognition, auxin responsive, gibberellin (GA) response, abscisic acid (ABA) responsive and E2F-binding site were found at the promoter regions of OsSET genes. It is well known that E2F transcription factors control the cell cycle by regulating transcription of genes required for DNA replication and cell cycle [62]. Many investigations show that the E2F targets have one or more consensus DNA sequence of E2F binding sites [63]–[65]. It was reported that ATX1/SDG27, ATXR5/SDG15, and ATXR6/SDG34 in Arabidopsis, OsSET6, and OsSET7 in rice, and lots of other cell cycle or DNA replication related genes were considered as E2F targets for their E2F binding cis-elements [54], [63], [64]. While, in our analysis, we found 32 OsSET genes had E2F binding site (E2FCONSENSUS, SITEIIBOSPCNA, SITEIOSPCNA, E2FANTRNR, E2F1OSPCNA, E2FAT, PE2FNTRNR1A) (Table. 1). Therefore, the analyses revealed that most of the OsSET genes might be regulated by E2F transcription factors.
Table 1. Conserved E2F binding cis-elements analysis of the OsSET gene promoters.
| Accession Numbera | IDb | Sequences |
| S000217 | SITEIIBOSPCNA | TGGTCCCAC |
| S000224 | SITEIOSPCNA | CCAGGTGG |
| S000366 | E2FANTRNR | TTTCCCGC |
| S000396 | E2F1OSPCNA | GCGGGAAA |
| S000417 | E2FAT | TYTCCCGCC; Y = T/C |
| S000455 | PE2FNTRNR1A | ATTCGCGC |
| S000476 | E2FCONSENSUS | WTTSSCSS; W = A/T; S = C/G |
Accession number of cis-element in PLACE database.
Locus identity number of cis-element in PLACE database.
Expression Profiling of OsSET Genes in Rice
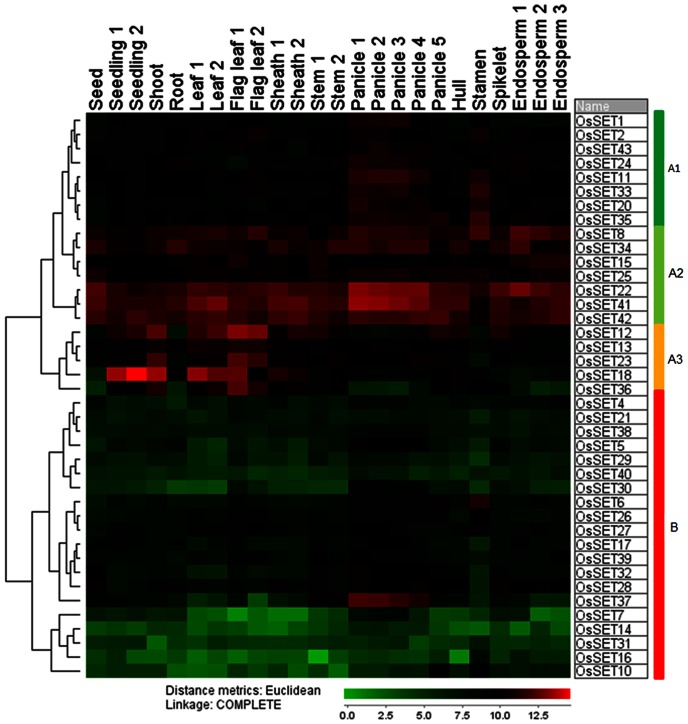
To investigate the transcript accumulation of OsSET genes in the entire life cycle, the expression profiling covering 24 developmental stages (Table S4) in Minghui 63 were analyzed using Affymetrix rice microarray data from CREP database. A hierarchical cluster displaying the logarithm of average signal values for the 40 OsSET genes were generated. Distinctly, the expression patterns of OsSET genes could be classified into two major groups ( Figure 5 ). 20 genes belonged to Group I, most of which showed high transcript accumulations (average expression signal from 777.3 to 4211.1) in the tissues analyzed. OsSET41 had the highest expression level in the entire life cycle. These genes could be further divided into three subgroups, subgroup A1–3. Subgroup A1 consists of 8 genes, which have high expression level in panicles and/or stamen. Subgroup A2 has 7 OsSET genes, all of which show relative high expression level in almost all tissues analyzed. Subgroup A3 has 5 OsSET genes, which display higher expression in vegetative tissues than in reproductive tissues. Group B contains 20 genes, exhibiting relative low expression signals in most tissues or preferential expressions in some tissues. OsSET5, OsSET6, OsSET10, OsSET17, OsSET27, OsSET28, OsSET32 and OsSET37 showed high expression in panicles; OsSET6, OsSET10 and OsSET27 expressed relatively higher in stamen, In addition, OsSET28 in spikelet and seed, OsSET32 in seed, OsSET37 in stem showed tissue-specific expressions.
Figure 5. Expression patterns of OsSET genes during the life cycle of the rice plant.
Hierarchical cluster displays the expression profile for 40 OsSET genes with matching probesets in the Affymetrix microarray. (Color bar at the base represents log2 expression values: green, representing low expression; black, medium expression; red, high expression).
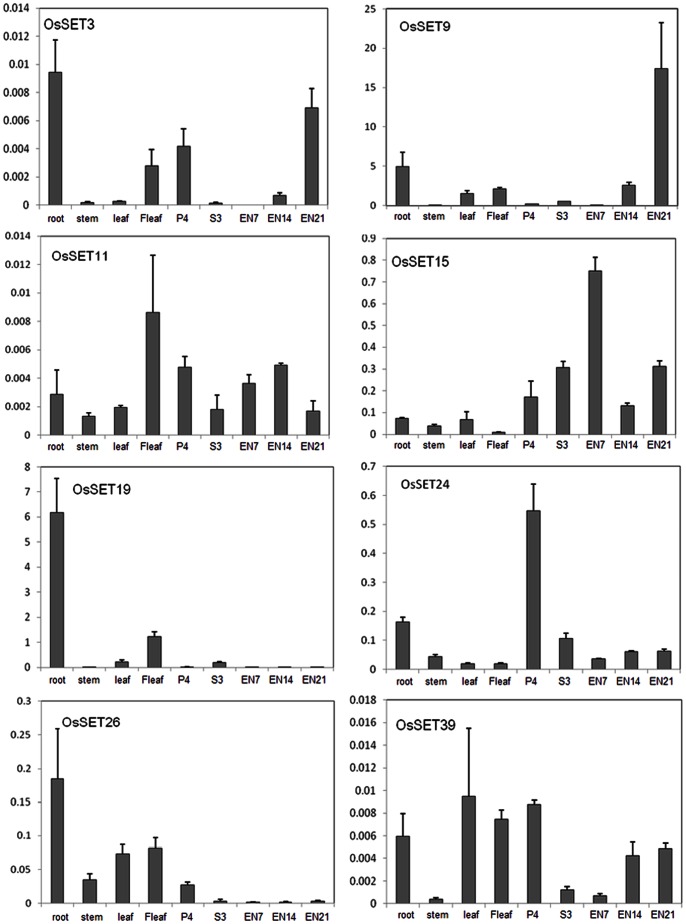
The expression patterns of some OsSET genes were further confirmed by real-time PCR analysis. The expression levels of OsSET3, OsSET9 and OsSET19, which have no probeset information in CREP database, were detected in different tissues ( Figure 6 ). OsSET3 shows a relative high expression in root, flag leafs, panicles and mature endosperm. Both OsSET9 and OsSET19 are included in class II, however, their expression patterns are divergent: OsSET9 are highly expressed in the later stage of endosperm (21 days after pollination), whereas the expression of OsSET19 enriched in young leaf and decreased in endosperm. The expression levels of OsSET11, OsSET24/OsCLF and OsSET15/OsiEZ1 are also detected, which are in accordance with the microarray data. OsSET26 has a low expression pattern in microarray analysis, whereas our quantitative PCR result shows a relative higher expression level in vegetative stage than reproductive stage. OsSET39 expresses relatively higher in root, leaf and panicles, and the expression is enriched in the developing endosperm, implying multiple functions in plants development.
Figure 6. Relative expression of eight OsSET genes in Zhonghua11.
root, roots at trefoil stage; stem, stems at heading stage; leaf, leafs at at trefoil stage; Fleaf, flag leafs at heading stage; P4, panicles at meiosis stage of young panicle development; S3, seeds of 3 days after pollination; EN, endosperm, the number followed it means the days after pollination.
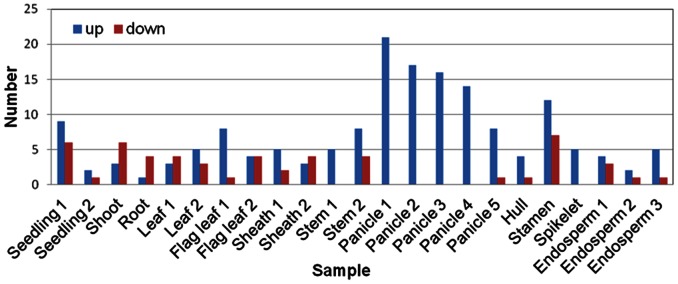
On the purpose of revealing more information in OsSET gene expression pattern, genes that showed differential expression during various developmental stages in comparison to seed were analyzed. Genes that considered as preferential expression in a given stage showed tremendous differences (Fig 7, Table S5). Up-regulated genes mainly accumulated in panicles and stamen, suggesting that OsSET genes may participate in various molecular pathways in flowering development. Surprisingly, although down-regulated genes accumulated in seedlings, they were activated in stamen, either. These microarray and real-time PCR results indicate that OsSET genes may play essential roles through the life cycle of rice.
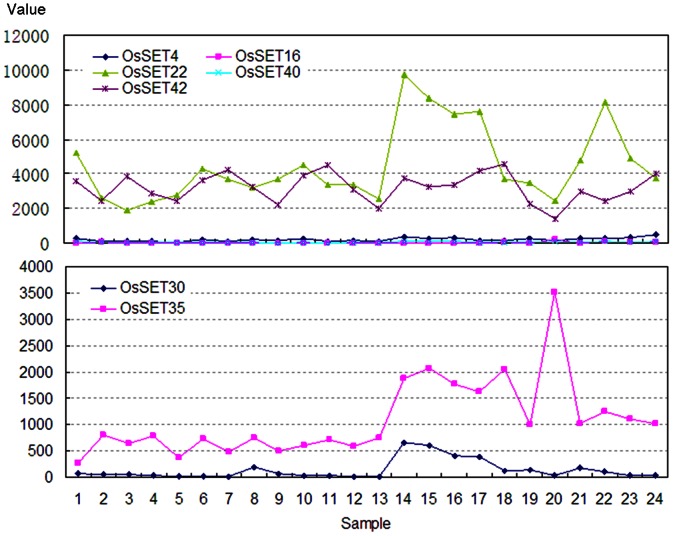
Figure 7. Expression patterns of OsSET genes found in segmentally duplicated regions of the rice genome.
X-axis represents the developmental stages as given in the following table. Y-axis represents the raw expression values obtained from microarray.
The expression patterns of segmentally duplicated OsSET genes were examined by Affymetrix microarray data. Probesets were available for all segmental duplication genes in microarray data. A comparison of expression level revealed that a pair of segmental duplicated genes always showed similar expression pattern, although one of the copy showed low expression level, or was not expressed at significant levels in most of the tissues ( Figure 8 ). In the group of OsSET4, OsSET16, OsSET22, OsSET40 and OsSET42 ( Figure 8A ), for instance, 2 out of 5 genes had a similarly high expression level. The resemblance also existed in the OsSET30 and OsSET35 group ( Figure 8B ). We might therefore infer that immediately after segmental duplication, the two copies of genes might be functionally redundant. However, only one of them is functional retained while the other degenerates into a pseudogene eventually.
Figure 8. Differential expressions of OsSET genes in different stages in Minghui 63 based on microarray analysis.
Differential expression genes have been taken p value less than 0.05 and fold change >2 or <0.5. When fold change >2, regulation is up, and when fold change <0.5, regulation is down.
Responses of OsSET genes under NAA, KT, and GA3 Treatments
Phytohormones play critical roles in plant growth and development. To investigate the OsSET genes in response to phytohormone treatment, differential expression analysis was performed. As a result, 9 OsSET genes that were differentially expressed under one or more of the phytohormone NAA, KT, GA3 in seedlings in trefoil stage, compared with the control without treatment. The fold change values with respect to control were given in Table S4. Three OsSET genes showed differential expression under all three phytohormone treatments, among which OsSET23 and OsSET36 were up-regulated, whereas OsSET18 was down-regulated. OsSET2 and OsSET16 were up-regulated to KT, and OsSET24 and OsSET34 were up-regulated to GA3 treatment. In contrast, OsSET17 and OsSET37 were down-regulated specifically to KT and GA3 treatment, respectively. The expression profile of the remaining genes in response to NAA, KT, and GA3 was not significant. These results partially in accordance with the cis-elements analysis, showing that the above 9 OsSET genes have one or more GA responsive elements (Table. S3).
Identification and Functional Annotation of Genes Co-expressed with OsSET Genes
Co-expression analysis has been successfully exploited to identify functional transcription regulators in Arabidopsis, rice and other organisms [66], [67]. Hence, in order to disinter more information of the OsSET genes, 40 OsSET genes with matching probeset were selected as “guide genes” to identify the co-expressed genes using expression data from CREP database, with an absolute value of the Pearson correlation coefficient (PCC) greater than 0.75 (α = 0.05) [68], [69]. As a result, 2390 genes whose expression pattern tightly correlated with 30 OsSET members were extracted (Table S6a).
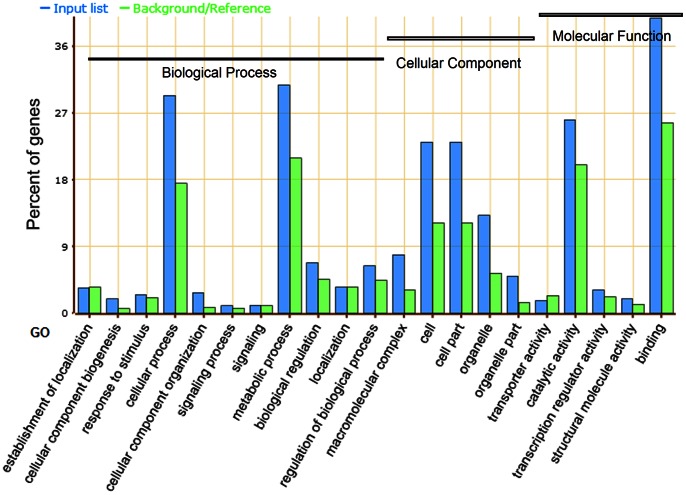
We next analyzed the GO annotations assigned to these genes by agriGO tools. The enriched GO annotations particularly concentrate on cellular process, cellular component biogenesis and organization, biological regulation and metabolic process ( Figure 9 , Table S6b). They encode proteins as macromolecular complex (protein complex, DNA polymerase, protein-DNA complex) in cell or organelle, substantially. The molecular functions tightly associated with them are catalytic activity, transcription regulator and binding. These results suggest that the functions of OsSET genes may be associated with DNA replication and gene transcription.
Figure 9. Enriched GO analysis of genes that co-expressed with OsSETs.
X-axis represents the GO annotation. Y-axis represents the percentage of GO annotation. Three categories of GO annotation are biological process, cellular component and molecular function. Input list represents genes analyzed, and the references/background represents all genes in agriGO database.
As is generally known, SET domain proteins have comprehensive impacts on the regulation of chromatin structure and function [5], [6]. Therefore, we focused on 503 out of 2390 co-expression genes which may be associated with epigenetic regulation (histone genes, cell cycle related genes, DNA replication, transcription factor, chromatin reassemble related genes and so on. Table S6c). After the recalculation and student-t test of PCC, 450 genes were co-expressed with 29 OsSET genes. In addition, 13 OsSET genes were co-expressed with each other tightly. Except for OsSET28, the other 12 OsSET genes have close expression relationships with histone genes, cell cycle control related genes and chromatin assemble factors, anther-specific proline-rich protein (APG) genes, DNA replication related genes and so on. The result gives informative clues in functional characterization of these OsSET genes.
OsSET Proteins May Be Involved in Cell Cycle Regulating by Histone Modification
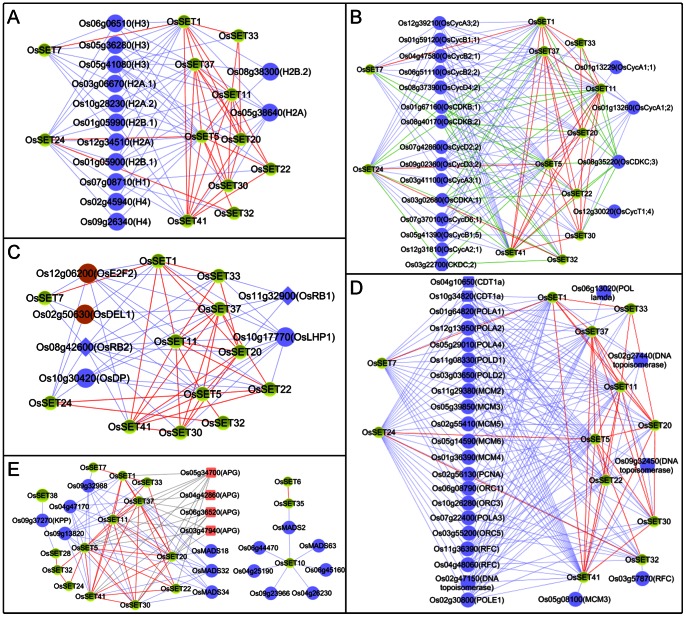
The visualized figure in Figure 10A shows the close relationships between 12 OsSET genes and 13 histone protein genes (containing H1, H2A, H2B.1, H2B.2, H3 and H4). In this network, OsSET5 is co-expressed with all of these histone genes, OsSET24 is co-expressed with 11 histone genes, OsSET37 has 9 co-expressed histone genes, OsSET7 has 7 ones, both OsSET11 and OsSET44 have 5 ones, while others has 1 or 2 co-expressed histone genes. OsSET5 and other five genes (OsSET20, OsSET22, OsSET30, OsSET32 and OsSET41) belong to class VB. OsSET24/OsCLF is a class I member. OsSET37 and other two genes (OsSET1, OsSET33) belong to class III. OsSET7 is a class IV gene. OsSET11 is a class VA gene. This co-expression network indicated that OsSET protein may not only modify histone lysine, but also be involved in multiple histone site modification directly or indirectly. Because histone proteins are essential for the packaging of newly synthesized DNA into chromosomes [70], we speculated that OsSET proteins may be relevant to cell cycle regulation.
Figure 10. Co-expression network of OsSET genes.
A. The co-expression relationship among OsSET genes and histone genes (H2A, H2B.1, H2B.2, H3 and H4). B. Network of OsSET genes and cell division related genes. C. Co-expression network of OsSET and RB-E2F/DB pathway genes. D. A co-expression network regarding the OsSET genes and DNA replication factors. E. A co-expression network concerning OsSET genes and genes involved in flower development.
Subsequently, we found that the 12 OsSET genes in Figure 9A were co-expressed with 19 cell cycle related genes, simultaneously ( Figure 10B ). These cell cycle related genes include 14 cyclin genes (Cycs, A-, B- and D- Type), and 5 cyclin-dependent kinase genes (CDKs, A-, B- and C-Type). It is noted that OsSET5, OsSET24 and OsSET37, which are co-expressed with most of histone genes, are also tightly co-expressed with various kinds of Cycs and CDKs. While OsSET22 and OsSET32 are co-expressed with A- and D-type Cycs, the OsSET33 is only co-expressed with D-type Cycs (OsCycD2;2 and OsCycD3;2). OsSET20 and OsSET33 are co-expressed with OsCDKB3;2 and OsCDKC;3. OsSET7 is co-expressed with OsCDKB;1 and OsCDKB;2. OsSET1 and OsSET32 are co-expressed with CDKC;3.
Usually, Cycs and CDKs act as complex at a precise time and drive the cell cycle progression by phosphorylating downstream target proteins. Cell cycle progression is critical for the maintenance of epigenetic marks and for allowing the daughter products to acquire a distinct epigenetic landscape [71]. Thus, the relevance might exist between SET, histone and cell cycle related protein. In human, it has been confirmed that Retinoblastoma (RB) can target H3K9 methylation to cyclin E promoter by SET-domain protein, Suv39H1, resulting in heterochromatin protein 1 (HP1) binding and silencing [72]. In higher eukaryotes, cell cycle is mainly controlled by E2F transcription factors, which acts through a conserved RB-E2F/DP pathway (DP, related to the E2F family that can dimerize with E2F members)[64], [73]–[75].
In Arabidopsis, CDKAs/CycDs complex can activate the expression of E2F/DP targets by phosphorylating RB and further releasing RB from its cooperator E2F/DP complex [76]–[78]. Genome-wide identification and expression analysis have found conserved E2F, RB, Cycs and CDKs in rice [79]–[81]. In our performances, two homologies of E2F (OsDEL1 and OsE2F2) and two RB homologies (OsRB1 and OsRB2) were found to be co-expressed with these 12 OsSET genes ( Figure 10C ). In consequence, these 12 OsSET proteins may be required for the RB-E2F/DP pathway during cell cycle progression. Although there is no direct evidence showing the connection between SET proteins and RB in vivo in plant, the CLF of Arabidopsis can bind the RB proteins both in maize and human [82], Shen et al proposed that HP1-HKMT-RB-E2F/DP complexes could repress E2F targets in plants [73]. In co-expression network of this study, the rice PcG gene OsSET24/OsCLF, a homology of Arabidopsis CLF, is co-expresses with OsE2F2, OsDEL1, OsRB2 and OsDP tightly. Thus, it is possible that a similar RB-PRC2 complex functions in the cell cycle regulation of rice. OsSETs are co-expressed with histone and cell cycle related genes simultaneously, implying that more HKMTs may be associated with the regulation of cyclins via histone modifications. Therefore, our co-expression results might provide light in the relationship between these OsSET proteins and RB-E2F/DB complex.
OsSETs May Function in Reproductive Development of Rice
Floral organ identity in plants are controlled by combinations of activities mediated by MADS box genes, some of which were identified in our co-expression analysis ( Figure 10E ). OsMADS18 is co-expressed with OsSET20 and OsSET37. OsMADS32 is co-expressed with OsSET1, OsSET5, OsSET11, OsSET20, OsSET30 and OsSET41. OsMADS34 is a member in SEPALLATA (SEP) subfamily [83], [84], which is co-expressed with OsSET5, OsSET11, OsSET20, OsSET30 and OsSET37. Recent research revealed that OsMADS18, one of APETALA1 (AP1)/FRUITFULL (FUL)-like genes, was induced in the shoot apical meristem (SAM) during meristem phase transition, which acted co-ordinately in the meristem to specify the identity of the inflorescence meristem (IM) downstream of the florigen signal [85]. The expression domains of OsMADS32 are mainly restricted to the marginal region of the palea and inner floral organs, showing its contribution on floral organ identity in rice [86]. OsMADS34 plays a role in the early development of spikelet formation [84]. In the above co-expression network, OsSET20 is co-expressed with three MADS box genes, while OsSET1 is co-expressed with one MADS box gene. Besides, another five SET genes, OsSET5, OsSET11, OsSET20, OsSET30 and OsSET37, are co-expressed with two MADS box genes. Therefore, it is possible that these OsSETs may take part in flowering transition and early floral development in rice.
In the co-expression network ( Figure 10E ), 8 OsSET genes are correlated with 4 APG-like protein genes, while OsSET5 and OsSET37 are co-expressed with four APG genes. Noticeably, the APG gene Os05g34700 is co-expressed with 8 OsSET genes. The transcripts of these 8 OsSET genes accumulate in young panicles. It was reported that APG transcript was confined to anther during microspore development in Brassica. napus flower buds [87]. It was also suggested that five APG genes in Silene latifolia were related to anther fertility, which were required for development of fertile pollen [88]. Hence, we might infer that these OsSET genes are involved in rice reproductive development though the regulation of OsAPGs during microsporogenesis stage.
Except for the above 13 OsSET genes, the other genes were also characterized by co-expression analysis. For example, OsSET6, OsSET10, OsSET16 and OsSET35 have a high expression level in stamen, and their co-expression genes include transcription factor, binding protein, pollen allergen and so on ( Figure 5 and Figure 10E ). Among them, OsSET6, OsSET10 and OsSET16 are co-expressed with a cyclin gene. Meanwhile, OsSET10 is co-expressed with five pollen allergen genes and 2 MADS-box genes (OsMADS2 and OsMADS63). OsSET35 is co-expressed with OsMADS2. The previous report showed that OsMADS2 transcript was first observed in the region where stamen primordia were formed, and then appeared in the lodicule primordia as well as the stamen primordia [89], [90]. OsMADS63 is the homolog of Arabidopsis AGL66, which encodes a MIKC*-type DNA binding factor as heterodimer affecting pollen viability, germination, and pollen tube growth [91]. Our analysis suggests that these four OsSET members might affect on the development of male gametophyte.
Conclusions
In conclusion, 43 OsSET genes can be classified into five classes as supported by phylogeny and conserved domains organization. Phylogenetic and structural analysis indicated that the domains beyond SET domain were significant for their specific functions. The expression analysis revealed that OsSET genes might participate in various molecular pathways both in vegetative and reproductive development. GO enrichment analysis showed that the above OsSET genes and their co-expressed genes seemed to particularly affect the same or similar GO categories. Promoter cis-elements identification and the combined analysis of expression correlation suggested that most of OsSET genes might be cell cycle regulated and were associated in the cell cycle progression by histone modifications via E2F. Moreover, we found that some MADS-box and APG proteins may be associated with OsSET on the regulation of cell differentiation and reproductive redevelopment in rice.
Although the studies of plant SET genes have received much progress, only a minority of OsSET genes has been verified in rice. The challenges still exist for the large number of genes in this family. It is a time-consuming process to molecular characterizes the functions and mechanisms of all OsSET genes in traditional approach. Thus our studies would provide valuable data for inferring the putative functions and pathways of the OsSET genes.
Materials and Methods
Identification of OsSET Members in Rice
Hidden Markov Model (HMM) profile of SET domain (PF00856) downloaded from Pfam (http://pfam.sanger.ac.uk/) were employed to identify the putative OsSET genes in rice (Oryza. sativa) [92]. The BlastP search was carried out using the HMM profile on website of MSU RGAP (http://rice.plantbiology.msu.edu/) and KOME (http://cdna01.dna.affrc.go.jp/cDNA/), followed by removal of redundant sequences from the two databases. Meanwhile, the keyword “SET” was also performed in these databases. Additionally, the Pfam and SMART database (http://smart.embl-heidelberg.de/smart/batch.pl ) were used to confirm and make classification of each predicted SET protein.
Chromosomal Localization and Gene Duplication
OsSET genes were mapped on rice chromosomes according to their positions available in MSU RGAP. The distribution of OsSET genes was drawn by MapInspect (http://www.plantbreeding.wur.nl/UK/software_mapinspect.html ). The duplicated genes were elucidated from the segmental genome duplication of rice (http://rice.plantbiology.msu.edu/segmental dup/500 kb/segdup 500 kb.shtml ), with the maximal length distance permitted between collinear gene pairs of 500 kb [93]. Tandem duplicates were defined as genes separated by five or fewer genes. The distances between these genes on the chromosomes were calculated and the percentage of sequence similarities between the proteins encoded by these genes were determined by MegAlign software 4.0 (MEGA4) [94].
Phylogenetic Analysis of OsSET Family
The protein sequences of OsSET family and Arabidopsis SET domain group (SDG) were aligned using ClustalX (version 2.0) program. An un-rooted neighbor-joining [93] phylogenetic tree was constructed in ClustalX based on the full sequences of the proteins with default parameters from rice and Arabidopsis. Bootstrap analysis was performed using 1,000 replicates. The phylogenetic tree thus obtained was viewed using MEGA 4 software.
Structural and Sequence Analysis of OsSET Genes
Information in gene structures, transcripts, full-length cDNA, BAC accessions for each gene and characteristics of corresponding proteins were procured from MSU RGAP, KOME and GRAMENE. Protein sequences of putative OsSET members collected from the MSU RGAP and KOME were analyzed by EXPASY PROTOPARAM tool (http://www.expasy.org/tools/protparam.html ). Information in the number of amino acids, molecular weight, theoretical isoelectric point (pI), amino acid composition, and instability index (instability index of >40 was considered as unstable) were obtained [95]. The conserved domains of the OsSET protein in rice were determined by PFam program.
Protein sequences were analyzed in the MEME program (http://meme.sdsc.edu/meme/cgi-bin/meme.cgi ) to confirm the conserved motifs. The MEME program was employed using the following parameters: number of repetitions-any, maximum number of motifs-200, optimum motif width set to >2 and <200.
Promoter sequences (−2000 bps) of OsSET family genes were obtained from the Rice Annotation Project (RAP) database (http://rapdb.dna.affrc.go.jp/tools/dump ). The cis-elements of promoters were identified using the PLACE Web Signal ScanPLACE (http://www.dna.affrc.go.jp/PLACE/signalup.html ) [96], [97].
Genome-wide Expression analysis of OsSET Family
Expression profile data of OsSET gene family in 24 tissues for Minghui 63 were extracted from CREP database (http://crep.ncpgr.cn, Microarray data sets: GSE19024) [98]. Expression values of each gene were logarithm in Microsoft excel 2007 and cluster analyses were performed using J-express 2011 with euclidean distances and hierarchical cluster method of “complete linkage”. The average signal value of biological replicates for each sample was used for analysis. When more than one probeset was available for one gene, the higher signal value of the probesets was used for analysis. Expression level in each of the tissues was compared against the expression in seed using a student-t test. The genes up- or down-regulated by more than two-fold and with p values <0.05 were considered to be differentially expressed. The average expression of more than two biological replicates for each sample was used for analysis.
Identification of Correlated Genes and Network Construction
The co-expression data were downloaded from the CREP database. The standard deviations for the expression level of each OsSET gene in 24 tissues were calculated. First, we ranked the genes according to the correlation coefficients and screen ones that were greater than 0.75 positively correlated with OsSET gene expression. Then the Pearson correlation coefficient (PCC) and the student-t test of candidate genes that we interested in were recalculated with R project (version 2. 14.1). As the permutation test done by Ouyang et al., PCC ≥0.7 were significant (α = 0.05), We mapped the correlated genes (at a more strict level, PCC ≥0.75, p value ≤0.05) to the network with Cytoscape v2.8.1 [69], [99]. GO enrichment was performed by Singular Enrichment Analysis (SEA) tool in agriGO (http://bioinfo.cau.edu.cn/agriGO/index.php ) with default parameters using the rice MSU6.1 genome annotation as background [100]. Statistical significance was determined using Fisher’s exact test and Yekutieli multi-test adjustment.
Real-time PCR Analysis of Representative Genes in OsSET Family
Primers designed for the RT-PCR analysis were listed in Table S6. Samples were ground in liquid nitrogen using a mortar and pestle. Total RNA (4 µg) was isolated using a RNAiso (Takara) and treated with RNase-free DNase I (Takara) for 15 min to eliminate possible contaminating DNA. First strand cDNA was then reverse transcribed from total RNA with an oligo(dT)18 primer in a 20 µl reaction (diluted to 40 µl before use) using an M-MLV Reverse Transcriptase (Promega) according to the manufacturer’s instructions. Real-time quantitative PCR was carried out on ABI StepOneTM Real-time PCR instrument (Applied Biosystems), containing 5 µl of 2× SYBR Premix EX Taq (Takara), 0.5 µl of Rox Reference Dye II (Takara), 0.5 µl of the cDNA sample, 2 µM of each gene-specific primer, in a final volume of 10 µl. The reactions were carried out according to the following temperature profile: 95°C for 30 seconds, 40 cycles of 95°C for 5 seconds, and 60°C for 34 seconds.
Plant Materials and Growth Conditions
A japonica rice variety of Zhonghua11 was used in this study. Plants were grown at long day under natural light.
Supporting Information
A list of 43 OsSET genes identified in rice and their sequences and protein characteristics.
(XLS)
OsSET genes that localized on duplicated segments of the rice genome.
(XLS)
Cis-elements analysis of OsSET gene promoters.
(XLS)
Average signal values in 24 samples of 40 OsSET genes in Minghui 63.
(XLS)
Table S5a. Results of differential expression analysis using seed as reference (Minghui63); Table S5b. Results of differential expression analysis in 7 d-old seedlings subjected to three phytohormone (NAA, GA3 and KT) treatments and plumule or radicle with light/dark regulation in Minghui63. Differential expression genes have been taken p value less than 0.05 and fold change >2 or <0.5. When fold change >2, regulation is up, and when fold change <0.5, regulation is down.
(XLS)
A list of co-expression genes mentioned in network construction with p value less than 0.05.
(XLS)
Primers used in the research.
(XLS)
Acknowledgments
We would like to thank Prof. Chungen Hu for helpful advice.
Funding Statement
This research was supported by grants from the National Natural Science Foundation of China (http://www.nsfc.gov.cn/e_nsfc/desktop/zn/0101.htm; grant number 30971551), the Fundamental Research Funds for the Central Universities (http://www.dost.moe.edu.cn/; grant number 2012MBDX012), and the Specialized Research Fund for the Doctoral Program of Higher Education (http://www.cutech.edu.cn/cn/index.htm; grant number 20100146120034). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Baumbusch LO, Thorstensen T, Krauss V, Fischer A, Naumann K, et al. (2001) The Arabidopsis thaliana genome contains at least 29 active genes encoding SET domain proteins that can be assigned to four evolutionarily conserved classes. Nucleic Acids Res 29: 4319–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gendler K, Paulsen T, Napoli C (2008) ChromDB: the chromatin database. Nucleic Acids Res 36: 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jenuwein T, Laible G, Dorn R, Reuter G (1998) SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol Life Sci 54: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marmorstein R (2003) Structure of SET domain proteins: a new twist on histone methylation. Trends Biochem Sci 28: 59–62. [DOI] [PubMed] [Google Scholar]
- 5. Schneider R, Bannister AJ, Kouzarides T (2002) Unsafe SETs: histone lysine methyltransferases and cancer. Trends Biochem Sci 27: 396–402. [DOI] [PubMed] [Google Scholar]
- 6. Yu Y, Bu ZY, Shen WH, Dong AW (2009) An update on histone lysine methylation in plants. Prog Nat Sci 19: 407–413. [Google Scholar]
- 7. Trievel RC, Flynn EM, Houtz RL, Hurley JH (2003) Mechanism of multiple lysine methylation by the SET domain enzyme Rubisco LSMT. Nat Struct Biol 10: 545–552. [DOI] [PubMed] [Google Scholar]
- 8. Hu P, Wang S, Zhang Y (2008) How do SET-domain protein lysine methyltransferases achieve the methylation state specificity? Revisited by ab initio QM/MM molecular dynamics simulations. J Am Chem Soc 130: 3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Springer NM, Napoli CA, Selinger DA, Pandey R, Cone KC, et al. (2003) Comparative analysis of SET domain proteins in maize and Arabidopsis reveals multiple duplications preceding the divergence of monocots and dicots. Plant Physiol 132: 907–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng DW, Wang T, Chandrasekharan MB, Aramayo R, Kertbundit S, et al. (2007) Plant SET domain-containing proteins: structure, function and regulation. Biochim Biophys Acta 1769: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pontvianne F, Blevins T, Pikaard CS (2010) Arabidopsis Histone Lysine Methyltransferases. Advances in botanical research. London: Academic Press Ltd-Elsevier Science Ltd. 1–22. [DOI] [PMC free article] [PubMed]
- 12. Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, et al. (1997) A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386: 44–51. [DOI] [PubMed] [Google Scholar]
- 13. Kim GT, Tsukaya H, Uchimiya H (1998) The CURLY LEAF gene controls both division and elongation of cells during the expansion of the leaf blade in Arabidopsis thaliana. Planta 206: 175–183. [DOI] [PubMed] [Google Scholar]
- 14. Köhler C, Hennig L (2010) Regulation of cell identity by plant Polycomb and trithorax group proteins. Curr Opin Genet Dev 20: 541–547. [DOI] [PubMed] [Google Scholar]
- 15. Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, et al. (2006) Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. The EMBO journal 25: 4638–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang D, Wang Y, He Y (2008) Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis Polycomb repressive complex 2 components. PLoS One 3: e3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schatlowski N, Stahl Y, Hohenstatt ML, Goodrich J, Schubert D (2010) The CURLY LEAF Interacting Protein BLISTER Controls Expression of Polycomb-Group Target Genes and Cellular Differentiation of Arabidopsis thaliana. Plant Cell 22: 2291–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu L, Shen WH (2008) Polycomb Silencing of KNOX Genes Confines Shoot Stem Cell Niches in Arabidopsis. Curr Biol 18: 1966–1971. [DOI] [PubMed] [Google Scholar]
- 19.Grossniklaus U, Jean-Philippe Vielle-Calzada, Marilu A. Hoeppner WBG (1998) Maternal Control of Embryogenesis by MEDEA, a Polycomb Group Gene in Arabidopsis. Science: 446–450. [DOI] [PubMed]
- 20. Baroux U, Gagliardini V, Page DR, Grossniklaus U (2006) Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20: 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL (1999) Imprinting of the MEDEA Polycomb Gene in the Arabidopsis Endosperm. Plant Cell 11: 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Köhler C, Page DR, Gagliardini V, Grossniklaus U (2005) The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37: 28–30. [DOI] [PubMed] [Google Scholar]
- 23. Erilova A, Brownfield L, Exner V, Rosa M, Twell D, et al. (2009) Imprinting of the Polycomb Group Gene MEDEA Serves as a Ploidy Sensor in Arabidopsis. PLoS Genet 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pontvianne F, Blevins T, Chandrasekhara C, Feng W, Stroud H, et al. (2012) Histone methyltransferases regulating rRNA gene dose and dosage control in Arabidopsis. Genes Dev 26: 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson JP, Johnson L, Jasencakova Z, Zhang X, PerezBurgos L, et al. (2004) Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112: 308–315. [DOI] [PubMed] [Google Scholar]
- 26. Ebbs ML, Bartee L, Bender J (2005) H3 lysine 9 methylation is maintained on a transcribed inverted repeat by combined action of SUVH6 and SUVH4 methyltransferases. Molecular and Cellular Biology 25: 10507–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ebbs ML, Bender J (2006) Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18: 1166–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alvarez-Venegas R, Pien S, Sadder M, Witmer X, Grossniklaus U, et al. (2003) ATX-1, an Arabidopsis homolog of trithorax, activates flower homeotic genes. Curr Biol 13: 627–637. [DOI] [PubMed] [Google Scholar]
- 29. Saleh A, Al-Abdallat A, Ndamukong I, Alvarez-Venegas R, Avramova Z (2007) The Arabidopsis homologs of trithorax (ATX1) and enhancer of zeste (CLF) establish ‘bivalent chromatin marks’ at the silent AGAMOUS locus. Nucleic Acids Res 35: 6290–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saleh A, Alvarez-Venegas R, Yilmaz M, Oahn L, Hou G, et al. (2008) The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell 20: 568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang YK, Wang Y, Zhang Y, Li SG, Lu XC, et al. (2003) OsSET1, a novel SET-domain-containing gene from rice. J Exp Bot 54: 1995–1996. [DOI] [PubMed] [Google Scholar]
- 32. Luo M, Platten D, Chaudhury A, Peacock WJ, Dennis ES (2009) Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Molecular Plant 2: 711–723. [DOI] [PubMed] [Google Scholar]
- 33. Thakur JK, Malik MR, Bhatt V, Reddy MK, Sopory SK, et al. (2003) A POLYCOMB group gene of rice (Oryza sativa L. subspecies indica), OsiEZ1, codes for a nuclear-localized protein expressed preferentially in young seedlings and during reproductive development. Gene 314: 1–13. [DOI] [PubMed] [Google Scholar]
- 34. Qin F-J, Sun Q-W, Huang L-M, Chen X-S, Zhou D-X (2010) Rice SUVH Histone Methyltransferase Genes Display Specific Functions in Chromatin Modification and Retrotransposon Repression. Mol Plant 3: 1674–2052. [DOI] [PubMed] [Google Scholar]
- 35. Ding Y, Wang X, Su L, Zhai JX, Cao SY, et al. (2007) SDG714, a histone H3K9 methyltransferase, is involved in Tos17 DNA methylation and transposition in rice. Plant Cell 19: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun C, Fang J, Zhao T, Xu B, Zhang F, et al. (2012) The Histone Methyltransferase SDG724 Mediates H3K36me2/3 Deposition at MADS50 and RFT1 and Promotes Flowering in Rice. Plant Cell 24: 3235–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC plant biology 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horton JR, Elgar SJ, Khan SI, Zhang X, Wade PA, et al. (2007) Structure of the SANT domain from the Xenopus chromatin remodeling factor ISWI. Proteins 67: 1198–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boyer LA, Latek RR, Peterson CL (2004) The SANT domain: a unique histone-tail-binding module? Nature reviews Molecular cell biology 5: 158–163. [DOI] [PubMed] [Google Scholar]
- 40. Valencia-Morales Mdel P, Camas-Reyes JA, Cabrera-Ponce JL, Alvarez-Venegas R (2012) The Arabidopsis thaliana SET-domain-containing protein ASHH1/SDG26 interacts with itself and with distinct histone lysine methyltransferases. J Plant Res 125: 679–692. [DOI] [PubMed] [Google Scholar]
- 41.Grini PE, Thorstensen T, Alm V, Vizcay-Barrena G, Windju SS, et al.. (2009) The ASH1 HOMOLOG 2 (ASHH2) Histone H3 Methyltransferase Is Required for Ovule and Anther Development in Arabidopsis. PLoS One 4. [DOI] [PMC free article] [PubMed]
- 42. Thorstensen T, Grini PE, Mercy IS, Alm V, Erdal S, et al. (2008) The Arabidopsis SET-domain protein ASHR3 is involved in stamen development and interacts with the bHLH transcription factor ABORTED MICROSPORES (AMS). Plant Mol Biol 66: 47–59. [DOI] [PubMed] [Google Scholar]
- 43. Lee JS, Shilatifard A (2007) A site to remember: H3K36 methylation a mark for histone deacetylation. Mutat Res 618: 130–134. [DOI] [PubMed] [Google Scholar]
- 44. Avramova Z (2009) Evolution and pleiotropy of TRITHORAX function in Arabidopsis. Int J Dev Biol 53: 371–381. [DOI] [PubMed] [Google Scholar]
- 45. Stec I, Nagl SB, van Ommen GJ, den Dunnen JT (2000) The PWWP domain: a potential protein-protein interaction domain in nuclear proteins influencing differentiation? FEBS Lett 473: 1–5. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Reddy B, Thompson J, Wang H, Noma K, et al. (2009) Regulation of Set9-mediated H4K20 methylation by a PWWP domain protein. Mol Cell 33: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu H, Zeng H, Lam R, Tempel W, Amaya MF, et al. (2011) Structural and histone binding ability characterizations of human PWWP domains. PLoS One 6: e18919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schuettengruber B, Martinez AM, Iovino N, Cavalli G (2011) Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol 12: 799–814. [DOI] [PubMed] [Google Scholar]
- 49. Papp B, Muller J (2006) Histone trimethylation and the maintenance of transcriptional ON and OFF states by trxG and PcG proteins. Genes Dev 20: 2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aasland R, Gibson TJ, Stewart AF (1995) The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci 20: 56–59. [DOI] [PubMed] [Google Scholar]
- 51. Pena PV, Davrazou F, Shi X, Walter KL, Verkhusha VV, et al. (2006) Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Ingen H, van Schaik FM, Wienk H, Ballering J, Rehmann H, et al. (2008) Structural insight into the recognition of the H3K4me3 mark by the TFIID subunit TAF3. Structure 16: 1245–1256. [DOI] [PubMed] [Google Scholar]
- 53. Spiliotopoulos D, Spitaleri A, Musco G (2012) Exploring PHD Fingers and H3K4me0 Interactions with Molecular Dynamics Simulations and Binding Free Energy Calculations: AIRE-PHD1, a Comparative Study. PLoS One 7: e46902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Raynaud C, Sozzani R, Glab N, Domenichini S, Perennes C, et al. (2006) Two cell-cycle regulated SET-domain proteins interact with proliferating cell nuclear antigen (PCNA) in Arabidopsis. Plant J 47: 395–407. [DOI] [PubMed] [Google Scholar]
- 55. Veiseth SV, Rahman MA, Yap KL, Fischer A, Egge-Jacobsen W, et al. (2011) The SUVR4 histone lysine methyltransferase binds ubiquitin and converts H3K9me1 to H3K9me3 on transposon chromatin in Arabidopsis. PLoS Genet 7: e1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, et al. (2007) The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 450: 908–912. [DOI] [PubMed] [Google Scholar]
- 57. Unoki M, Nishidate T, Nakamura Y (2004) ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG through its SRA domain. Oncogene 23: 7601–7610. [DOI] [PubMed] [Google Scholar]
- 58. Achour M, Jacq X, Ronde P, Alhosin M, Charlot C, et al. (2008) The interaction of the SRA domain of ICBP90 with a novel domain of DNMT1 is involved in the regulation of VEGF gene expression. Oncogene 27: 2187–2197. [DOI] [PubMed] [Google Scholar]
- 59. Johnson LM, Bostick M, Zhang X, Kraft E, Henderson I, et al. (2007) The SRA methyl-cytosine-binding domain links DNA and histone methylation. Current biology : CB 17: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ding B, Zhu Y, Gao J, Yu Y, Cao KM, et al. (2007) Molecular characterization of three rice SET-domain proteins. Plant Sci 172: 1072–1078. [Google Scholar]
- 61. Caro E, Stroud H, Greenberg MV, Bernatavichute YV, Feng S, et al. (2012) The SET-Domain Protein SUVR5 Mediates H3K9me2 Deposition and Silencing at Stimulus Response Genes in a DNA Methylation-Independent Manner. PLoS Genet 8: e1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Helin K (1998) Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev 8: 28–35. [DOI] [PubMed] [Google Scholar]
- 63. Vandepoele K, Vlieghe K, Florquin K, Hennig L, Beemster GT, et al. (2005) Genome-wide identification of potential plant E2F target genes. Plant Physiol 139: 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kosugi S, Ohashi Y (2002) E2F sites that can interact with E2F proteins cloned from rice are required for meristematic tissue-specific expression of rice and tobacco proliferating cell nuclear antigen promoters. The Plant journal : for cell and molecular biology 29: 45–59. [DOI] [PubMed] [Google Scholar]
- 65. de Jager SM, Menges M, Bauer UM, Murra JA (2001) Arabidopsis E2F1 binds a sequence present in the promoter of S-phase-regulated gene AtCDC6 and is a member of a multigene family with differential activities. Plant Mol Biol 47: 555–568. [DOI] [PubMed] [Google Scholar]
- 66. Stuart JM, Segal E, Koller D, Kim SK (2003) A gene-coexpression network for global discovery of conserved genetic modules. Science 302: 249–255. [DOI] [PubMed] [Google Scholar]
- 67. Fu F-F, Xue H-W (2010) Coexpression Analysis Identifies Rice Starch Regulator1, a Rice AP2/EREBP Family Transcription Factor, as a Novel Rice Starch Biosynthesis Regulator. Plant Physiol 154: 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aoki K, Ogata Y, Shibata D (2007) Approaches for extracting practical information from gene co-expression networks in plant biology. Plant Cell Physiol 48: 381–390. [DOI] [PubMed] [Google Scholar]
- 69. Ouyang Y, Huang X, Lu Z, Yao J (2012) Genomic survey, expression profile and co-expression network analysis of OsWD40 family in rice. BMC genomics 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rattray AMJ, Mueller B (2012) The control of histone gene expression. Biochem Soc Trans 40: 880–885. [DOI] [PubMed] [Google Scholar]
- 71. Sanchez MdlP, Caro E, Desvoyes B, Ramirez-Parra E, Gutierrez C (2008) Chromatin dynamics during the plant cell cycle. Seminars in cell & developmental biology 19: 537–546. [DOI] [PubMed] [Google Scholar]
- 72. Nielsen SJ, Schneider R, Bauer UM, Bannister AJ, Morrison A, et al. (2001) Rb targets histone H3 methylation and HP1 to promoters. Nature 412: 561–565. [DOI] [PubMed] [Google Scholar]
- 73. Shen WH (2002) The plant E2F-Rb pathway and epigenetic control. Trends Plant Sci 7: 505–511. [DOI] [PubMed] [Google Scholar]
- 74. van den Heuvel S, Dyson NJ (2008) Conserved functions of the pRB and E2F families. Nature reviews Molecular cell biology 9: 713–724. [DOI] [PubMed] [Google Scholar]
- 75. Dimova DK, Stevaux O, Frolov MV, Dyson NJ (2003) Cell cycle-dependent and cell cycle-independent control of transcription by the Drosophila E2F/RB pathway. Genes Dev 17: 2308–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qi R, John PC (2007) Expression of genomic AtCYCD2;1 in Arabidopsis induces cell division at smaller cell sizes: implications for the control of plant growth. Plant Physiol 144: 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao XA, Harashima H, Dissmeyer N, Pusch S, Weimer AK, et al.. (2012) A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant Arabidopsis thaliana. PLoS Genet 8. [DOI] [PMC free article] [PubMed]
- 78. Nowack MK, Harashima H, Dissmeyer N, Zhao XA, Bouyer D, et al. (2012) Genetic Framework of Cyclin-Dependent Kinase Function in Arabidopsis. Developmental Cell 22: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 79. Guo J, Song J, Wang F, Zhang XS (2007) Genome-wide identification and expression analysis of rice cell cycle genes. Plant Mol Biol 64: 349–360. [DOI] [PubMed] [Google Scholar]
- 80. La H, Li J, Ji Z, Cheng Y, Li X, et al. (2006) Genome-wide analysis of cyclin family in rice (Oryza Sativa L.). Molecular genetics and genomics : MGG 275: 374–386. [DOI] [PubMed] [Google Scholar]
- 81. Shultz RW, Tatineni VM, Hanley-Bowdoin L, Thompson WF (2007) Genome-wide analysis of the core DNA replication machinery in the higher plants Arabidopsis and rice. Plant Physiol 144: 1697–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Williams L, Grafi G (2000) The retinoblastoma protein - a bridge to heterochromatin. Trends Plant Sci 5: 239–240. [DOI] [PubMed] [Google Scholar]
- 83. Gao X, Liang W, Yin C, Ji S, Wang H, et al. (2010) The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiol 153: 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kobayashi K, Maekawa M, Miyao A, Hirochika H, Kyozuka J (2010) PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant & cell physiology 51: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kobayashi K, Yasuno N, Sato Y, Yoda M, Yamazaki R, et al. (2012) Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 24: 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sang X, Li Y, Luo Z, Ren D, Fang L, et al. (2012) CHIMERIC FLORAL ORGANS1, encoding a monocot-specific MADS box protein, regulates floral organ identity in rice. Plant Physiol 160: 788–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roberts MR, Foster GD, Blundell RP, Robinson SW, Kumar A, et al. (1993) Gametophytic and sporophytic expression of an anther-specific Arabidopsis thaliana gene. Plant J 3: 111–120. [PubMed] [Google Scholar]
- 88. Ageez A, Kazama Y, Sugiyama R, Kawano S (2005) Male-fertility genes expressed in male flower buds of Silene latifolia include homologs of anther-specific genes. Genes Genet Syst 80: 403–413. [DOI] [PubMed] [Google Scholar]
- 89. Yao SG, Ohmori S, Kimizu M, Yoshida H (2008) Unequal genetic redundancy of rice PISTILLATA orthologs, OsMADS2 and OsMADS4, in lodicule and stamen development. Plant & cell physiology 49: 853–857. [DOI] [PubMed] [Google Scholar]
- 90. Kyozuka J, Kobayashi T, Morita M, Shimamoto K (2000) Spatially and temporally regulated expression of rice MADS box genes with similarity to Arabidopsis class A, B and C genes. Plant & cell physiology 41: 710–718. [DOI] [PubMed] [Google Scholar]
- 91. Adamczyk BJ, Fernandez DE (2009) MIKC* MADS domain heterodimers are required for pollen maturation and tube growth in Arabidopsis. Plant Physiol 149: 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ouyang Y, Chen J, Xie W, Wang L, Zhang Q (2009) Comprehensive sequence and expression profile analysis of Hsp20 gene family in rice. Plant Mol Biol 70: 341–357. [DOI] [PubMed] [Google Scholar]
- 93. Haas BJ, Delcher AL, Wortman JR, Salzberg SL (2004) DAGchainer: a tool for mining segmental genome duplications and synteny. Bioinformatics 20: 3643–3646. [DOI] [PubMed] [Google Scholar]
- 94. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 95. Guruprasad K, Reddy BV, Pandit MW (1990) Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng 4: 155–161. [DOI] [PubMed] [Google Scholar]
- 96. Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prestridge DS (1991) SIGNAL SCAN: a computer program that scans DNA sequences for eukaryotic transcriptional elements. Computer applications in the biosciences : CABIOS 7: 203–206. [DOI] [PubMed] [Google Scholar]
- 98. Wang L, Xie WB, Chen Y, Tang WJ, Yang JY, et al. (2010) A dynamic gene expression atlas covering the entire life cycle of rice. Plant J 61: 752–766. [DOI] [PubMed] [Google Scholar]
- 99. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, et al. (2003) Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of 43 OsSET genes identified in rice and their sequences and protein characteristics.
(XLS)
OsSET genes that localized on duplicated segments of the rice genome.
(XLS)
Cis-elements analysis of OsSET gene promoters.
(XLS)
Average signal values in 24 samples of 40 OsSET genes in Minghui 63.
(XLS)
Table S5a. Results of differential expression analysis using seed as reference (Minghui63); Table S5b. Results of differential expression analysis in 7 d-old seedlings subjected to three phytohormone (NAA, GA3 and KT) treatments and plumule or radicle with light/dark regulation in Minghui63. Differential expression genes have been taken p value less than 0.05 and fold change >2 or <0.5. When fold change >2, regulation is up, and when fold change <0.5, regulation is down.
(XLS)
A list of co-expression genes mentioned in network construction with p value less than 0.05.
(XLS)
Primers used in the research.
(XLS)