Abstract
Aberrant activation of the canonical Wnt signal transduction pathway is involved in many diseases including cancer and is especially implicated in the development and progression of colorectal cancer. The key effector protein of the canonical Wnt pathway is β-catenin, which functions with T-cell factor/lymphoid enhancer factor to activate expression of Wnt target genes. In this study, we used a new functional screen based on cell survival in the presence of cDNAs encoding proteins that activate the Wnt pathway thus identifying novel Wnt signaling components. Here we identify carboxypeptidase E (|CPE) and its splice variant, ΔN-CPE, as novel regulators of the Wnt pathway. We show that whereas ΔN-CPE activates the Wnt signal, the full-length CPE (F-CPE) protein is an inhibitor of Wnt/β-catenin signaling. F-CPE forms a complex with the Wnt3a ligand and the Frizzled receptor. Moreover, F-CPE disrupts disheveled-induced signalosomes that are important for transducing the Wnt signal and reduces β-catenin protein levels and activity. Taken together, our data indicate that F-CPE and ΔN-CPE regulate the canonical Wnt signaling pathway negatively and positively, respectively, and demonstrate that this screening approach can be a rapid means for isolation of novel Wnt signaling components.
Keywords: Wnt signaling, carboxypeptidase E (CPE), β-catenin, functional screen
Introduction
The Wnt/β-catenin pathway is a key developmental pathway controlling both embryonic development and tissue maintenance in adults. However, aberrant constitutive activation of the Wnt pathway leads to uncontrolled cell proliferation, growth and survival, promoting the progression of various types of human cancers, especially the human colorectal cancers (CRCs).1–3 In the absence of a Wnt signal, the levels of β-catenin, the key effector of the Wnt signaling pathway, are kept low by the ‘β-catenin destruction complex’ that contains, among others, the tumor suppressor protein adenomatous polyposis coli and Axin. This complex promotes phosphorylation of β-catenin by casein kinase 1α and glycogen synthase kinase 3β, marking it suitable for ubiquitylation and subsequent proteasomal degradation. In many cancers, mutations in adenomatous polyposis coli, Axin, or β-catenin disrupt the degradation of the latter, leading to uncontrolled activation of Wnt target genes. When the Wnt pathway is activated, the Wnt ligand binds to the Frizzled (Fz) transmembrane receptor and the coreceptor low-density lipoprotein receptor-related 5 or LRP64 leading LRP6 into caveolincontaining vesicles for endocytosis.5 The cytoplasmic tail of LRP6 is then phosphorylated by casein kinase 1α and GSK-3β, which are recruited together with Axin from the ‘β-catenin destruction complex’.6 These events trigger the polymerization of Disheveled (Dvl), promoting the formation of punctate protein clusters that are membrane-associated signalosomes and the disassembly of the ‘β-catenin destruction complex’.7–11 As a result, unphosphorylated β-catenin accumulates in the cytoplasm and translocates into the nucleus where it associates with TCF transcription factors and upregulates the transcription of Wnt target genes. Abnormal expression of Wnt signaling target gene is one of many aberrations that likely contribute to neoplastic transformation. The Wnt cascade is extremely complex, regulated by various other signaling pathways and feedback loops and comprised of numerous components, some of which are yet unknown.
In an attempt to identify new Wnt signaling components we have utilized a novel screening technique. The screen is based on cell survival only in the presence of cDNAs that activate the Wnt pathway. Here we identified carboxypeptidase E (CPE) and its splice variant, ΔN-CPE, as novel Wnt signaling regulators. CPE is a multifunctional protein harboring both enzymatic and nonenzymatic functions, (reviewed in Cawley et al.12) largely expressed in endocrine cells and peptidergic neurons in the nervous system. CPE is primarily known as a prohormone processing enzyme that also functions in prohormone sorting, vesicle transport and secretion.12–14 Different forms of CPE are expressed in different subcellular localizations exhibiting distinct functions: for example, soluble CPE found in secretory granules functions as an exopeptidase that cleaves C-terminal basic amino-acid residues to produce mature neuropeptides and hormones,14 whereas membrane-bound CPE in the trans-Golgi network functions as a sorting receptor.13 Recent studies analyzing CPE mRNA in microarrays suggest that it might be involved in the development or progression of cancer. Reduced CPE activity was seen in neuroblastoma,15 whereas elevated levels of CPE in neuroendocrine tumors are a predictor of good prognosis.16 In glioma cell lines, high CPE levels were associated with increased proliferation rate, whereas CPE knockdown enhanced migration and invasion.17 CPE was also present in non-endocrine-derived cancerous cells where its expression is normally absent, including epithelia-derived cancer cells such as breast,18 cervical and kidney cancers.19 These studies suggest that elevated levels of CPE mRNA correlate with metastatic tumors when compared with normal tissue or benign tumors.19 The apparent contradictory findings as to whether CPE promotes or inhibits metastasis were clarified by the recent discovery of the splice variant, CPE-ΔN (also called ΔN-CPE). Examining the expression of CPE in epithelial-derived tumors, such as hepatocellular carcinoma and colorectal carcinoma, reveal that only the splice variant, and not full-length CPE (F-CPE), is expressed.20 This alternative spliced variant, ΔN-CPE, lacks the signal peptide that normally directs it into the secretory pathway and is expressed in highly metastatic tumors.20 Cytoplasmic ΔN-CPE translocates into the nucleus and increases the expression of the metastatic gene, ‘neural precursor cell expressed developmentally downregulated’ (NEDD 9), in a histone deacetylase 1 and 2 activity-dependent manner. Other metastatic and anti-apoptotic genes are also upregulated leading to ΔN-CPE-induced promotion of tumor metastasis.21
In the present study, we show that whereas ΔN-CPE protein activates the oncogenic Wnt pathway, the F-CPE protein decreases both Wnt signaling and β-catenin levels. Additionally, F-CPE colocalizes with Wnt3a whereas the ΔN-CPE colocalizes in the nucleus with β-catenin. Finally, F-CPE interacts with both the Wnt3a ligand and its receptor Fz, resulting in disruption of the Dvl induced signalosomes. These findings indicate that CPE and ΔN-CPE function as novel regulators of the canonical Wnt signaling pathway.
Results
CPE—a novel Wnt regulator
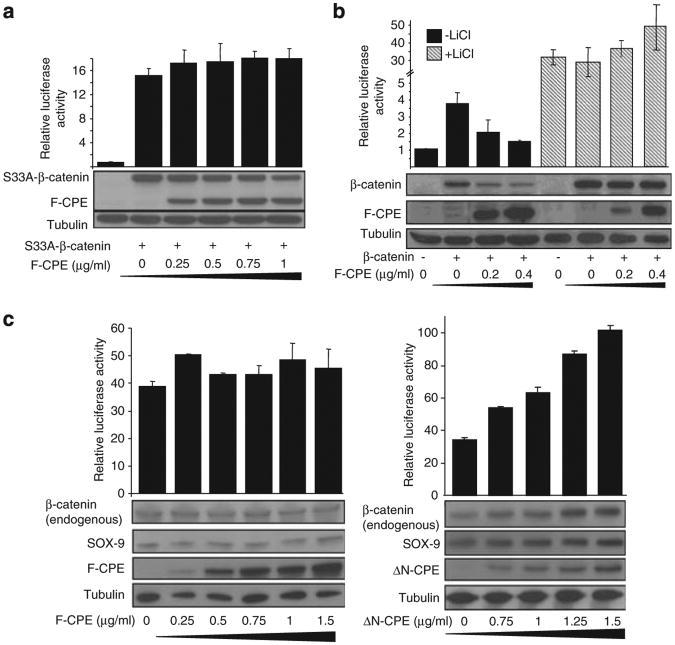
A screen aimed at identification of novel Wnt signaling activators led to the isolation of 104 genes. A secondary screen narrowed down these candidates to 32 putative novel Wnt signaling activators (Supplementary Figures 1 and 2; Supplementary Table 1). One of the genes identified in our screen was CPE. CPE was isolated in three independent screening rounds, exhibiting a relatively strong activation of β-catenin/TCF-dependent gene transcription (Supplementary Figure 2). The CPE cDNA isolated in our screen contained only the C-terminal 140 amino acids (as the cDNA library is an oligo dT library). To investigate the effect of CPE on the Wnt pathway, the F-CPE cDNA and the 365 C-terminus CPE residues (ΔN-CPE) fused to either green fluorescent protein (GFP) or FLAG tag were used. The CPE-encoding plasmids were first tested for their ability to specifically affect the Wnt pathway by measuring the β-catenin/TCF-mediated transcription (using the pTOPFLASH/pFOPFLASH reporter assay). The effect on β-catenin protein levels was also examined by SDS–polyacrylamide gel electrophoresis and western blotting. HEK293T cells were co-transfected with the pTOPFLASH/pFOPFLASH reporter plasmids, GFP-β-catenin and the two CPE constructs. In agreement with our screen results, the ΔN-CPE protein led to increased β-catenin/TCF-mediated transcription and β-catenin protein levels (Figure 1a). Surprisingly, expression of F-CPE resulted in decreased β-catenin/TCF-mediated transcription and reduced β-catenin protein levels (Figure 1a). The ability of F-CPE to reduce β-catenin/TCF-mediated transcription and β-catenin protein levels was re-enforced using a dose-dependent assay (Figure 1b). Similar results were obtained when myc or FLAG-β-catenin were used (not shown). Next, we upregulated the Wnt pathway using ectopic expression of other known Wnt activators: Dvl or the ligand Wnt3a and its receptor Fz1. Again, F-CPE protein reduced the levels of β-catenin/TCF-mediated transcription as well as endogenous β-catenin protein levels (using an antibody that detects the active form of β-catenin)22 (Figure 1c). Specificity of the effect was confirmed by using RNA interference to reduce CPE expression in PC-12 cells that express endogenous F-CPE (Figure 7d), but no detectable ΔN-CPE. As shown in Figure 1d, depletion of CPE led to increased Wnt signaling levels and upregulation of the active form of β-catenin and the Wnt target genes c-myc and SOX-9.
Figure 1.
CPE affects the Wnt signaling pathway. (a) HEK293T cells were co-transfected with the pTOPFLASH/pFOPFLASH reporters and the β-gal plasmid, along with GFP-β-catenin and F-CPE-GFP or ΔN-CPE-GFP as indicated (0.5 μg/ml). Forty-eight hours later the cells were harvested and luciferase levels were determined (upper panels). The same samples were subjected to western blot analysis using an anti-GFP antibody. β-Galactosidase activity was used to normalize transfection efficiency. Tubulin served as a loading control. (b) As in a, showing dose-dependent activity of the F-CPE-GFP construct on β-catenin and Wnt signaling. (c) HEK293T cells were co-transfected with the pTOPFLASH/pFOPFLASH reporters, the β-gal plasmid and F-CPE-GFP, Fz1 + Wnt3a or Dvl as indicated. Forty-eight hours later the cells were harvested and luciferase levels were determined (upper panel). The same samples were subjected to western blot analysis. F-CPE-GFP protein was detected using an anti-GFP antibody, and endogenous active β-catenin was detected using an anti-active β-catenin antibody. Tubulin served as a loading control. (d) Using transient transfection of specific CPE siRNA-encoding oligos, the expression of the endogenous F-CPE protein in PC-12 cells was decreased. Twenty-four hours post siRNA transfection the cells were transfected with pTOPFLASH/pFOPFLASH and β-gal plasmids for luciferase detection. At 48 h post transfection, the cells were harvested and subjected to luciferase assay according to the manufacturer's instructions followed by western blot analysis as described above. The endogenous CPE, c-myc and SOX-9 were detected using specific antibodies. Endogenous active β-catenin was detected using an anti-active β-catenin antibody. Tubulin served as a loading control.
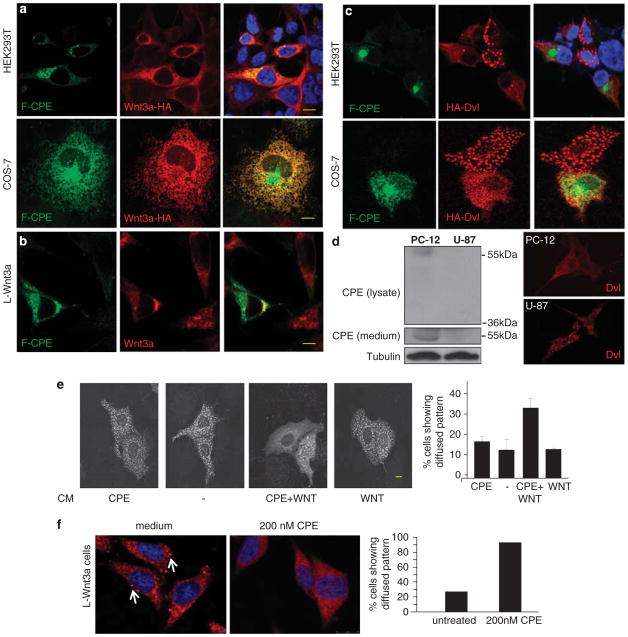
Figure 7.
CPE partly colocalizes with Wnt3a and affects the Dvl protein assemblies. (a) HEK293TorCOS-7 cells were co-transfected with F-CPE-GFP and HA-Wnt3a. Forty-eight hours later the cells were fixed, stained with an anti-HA antibody that was visualized using a rhodamine-conjugated antibody. Images were taken using a confocal microscope. (b) L-Wnt 3a cells were transfected with F-CPE-GFP. Forty-eight hours later the cells were fixed, stained with an anti-Wnt 3a antibody that was visualized using a rhodamine-conjugated antibody. Images were taken using a confocal microscope. (c) HEK293T or COS-7 cells were co-transfected with F-CPE-GFP and HA-Dvl. Forty-eight hours later the cells were fixed, stained with an anti-HA antibody that was visualized using a rhodamine-conjugated antibody. Images were taken using a confocal microscope. (d, left panel) Medium from PC-12 or U-87 cells was collected and cells were harvested and subjected to western blot analysis. Endogenous F-CPE but not ΔN-CPE was detected in PC-12 cells and media, and neither was detected in U-87 cells or media using an anti-CPE antibody. Tubulin served as a loading control. Right panel: PC-12 and U-87 cells were transfected with HA-Dvl and subjected to IF imaging as described. (e) COS-7 cells were transfected with HA-Dvl and incubated with CM as indicated. Representative staining are shown (left). The percentage of cells containing punctate versus diffused pattern of HA-Dvl staining was calculated in a large number of cells (n = 50) (right panel). (f) L-Wnt3a cells were incubated with 200 nm purified F-CPE for 12 h. Representative endogenous Dvl staining (left panels) in L-wnt3a cells with or without CPE treatment (200 nm) is shown. Arrows point to the punctate expression pattern of Dvl. Bar graphs (right) showing the percentage of cells expressing Dvl in a punctate pattern (versus diffused pattern) calculated in a large number of cells (n =80). Bar = 10μm.
A functional β-catenin destruction complex is required for the activity of CPE
To better understand the mechanism by which CPE regulates the Wnt pathway, we tested the effect of F-CPE on a constitutively active β-catenin mutant S33A-β-catenin that cannot be phosphorylated by GSK-3β23 (Figure 2a). Alternatively, we used LiCl that inhibits the function of GSK-3β and activates the Wnt pathway (Figure 2b). Results show that under both of these conditions F-CPE had little effect on either β-catenin/TCF-mediated transcription or β-catenin protein levels. Next we tested the ability of CPE to modulate Wnt signaling independently of the ‘β-catenin destruction complex’. To this end, we used the CRC cell line SW480 that contains a non-functional β-catenin degradation complex.24 The F-CPE had no effect on β-catenin/TCF-mediated transcription, on the endogenous β-catenin levels or the Wnt target gene SOX-9. However, the ΔN-CPE construct increased β-catenin/TCF-mediated transcription in a dose-dependent manner and enhanced the levels of β-catenin and to a lesser extent the Wnt signaling target gene SOX-9 (Figure 2c). Taken together, these results suggest that a functional β-catenin degradation complex is needed for the ability of CPE to affect the Wnt signal.
Figure 2.
A functional β-catenin degradation complex is required for the activity of CPE. (a) HEK293T cells were co-transfected with the pTOPFLASH/pFOPFLASH reporters, the β-gal plasmid, a constitutively active form of GFP-β-catenin (S33A-β-catenin) and different amounts of F-CPE-GFP. Forty-eight hours later the cells were harvested and luciferase levels were determined. The samples were subjected to western blot analysis using an anti-GFP antibody. Tubulin served as a loading control. (b) HEK293T cells were transected with the pTOPFLASH/pFOPFLASH reporters, the β-gal plasmid, GFP-β-catenin (0.3 μg/ml) and different amounts of F-CPE-GFP as indicated. Twenty-four hours later the cells were treated with 30 mm LiCl for 24 h. The samples were harvested and subjected to luciferase assay and western blot analysis using an anti-GFP antibody. Tubulin served as a loading control. (c) SW480 cells were transfected with the pTOPFLASH/pFOPFLASH reporters, renilla luciferase and F-CPE-GFP (left) or ΔN-CPE-GFP (right). Forty-eight hours later the cells were harvested and luciferase levels were determined. Renilla luciferase levels were used to normalize transfection efficiency. The samples were subjected to western blot analysis using an anti-GFP antibody. Levels of endogenous β-catenin and SOX-9 were determined using specific antibodies. Tubulin served as a loading control.
CPE inhibits the Wnt pathway in a proteasome-dependent manner
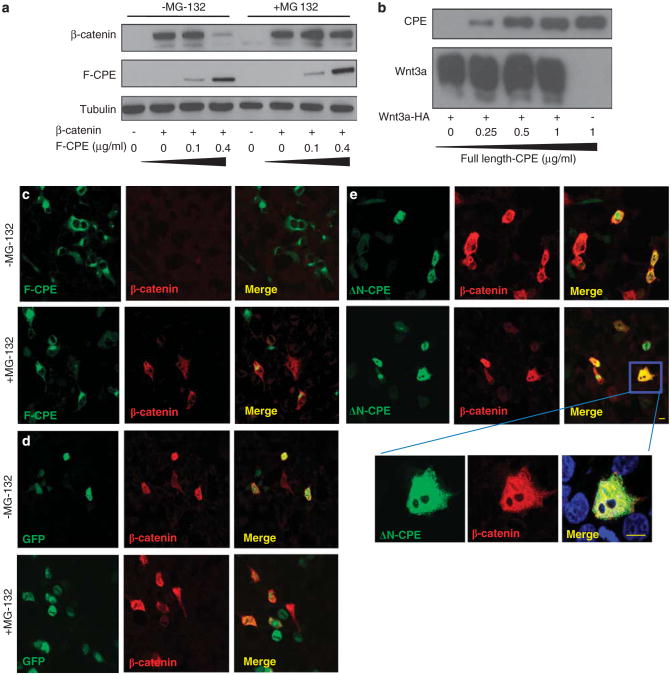
Our results indicate that expression of CPE leads to reduced levels of the β-catenin protein. To test whether the effect of CPE on β-catenin was proteasome dependent, we used the proteasome inhibitor MG132. Western blot analysis clearly shows that the effect of F-CPE on β-catenin protein levels was reversed when the cells were treated with MG132 (Figure 3a). As CPE did not affect Wnt3a levels, (Figure 3b) the decrease in Wnt signaling is not attributed to reduced levels of the Wnt3a protein. Next, myc-β-catenin was coexpressed in HEK293T cells together with the ΔN-CPE or F-CPE constructs, and immunofluorescence (IF) assays were performed. As expected, myc-β-catenin staining was abolished when co-transfected with F-CPE. However, treating the cells with the proteasome inhibitor MG132 reversed this effect (Figure 3c). Intriguingly, overexpression of F-CPE-GFP resulted in depletion of myc-β-catenin in both transfected and untransfected cells, suggesting that secreted CPE affected β-catenin levels in the latters (Figure 3c). In the absence of CPE (GFP control), β-catenin was detected in transfected cells and somewhat stabilized by MG132 as expected (Figure 3d). Coexpressing the ΔN-CPE construct with myc-β-catenin did not diminish β-catenin levels regardless of MG132 treatment (Figure 3e). In the co-transfected cells the two proteins partly colocalized in both the cytoplasm and the nucleus (Figure 3e enlargement). These results indicate that F-CPE induces proteasome-mediated degradation of β-catenin.
Figure 3.
The effect of CPE on the Wnt signal depends on the proteasome activity. (a) HEK293T cells were co-transfected with GFP-β-catenin and F-CPE-GFP as indicated. Twenty-four hours later, 25μm MG132 was added to the cells for an additional 24 h. Cells were harvested and subjected to western blot analysis using an anti-GFP antibody. Tubulin served as a loading control. (b) HEK293T cells were co-transfected with Wnt3a-HA and increasing amounts of F-CPE-GFP as indicated. Western blot analysis was performed using anti-HA and anti-GFP antibodies, respectively. (c, d and e) HEK293T cells were co-transfected with F-CPE-GFP, GFP or the ΔN-CPE-GFP constructs along with myc-β-catenin (as indicated). Twenty-four hours post transfection the cells were treated overnight with 25 μm MG132. The cells were fixed and reacted with an anti-myc antibody and rhodamine-conjugated antibody. Images were taken using a confocal microscope. Note the ΔN-CPE immunostaining in the cytoplasm and the nucleus in some cells. Bar = 10μm.
Secreted F-CPE affects Wnt signaling
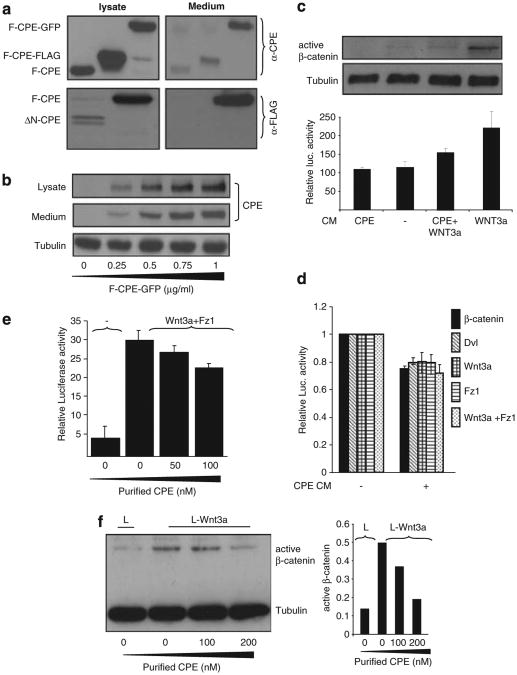
As CPE is a secreted protein,25 we examined whether the different CPE constructs used in this study are secreted from transfected cells. Our results show that F-CPE, either untagged or tagged with a GFP or FLAG epitope, is efficiently secreted into the medium (Figure 4a, top panel). ΔN-CPE that showed relatively lower levels of expression was not secreted (Figure 4a, bottom panel) even when larger amounts of plasmids were used. This is not surprising as the ΔN-CPE protein lacks a signal peptide.20 Figure 4b depicts a clear correlation between the transfected and secreted amounts of F-CPE protein. To explore whether secreted CPE affects the Wnt signal conditioned media (CM) from cells transfected with F-CPE-GFP or from Wnt-secreting cells (L-Wnt3a)26 were used. HEK293T cells transfected with the pTOPFLASH/pFOPFLASH reporters were grown in different CM for 18 h. As expected, the Wnt CM increased TCF/β-catenin-dependent transcription and led to amplified β-catenin protein levels (Figure 4c, lane 4). Supplementing the cells with CPE CM resulted in a decreased TCF/β-catenin-mediated transcription and β-catenin protein levels (Figure 4c, lane 3). Reduced levels of TCF/β-catenin-mediated transcription were also obtained when CPE CM was added to cells transfected with different activators of the Wnt cascade (Figure 4d). To establish the specificity of our results purified CPE recombinant protein was used. Wnt signaling activated by overexpressing Wnt3a + Fz1 was reduced in the presence of purified CPE protein (Figure 4e). We also show that treatment of L-Wnt3a cells with the purified CPE protein led to a decrease in endogenous active β-catenin levels (Figure 4f).
Figure 4.
Secreted CPE affects Wnt signaling. (a; upper panel) HEK293T cells were transfected with F-CPE-GFP, F-CPE-FLAG and untagged-F-CPE. Forty-eight hours post transfection the cell media was collected and the transfected cells were harvested. The media and lysates were subjected to western blot analysis using an anti-CPE antibody. HEK293T cells were transfected with FLAG-tagged F-CPE and ΔN-CPE (lower panel). Forty-eight hours post transfection the cell media was collected and the transfected cells were harvested. The media and lysates were subjected to western blot analysis using an anti-FALG antibody. Tubulin served as a loading control. (b) HEK293T cells were transfected with different amounts of F-CPE-GFP. Media and lysates were subjected to western blot analysis using an anti-CPE antibody. (c) HEK293T cells were transfected with F-CPE-GFP. Twenty-four hours later, CM was collected from transfected cells. Wnt CM was collected from L-Wnt3a cells. The different media was added to HEK293T cells transfected with the pTOPFLASH/pFOPFLASH reporter plasmids. Luciferase levels were detected 24 h later. The upper panel shows the levels of endogenous, active β-catenin as detected using a specific antibody. (d) HEK293T cells were co-transfected with β-catenin-GFP, HA-Wnt3a, HA-Dvl and Fz1 along with the pTOPFLASH/pFOPFLASH reporter plasmids. CPE CM (as in c) was added to transfected cells for 24 h and luciferase levels were then detected. (e) HEK293T cells were co-transfected with Wnt3a and Fz1 along with the pTOPFLASH/pFOPFLASH reporter plasmids. Twelve hours later the cells were supplemented with media containing purified CPE as indicated. Twelve hours later luciferase levels were determined. (f) L and L-Wnt3a cells were supplemented with media containing purified CPE as indicated. The cells were harvested 12 h later and active β-catenin levels were detected using a specific antibody. Tubulin served as a loading control (left). Densitometric analysis of active β-catenin was performed using TINA software (right).
CPE interacts with both Wnt3a and Fz1 but does not disrupt the Wnt−Fz complex
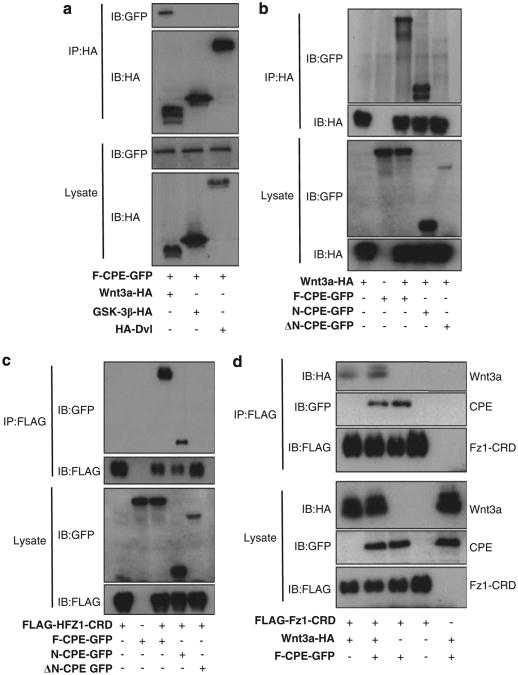
The next experiments were aimed at determining whether there is a cellular interaction between F-CPE and members of the Wnt pathway. HEK293T cells were co-transfected with constructs encoding HA-GSK-3β, Dvl or Wnt3a and F-CPE-GFP. As seen in Figure 5a, F-CPE specifically coimmunoprecipitated with Wnt3a but not with GSK-3β or Dvl. To map the interacting domains we tested a construct that expresses the first 113 amino-acid residues of CPE (N-CPE) in addition to ΔN-CPE and F-CPE constructs. Only the F-CPE and the N-terminal domain interacted with Wnt3a (Figure 5b). We also show that both constructs interact with the cysteine-rich domain (CRD), which is the Wnt-binding domain of Fz1 (Figure 5c). As activation of the Wnt pathway is dependent on binding of the Wnt ligand to its receptor Fz, we examined whether CPE interferes with the ability of Wnt3a to bind Fz1. HEK293T cells were co-transfected with HA-Wnt3a, FLAG-HFz1-CRD and either F-CPE-GFP or empty vector. Data show that the same amounts of Wnt3a coimmunoprecipitated with HFz1-CRD in the presence and absence of F-CPE, indicating that F-CPE does not disrupt the Wnt− Fz complex (Figure 5d).
Figure 5.
CPE forms a complex with Wnt3a and Fz1 but does not interrupt the Wnt–FZ complex. (a) HEK293T cells were co-transfected with F-CPE-GFP, HA-Wnt3a, HA-GSK-3β or HA-Dvl as indicated. Total cellular lysate was immunoprecipitated using an anti-HA antibody and analyzed by western blotting using an anti-HA and an anti-GFP antibodies. (b) HEK293T cells were co-transfected with Wnt3a and the different CPE constructs as indicated. IP was performed as described in a. (c) HEK293T cells were co-transfected with FLAG-Hfz1-CRD and the CPE constructs as indicated. IP was performed using an anti-FLAG antibody. (d) HEK293T cells were co-transfected with Wnt3a, FLAG-Hfz1-CRD and F-CPE-GFP as indicated. IP was performed as described in c.
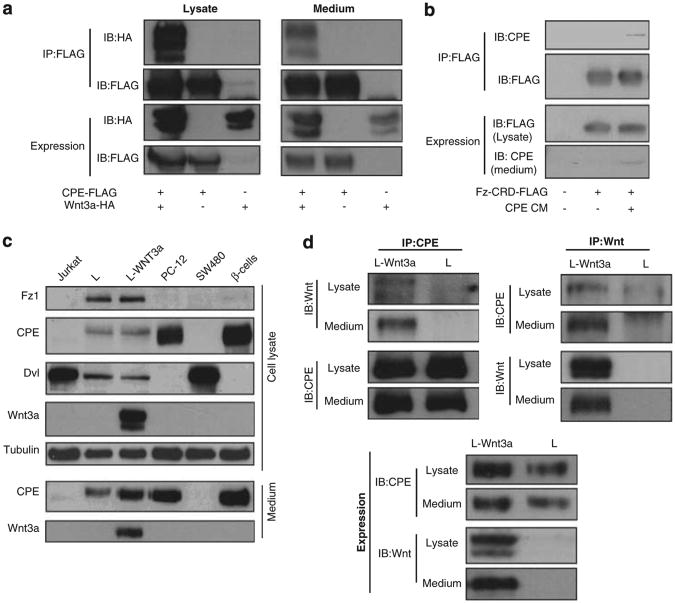
Secreted CPE forms a complex with secreted Wnt3a and Fz1-CRD
As we hypothesized that extracellular F-CPE affects the Wnt cascade, we tested whether F-CPE and Wnt3a can interact outside the cells. To this end we ectopically expressed CPE and Wnt3a and collected both CM and cell extracts for immunoprecipitation (IP) assays. Indeed, CPE and Wnt3a interact in both the cell lysates and media of the transfected cells (Figure 6a). Cells were then transfected with FLAG-HFz1-CRD and supplemented with CPE CM to test for complex formation. As depicted in Figure 6b secreted CPE can interact with the extracellular domain of the Fz1 receptor—CRD. To study the endogenous interactions we used L-Wnt3a cells, as they are the only cells expressing and secreting both CPE and Wnt3a in a number of cell lines tested (Figure 6c). As seen in Figure 6d, a protein complex containing CPE and Wnt3a was precipitated from both lysate and media of L-Wnt3a cells using either anti-Wnt3a or anti-CPE antibodies.
Figure 6.
CPE and Wnt3a form an extracellular complex. (a) HEK293T cells were co-transfected with F-CPE-FLAG and HA-Wnt3a as indicated. Twenty-four hours later, media and cell lysates were subjected to IP assay using FLAG-conjugated agarose beads. Western blot analysis was then performed using anti-FLAG and anti-HA antibodies. (b) HEK293T cells were transfected with FLAG-Hfz1-CRD. Twelve hours post transfection the cells were supplemented with CPE CM for an additional 12 h. The cells were then harvested and subjected to an IP assay using FLAG-conjugated agarose beads. Western blot analysis was then performed using anti-FLAG and anti-CPE antibodies. (c) The expression pattern of different Wnt signaling components in media and lysates were determined in different cell lines by western blot analysis using specific antibodies. (d) L and L-Wnt3a cells were used to determine complex formation between CPE and Wnt3a by IP assays. Both media and lysates were collected and specific antibodies were used for IP and western blot analysis.
CPE partly colocalizes with Wnt3a and disrupts the protein assemblies of Dvl
To try and identify the mechanism by which F-CPE affects the Wnt cascade, we examined its subcellular localization along with several other Wnt signaling members. According to our results, transfected CPE did not colocalize with Axin and GSK-3β, nor did it alter their subcellular localization (data not shown). However, in both COS-7 and HEK293T cells, F-CPE showed some colocalization with Wnt3a (Figure 7a). Importantly, ectopically expressed CPE colocalized with endogenous Wnt3A (Figure 7b). As CPE and Wnt traffic together to the Golgi complex27,28 they are likely packaged in the same vesicles for secretion.29 More interestingly, when coexpressed with Dvl, F-CPE appears to disrupt the Dvl protein assemblies (Figure 7c). These protein puncti, termed as signalosomes, were reported to be essential for Dvl's activity as a positive regulator of the Wnt pathway.7,8,30 The effect of CPE on Dvl was seen only in cells overexpressing both proteins. Perhaps the local concentration of CPE around the transfected cells was higher than neighboring untransfected cells hence insufficient to affect them. It has been shown that overexpressed Dvl forms large cytoplasmic puncti whereas the endogenous Dvl forms low-molecular weight oligomers.31 These large puncti were also shown to activate the Wnt pathway (reviewed in Reznik and Fricker32 It is possible that overexpressed CPE may strongly affect overexpressed Dvl (that forms large signalosomes) in cells that ectopically express both proteins. We compared the ectopic expression pattern of Dvl in two neural cell lines. Indeed, in PC-12 cells that express and secrete F-CPE, the staining pattern of ectopically expressed Dvl is largely cytoplasmic and diffused. In contrast, Dvl staining in U-87 cells that do not have detectable CPE shows typical punctate clusters (Figure 7d). We then tested whether secreted F-CPE can elicit the same effect. COS-7 cells were transfected with HA-Dvl and supplemented with CPE and Wnt3a CM before IF assays (Figure 7e). The expression pattern of exogenous Dvl in cells that were exposed to both CPE and Wnt3a CM was more diffused as compared with the characteristic punctate pattern usually seen when Dvl is overexpressed. The cells exhibiting diffused Dvl staining pattern were counted. Results show that exposing cells to CM containing both CPE and Wnt3a resulted in a higher number of cells presenting a cytoplasmic, diffused Dvl expression (Figure 7e, right). A similar experiment was performed in L-Wnt3a cells supplemented with purified F-CPE. A punctate pattern of endogenous Dvl is clearly observed in the non-treated cells, whereas treatment with soluble F-CPE resulted in a more diffused pattern of Dvl (Figure 7f). These results suggest that CPE disrupts the signalosomes, enhancing the proteasomal degradation of β-catenin.
Discussion
In the present study, we have taken advantage of our ability to activate Wnt signaling under defined conditions to establish a functional screening system in mammalian cells. This screening method allows a rapid and efficient way to identify genes whose expression specifically modulates the Wnt pathway. One of the genes isolated in our screen encodes the CPE protein. The carboxypeptidase family of proteins participates in many diverse functions, however, only carboxypeptidase Z (CPZ) has been previously connected to the Wnt cascade as it was shown to bind the non-canonical Wnt4 ligand.33,34 Unlike CPE, CPZ contains a CRD similar to that found in the Fz family of Wnt receptors that mediates its binding to Wnt4.35 Although CPE has a number of defined functions, there are no current indications for its involvement in any specific cellular signaling pathways. Here, we provide evidence that CPE participates in regulating the canonical Wnt signaling pathway. This pathway is extremely complex and is regulated at all levels from the secretion of the Wnt ligand to the reception of the signal in the nucleus by TCF/LEF family members. Our studies indicate that whereas ΔN-CPE, which is intracellular and partly nuclear, induces β-catenin accumulation and activates Wnt target gene expression, the F-CPE is a potent negative regulator of this cascade. The antagonistic effect of CPE on the Wnt pathway depends on an intact ‘β-catenin destruction complex’ and proteasomal degradation. Our results also show that CPE forms a complex probably through sequences located at its N-terminal domain, with Wnt3a and the extracellular CRD of Fz1.
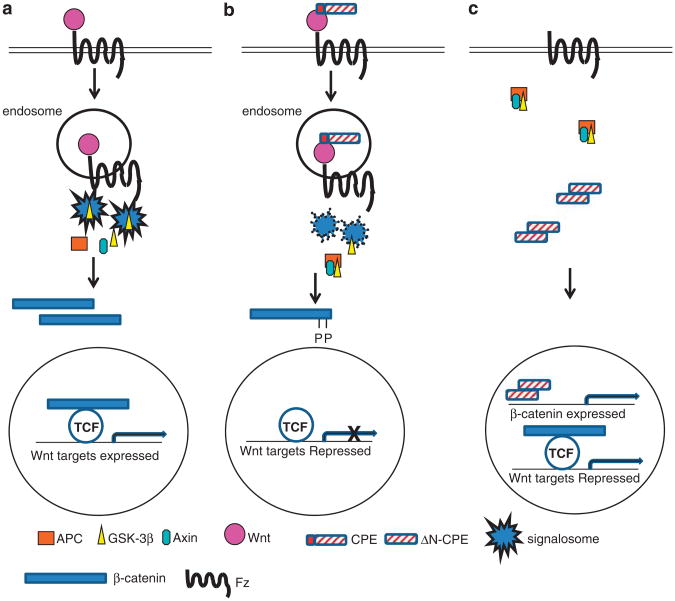
There are currently two main models describing the activation of the Wnt signaling cascade. According to the first one, binding of Wnt to its receptors leads to internalization of the complex and the formation of intracellular early endosome vesicles. GSK-3β is recruited to these endosomes along with other members of the ‘β-catenin degradation complex’. This leads to both sequestration of GSK-3β from β-catenin and disruption of the ‘β-catenin degradation complex’. The latter is then disrupted resulting in β-catenin accumulation.5 A second model proposes that binding of the Wnt ligand to its receptors leads to recruitment and polymerization of Dvl at the plasma membrane. In turn, Dvl interacts with Axin promoting the formation of ‘signalosomes’ in which GSK-3β-mediated phosphorylation of b-catenin is blocked (reviewed in Reznik and Fricker32). It has also been suggested that when the Wnt extracellular inhibitor Dickkopf homolog 1 (dkk1) binds LRP6, the complex is internalized into endosomes preventing the formation of the Dvl-induced signalosomes.36 Although the findings presented in this study do not fully describe the mechanism by which CPE functions to inhibit the Wnt pathway, we hypothesize that, similarly to dkk1, secreted F-CPE may be internalized together with the Wnt-receptor complex into endosomes and in turn lead to disruption of the signalosomes thus reducing Wnt signaling levels (Figures 8a and b). Our findings show that F-CPE colocalizes with the Wnt3a ligand. As CPE functions as a sorting receptor helping direct pro-hormones from the trans-Golgi network into regulated secretory granules27 and the Wnt proteins are also known to use the trans-Golgi to secretory granule route,28 one assumption may be that both CPE and Wnt3a are expressed in the same Golgi compartments, and the same secretory vesicles as seen in cases of other proteins.29
Figure 8.
Hypothetic models for the role of F-CPE and ΔN-CPE in Wnt signaling. (a) Following binding of the Wnt ligand to its receptor, a complex containing different Wnt signaling components forms in cytoplasmic endosomes resulting in sequestration of GSK-3β and signalosome formation. The ‘β-catenin degradation complex’ is disassembled and β-catenin accumulates and translocates into the nucleolus. (b) Binding of F-CPE to Wnt and Fz affects signalosome formation and β-catenin can be efficiently degraded. (c) The ΔN-CPE splice variant expressed in cancer cells translocates into the nucleus and upregulates β-catenin mRNA transcription and protein levels to promote Wnt signaling.
The Wnt/β-catenin signaling pathway is a major determinant of gene expression patterns along the colonic crypt axis and controls proliferation versus differentiation in healthy and malignant intestinal epithelial cells. It has been shown that while the Wnt pathway is highly active at the crypt bottom, as the cells move up the crypt and start to differentiate, the Wnt pathway is shut down.37,38 Interestingly the latter study also shows that the expression pattern of CPE is altered in ΔnTCF4-expressing CRC cells. Previous studies have described the expression of CPE in different epithelia-derived cancers.19 However, recent work indicates that in these cancer types only the CPE splice variant— ΔN-CPE, but not F-CPE, is expressed. Moreover, ΔN-CPE is expressed in highly metastatic cancers as compared with normal tissue or benign tumors.20 In these cells, ΔN-CPE may also contribute to cancer development by inducing both Wnt signaling activity (perhaps by the hypothetical mechanism shown in Figure 8c) and NEDD9 gene expression, leading to transformation and tumorogenesis.20 Thus, it is plausible to speculate that CPE and its splice variant ΔN-CPE are important modulators of the Wnt cascade affecting both normal and aberrant cell processes. According to this scenario, in the first stages of CRC development the expression of F-CPE is lost and as the disease progresses ΔN-CPE begins to be expressed. ΔN-CPE may promote tumor development in both Wnt-dependent and -independent manners, whereas the F-CPE acts extracellularly to antagonize the Wnt pathway in normal colonic development, as well as in malignant transformation.
Materials and Methods
Cell culture, transfection
HEK293T, HEK293-EBNA (stably expressing the EBNA-1 gene; Invitrogen, Grand Island, NY, USA), L-Wnt3a cells, L cells, COS-7, U-87, Jurkat and the human CRC cell line SW480 were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 100 U/ml penicillin/streptomycin at 37 °C in a humidified 5% CO2 atmosphere. PC-12 and β-cells were maintained as described above with additional 10% horse serum. Transfection of HEK293T cells was performed by CaPO4 precipitation. All other cells were transfected using the DNA transfection reagents jetPEI (Polyplus Transfection, Illkirch, France) or Lipofectamine 2000 (Invitrogen), following the manufacturers' protocols.
Plasmids and reagents
CPE constructs: CPE-GFP (pEGFP-N3) and hCPE (pcDNA3.1(+)) were kindly provided by YP Loh (NICHD, NIH, Bethesda, MD, USA). DN-CPE-GFP and N-terminal CPE-GFP were constructed by inserting the cDNA into pEGFP-N3 (Clontech, Oxon, UK) using BamHI and XhoI restriction sites. FLAG-CPE constructs were prepared by inserting the different CPE cDNAs into the FLAGX3-pcDNA3.1(−) using ApaI and XhoI restriction sites. GFP-β-catenin was constructed by inserting the β-catenin cDNA into pEGFP-C1 (Clontech) using BamHI and XbaI restriction sites. This expression vector was used as a template for mutagenesis of β-catenin by the QuikChange site-directed mutagenesis kit (Stratagene, Santa Clara, CA, USA) to replace serine 33 with alanine to generate GFP-S33A-b-catenin. The HA-GSK-3β expression vector was kindly provided by DrTC Dale (Developmental Biology, Chester Beatty Laboratories, Institute of Cancer Research, London, UK). FLAG-GSK-3β was kindly provided by Dr Hagit Eldar-Finkelman (Tel-Aviv University, Tel Aviv, Israel). FLAG-Dvl, FLAG-Fz1-CRD and HA-β-catenin were kindly provided by Dr Arnona Gazit and Dr Abraham Yaniv (Tel-Aviv University). Human Frizzled 1 (Fz1), described previously, was kindly provided by Dr SA Aaronson (Mount Sinai Medical Center, New York, NY, USA). HA-Wnt3a was purchased from Upstate Biotechnology (New York, NY, USA). HA-Dvl was constructed in the pcDNA3 vector. The Wnt-responsive TCF-dependent luciferase constructs pTOPFLASH (containing multi TCF-binding sites linked to a luciferase reporter) and its mutated version pFOPFLASH were kindly provided by Dr H Clevers (Center for Biomedical Genetics, Hubrecht, The Netherlands) and were described previously.39 pCMV-Renilla and pCMV-β-galactosidase expression plasmids, used to evaluate transfection efficiency, were purchased from Promega (Madison, WI, USA) and Clontech, respectively. MG132 (Calbiochem, San Diego, CA, USA), LiCl (Sigma, Rehovot, Israel), puromycin and hygromycin B (Sigma) were used at concentrations as indicated. Recombinant Human CPE (10069-H08H; Sino Biological Inc., Beijing, China) diluted in Dulbecco's modified Eagle's medium and added to cells as indicated.
Luciferase reporter assays
To assay TCF-mediated transcription, cells were seeded at 1 × 105 cells per well in a 24-well plate 24 h before transfection. Cells were transfected with the indicated vectors, along with pTOPFLASH/pFOPFLASH and β-gal or pCMV-Renilla plasmids. Forty-eight hours post transfection the cells were harvested and subjected to luciferase assay according to the manufacturer's instructions. In all assays, FOPFLASH activity was measured by replacing the pTOPFLASH with pFOPFLASH under equivalent conditions. Data are presented as mean values and s.d's for at least three independent experiments done in duplicate.
Western blot analysis and IP
Forty-eight hours following transfection, cells were washed with phosphate-buffered saline (PBS) and solubilized in reporter lysis buffer (luciferase buffer, Promega), lysis buffer (100mm NaCl, 50 mm Tris, pH7.5, 1% Triton X-100, 2 mm EDTA) or radioimmuno precipitation assay buffer (50mm Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium-deoxycholate, 150 mm NaCl, 1 mm EDTA) containing protease inhibitor cocktail (Sigma). Extracts were clarified by centrifugation at 12 000 g for 15 min at 4 °C. Following SDS–polyacrylamide gel electrophoresis separation, proteins were transferred to nitrocellulose membranes and blocked with 5% low-fat milk. Membranes were incubated with specific primary antibodies, washed with PBS containing 0.001% Tween-20 (PBST) and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. After washing in PBST, membranes were subjected to enhanced chemiluminescence detection analysis. For IP analysis, cells were solubilized in lysis buffer (see above). Cell lysates were incubated with anti-FLAG M2-agarose affinity gel (Sigma), with rotation for 2–18h at 4 °C. Alternatively, cell lysates were incubated with the specific antibody for 1–2 h at 4 °C before 2–18h rotated incubation with protein A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C. Beads were collected by slow centrifugation, washed four times with lysis or radioimmuno precipitation assay buffer and analyzed by SDS–polyacrylamide gel electrophoresis followed by detection with specific antibody. IP from medium was performed similarly. Cells were grown in medium without serum for 24 h, which was then collected and subjected to IP assays. Intensity of the protein bands was quantified by a computer-assisted densitometer (TINA 2.0c; Fuji BAS, Tokyo, Japan).
Immunofluorescence
HEK293T, PC-12, U-87, L, L-Wnt3a and COS-7 cells were grown on coverslips and fixed 48 h post transfection for 20 min in PBS containing 4% paraformaldehyde. After three washes with PBS, the fixed cells were permeabilized with 0.1% Triton X-100 for 10 min and blocked with bovine serum albumin for 1 h. Subsequently, cells were incubated at room temperature with primary and secondary antibodies for 60 and 30 min, respectively. 4-6′ diamidino-2 phenylindole (Sigma) was used to stain cell nuclei. GFP was detected without staining. Cells were visualized by confocal microscopy.
Antibodies
The following antibodies were used: mouse anti-β-catenin (IB: 1:5000; IF: 1:300; BD Transduction Laboratories, Franklin Lakes, NJ, USA), mouse anti-active β-catenin (Anti-ABC clone 8E7; Merck Millipore, Temecula, CA, USA) (IB: 1:1000; IF: 1:150; Millipore), rabbit anti-GFP (1:1000; Santa Cruz Biotechnology), rat anti-HA (IB: 1:2500; IF: 1:300; Roche, Indianapolis, IN, USA), anti-HA-probe (Y-11) antibody (IB: 1:1000; Santa Cruz Biotechnology), mouse anti-FLAG (1:5000; Sigma), rabbit anti-FLAG (1:400; Sigma), mouse anti-CPE (IB: 1:500; IF: 1:50; IHC 1:50; R&D Systems, Minneapolis, MN, USA), rabbit anti-Dvl2 (IB: 1:1000; Abcam, Cambridge, UK), rabbit anti-Wnt3a (IB: 1:2500; IF: 1:50; Cell Signaling, Temecula, CA, USA), rabbit anti-Fz1 (IB: 1:500; Santa Cruz Biotechnology), rabbit anti-sox-9 (IB: 1:2000; Millipore), mouse anti-myc (IB: 1:1000; IF:1:100; Santa Cruz Biotechnology) and rabbit anti-CPE.20 Mouse anti-actin (1:10 000; ImmunO, Aurora, OH, USA) and mouse anti-tubulin (1:10 000; Sigma) were used as loading controls. Anti-rat horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (1:5000). Anti-mouse and anti-rabbit secondary antibodies were obtained from Jackson Immuno Research (West Grove, PA, USA) (1:10 000). For IF Alexa red and green (1:500; Molecular Probes, Grand Island, NY, USA) were used.
Small interfering assay
CPE small interfering RNA (siRNA) oligonucleotides were purchased from Thermo Scientific Dharmacon (Chicago, IL, USA; siGENOME SMART pool M-005823-00-0005, Human CPE, NM_001873) as well as transfection reagent Dharmafect-1. Transfection was performed according to the manufacturer's protocol. All experiments were performed in PC-12 cells using 100 nM siRNA oligonucleotides for 72 h. Non targeting RNA oligonucleotides (Thermo Scientific Dharmacon) were used for scr control. PC-12 cells were transfected 24 h after siRNA transfection with pTOP-FLASH/pFOPFLASH and β-gal plasmids for luciferase detection. Forty-eight hours post transfection, the cells were harvested and subjected to either luciferase assay or western blot analysis as describe above.
CM effect
Transfection of HEK293T or COS-7 cells with the indicated plasmids was performed in a 24-well plate. Thirty hours later the medium was replaced with condition medium (CM) from HEK293T cells transfected with F-CPE-GFP or GFP and medium collected from L or L-Wnt3a cells. The two types of media were combined at a 1:1 ratio and added to the transfected cells for 18 h. Alternatively, purified recombinant CPE (Sino Biological Inc.) diluted in Dulbecco's modified Eagle's medium to the desired concentration (specified in the figure) was added to cells for 18 h. The HEK293T cells were then harvested and subjected to either western blotting or luciferase reporter assay. COS-7 cells transfected with HA-Dvl and L-Wnt3a cells were supplemented with CPE CM 12 h before IF assay as described above.
Supplementary Material
Acknowledgments
This work was supported by the Israel Science Foundation, by grant no. 20120016 from the Public Committee for Allocation of Estate Funds, Ministry of Justice, Israel, the Recanati Foundation, Israel Cancer Association through the Estate of the late Alexander Smidoda, US—Israel Binational Science Foundation and in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development to YPL.
Footnotes
Conflict Of Interest: The authors declare no conflict of interest.
References
- 1.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 5.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007;120(Pt 14):2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- 8.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 9.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe C, Mendoza-Topaz C, Mieszczanek J, Bienz M. Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization. J Cell Sci. 2010;123(Pt 9):1588–1599. doi: 10.1242/jcs.067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 12.Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. New roles of carboxypeptidase e in endocrine and neural function and cancer. Endocr Rev. 2012;33:216–253. doi: 10.1210/er.2011-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 14.Fricker LD, Snyder SH. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983;258:10950–10955. [PubMed] [Google Scholar]
- 15.Tang SS, Zhang JH, Liu HX, Li HZ. PC2/CPE-mediated pro-protein processing in tumor cells and its differentiated cells or tissues. Mol Cell Endocrinol. 2009;303:43–49. doi: 10.1016/j.mce.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, et al. Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol. 2004;35:1196–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Horing E, Harter PN, Seznec J, Schittenhelm J, Buhring HJ, Bhattacharyya S, et al. The ‘go or grow’ potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic stress. Acta Neuropathol. 2012;124:83–97. doi: 10.1007/s00401-011-0940-x. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Keegan BP, North WG. Key peptide processing enzymes are expressed by breast cancer cells. Cancer Lett. 2001;165:211–218. doi: 10.1016/s0304-3835(01)00409-8. [DOI] [PubMed] [Google Scholar]
- 19.Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cell Mol Neurobiol. 2010;30:1377–1381. doi: 10.1007/s10571-010-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Lee TK, Murthy SR, Cawley NX, Dhanvantari S, Hewitt SM, Lou H, et al. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. J Clin Invest. 2011;121:880–892. doi: 10.1172/JCI40433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Murthy SR, Lee TK, Cawley NX, Hewitt SM, Pacak K, Loh P. An N-terminal truncated carboxypeptidase E splice isoform induces metastasis by activating NEDD9 and other metastasis inducing genes AACR Annual Meeting 2012; Chicago, IL. p. 2012. [Google Scholar]
- 22.Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. beta-catenin signaling cascade. J Biol Chem. 2004;279:14879–14888. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- 24.Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, et al. APC mutations in sporadic colorectal tumors: a mutational ‘hotspot’ and interdependence of the ‘two hits’. Pro Natl Acad Sci USA. 2000;97(7):3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fricker LD, Devi L. Posttranslational processing of carboxypeptidase E, a neuro-peptide-processing enzyme, in AtT-20 cells and bovine pituitary secretory granules. J Neurochem. 1993;61:1404–1415. doi: 10.1111/j.1471-4159.1993.tb13634.x. [DOI] [PubMed] [Google Scholar]
- 26.Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 27.Park JJ, Koshimizu H, Loh YP. Biogenesis and transport of secretory granules to release site in neuroendocrine cells. J Mol Neurosci. 2009;37:151–159. doi: 10.1007/s12031-008-9098-y. [DOI] [PubMed] [Google Scholar]
- 28.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 29.Strous GJ, Willemsen R, van Kerkhof P, Slot JW, Geuze HJ, Lodish HF. Vesicular stomatitis virus glycoprotein, albumin, and transferrin are transported to the cell surface via the same Golgi vesicles. J Cell Biol. 1983;97:1815–1822. doi: 10.1083/jcb.97.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, et al. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 31.Liu YT, Dan QJ, Wang J, Feng Y, Chen L, Liang J, et al. Molecular basis of Wnt activation via the DIX domain protein Ccd1. J Biol Chem. 2011;286:8597–8608. doi: 10.1074/jbc.M110.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling—two contrasting models. J Cell Sci. 2011;124(Pt 21):3537–3544. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- 33.Moeller C, Swindell EC, Kispert A, Eichele G. Carboxypeptidase Z (CPZ) modulates Wnt signaling and regulates the development of skeletal elements in the chicken. Development. 2003;130:5103–5111. doi: 10.1242/dev.00686. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Shao YY, Ballock RT. Carboxypeptidase Z (CPZ) links thyroid hormone and Wnt signaling pathways in growth plate chondrocytes. J Bone Miner Res. 2009;24:265–273. doi: 10.1359/jbmr.081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reznik SE, Fricker LD. Carboxypeptidases from A to z: implications in embryonic development and Wnt binding. Cell Mol Life Sci. 2001;58:1790–1804. doi: 10.1007/PL00000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2012;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 38.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 39.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.