Abstract
Recent advances have increased excitement about the potential for therapeutic production of red blood cells (RBCs) in vitro. However, generation of RBCs in the large numbers required for transfusion remains a significant challenge. In this article, we summarize recent progress in producing RBCs from various cell sources, and discuss the hurdles that remain for translation into the clinical arena.
Unmet Transfusion Needs
Blood transfusion is an indispensable cell therapy. The safety and adequacy of the blood supply are national and international priorities. With over 93 million donations made every year worldwide, the blood supply in industrialized countries is adequate overall. The number of units collected exceeded those transfused by 13% in the USA in 2008. There are, however, several shortcomings to the current system.
Due to the substantial polymorphism of blood group antigens, there are, even in developed countries, chronic shortages of blood for some patient groups (Zimring et al., 2011). Immune reactivity problems are magnified when donors and recipients are from different ethnic groups. In the USA, more than 40% of Sickle Cell Anemia patients, who are largely of African descent, experience immune reactions when transfused with blood from donors, who are mostly of Caucasian descent. Targeted recruitment programs have aimed to balance the ethnicity of donors and recipients but disparities in supply and demand still exist for rare blood units. Data from Life-Share Blood Centers (http://www.lifeshare.org/facts/raretraits.htm; Shreveport, LA) indicate that screening of 17,603 donors identified only 101 donors with the rare U negative phenotype to serve a population of 30 chronically transfused U negative patients.
Sporadic shortages of blood can also occur in association with natural or man-made disasters. In emergencies, national plans call for sponsoring emergency blood drives, but the infrastructure to maintain blood collection and distribution systems may be disrupted during disasters of severe magnitude, such as the recent earthquake in Japan. For this reason, emergency plans also call for sharing of blood resources across geographical areas and accessing frozen blood inventories, which are limited.
There is also increasing concern that the blood supply may be curtailed by new restrictions on donor eligibility as new blood transmissible diseases are discovered and/or emerging diseases, such Dengue fever, spread to new geographical areas, increasing unit rejection due to positivity for transmissible disease. In addition, blood usage by the growing numbers of individuals >60 years of age is predicted to increase, leading to an insufficient blood supply by 2050.
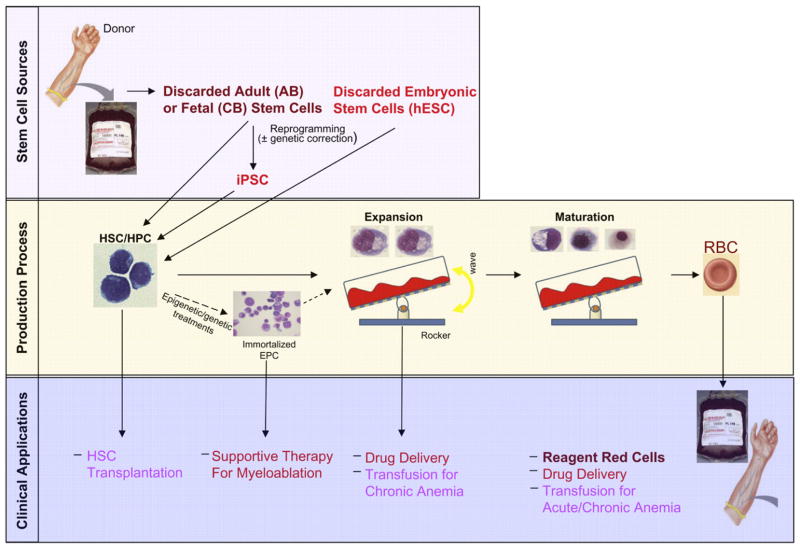
Over the years, the transfusion medicine community has evaluated several alternative transfusion products (TPs), including hemoglobin solutions, perfluorocarbons, and enzymatically/chemically modified RBCs to produce ORh-negative blood. Only hemoglobin solutions moved to phase 3 clinical trials. A meta-analysis of 16 clinical trials demonstrated that use of hemoglobin solutions leads to increased risk for myocardial infarction and death (Natanson et al., 2008). Against this backdrop, research on in vitro expanded RBCs as alternative TPs has gained new momentum (Figure 1).
Figure 1. The Pathway to Therapeutic Production of Red Blood Cells from Stem Cells.
Outline of the potential sources of stem cells for in vitro therapeutic products, including transfusion products (TPs) that are currently under investigation (top panel), the different phases of a production process (middle panel), and a list of possible intermediate and long-term clinical applications of in-vitro-generated erythroid cells (bottom panel). The diagram is color coded: intensity of red shades is inversely related to projected time for clinical application. Human hematopoietic stem/progenitor cells (HSC/HPC), immortalized erythroid precursor cells (EPC), and erythroblasts at different stages of maturation up to the RBC stage in the expansion and maturation phase of the production process are represented. The culture protocols used to promote proliferation, maturation, and enucleation of erythroid cells vary significantly. Therefore, the generation of TPs in vitro will require establishing sequential GMP conditions, the most challenging of which may be represented by those that favor enucleation because of the need to replace the function exerted by stromal cells of murine origin with defined components.
The Search for the Optimal Stem Cell Source
The concept of using in-vitro-generated RBCs as a TP arose when it was realized that currently discarded primary stem cell sources (low volume cord blood [CB] and cells discarded during the leukoreduction process of adult blood [AB] donations) have the potential to generate sufficient RBCs for several transfusions (Migliaccio et al., 2009; Peyrard et al., 2011). However, due to intrinsic differences in hematopoietic stem cell (HSC) content and in proliferation ability of hematopoietic progenitor cells, the number of RBCs generated from sources from different individuals varies over two-logs (Migliaccio et al., 2009). Understanding the factors underlying this variability will optimize donor selection, an important facet of in vitro production of TPs from primary sources.
Current technologies are able to produce sufficient RBCs for their functional evaluation in vivo (107). In 2011, the Douay laboratory provided evidence that autologous RBCs generated in vitro under good manufacturing practice (GMP) conditions from mobilized CD34pos cells collected by apheresis survive in vivo in man as long as their natural counterparts do (determined by 51Cr labeling, the only method accepted by the US Food and Drug Administration) when transfused into an autologous recipient (Giarratana et al., 2011). This study was received with both excitement and reservations by the hematological community. On one hand, it described the type of safety data that could support an investigational new drug application. On the other hand, however, its ultimate goal, therapeutic transfusion, is still unrealistic based on the protocol described.
Although extensive, the potential of primary stem cells for generating RBCs in vitro is limited. In principle, the ideal stem cell candidate for industrial production would have unlimited expansion potential to justify the costs associated with its characterization (viral screening; genomic, proteomic, and epigenomic profiling; etc.) for a production process that would meet current GMP criteria for medicinal products (those of the European Medicines Agency [http://www.ema.europa.eu] and those of the US Food and Drug Administration [http://www.fda.gov/BiologicsBloodVaccines]). Stem cell sources with unlimited proliferation potential include human ESCs (hESCs) and induced pluripotent stem cells (iPSCs) (Yamanaka and Blau, 2010). The potential genomic instability of these stem cells poses a reduced safety concern in this context as the final cell product, RBCs, does not contain a nucleus.
Seminal studies in 2006 from Dr. Nakamura and colleagues established methods for generating RBCs from murine ESCs (Miharada et al., 2006), and then in 2008 the same group demonstrated that transfusion of ex-vivo-produced RBCs protects mice from lethal hemolytic anemia (Hiroyama et al., 2008). Papayannopoulou and colleagues (Chang et al., 2006b) and Lanza and colleagues (Lu et al., 2008) established methods for generating RBCs from hESCs and provided extensive characterization of the biological properties of these hESC-derived RBCs. hESCs have been proposed as a source of stem cells to generate universal ORh negative RBC TPs in vitro. However, the genotype (O/ARh positive) of the three GMP-grade hESC lines currently available (H1, H7, and H9) is not suitable for generating universal donor RBCs. Genotype characterization and GMP-derivation of the additional 136 hESC lines currently available is needed to identify hESCs lines with the potential to generate ORh negative RBCs. It would be even more challenging to identify hESC lines suitable for generating RBCs for patients with rare blood types. In theory, it might be possible to engineer rare blood-specific hESC lines through homologous recombination (Zou et al., 2011). However, the genetic basis of some blood group polymorphisms (as an example, Rh) is still not completely understood (Zimring et al., 2011). Further studies on hESC biology and on the genetic basis of RBC antigen heterogeneity would therefore be needed to generate blood-group specific hESC lines.
As iPSCs can be generated from any donor, they are theoretically suitable for generating phenotypically matched RBCs. It has been calculated that iPSCs generated from donors with as few as 24 rare blood phenotypes could produce TPs to accommodate virtually all patient groups in France (Peyrard et al., 2011). However, identification of suitable donors is an intrinsic limitation of this approach, because these phenotypes are so rare that donors with the rarest of them may not exist. It is more likely that iPSCs from dedicated rare donors would be used to generate ex vivo TPs for their matched patients, relieving pressure for continuous donations from the public. A number of groups have published methods for generating RBCs from iPSCs (Chang et al., 2011; Papapetrou et al., 2011). In general, regardless of their origin, iPSCs generate RBCs expressing mostly fetal hemoglobin (hemoglobin F). RBCs expressing hemoglobin F are slightly less efficient than those expressing adult hemoglobin for oxygen delivery. However, patients who retain expression of hemoglobin F in adult life are not anemic. Thus, the fetal phenotype of RBCs derived from iPSCs derived from patients with hemoglobinopathies is not necessarily a barrier to their use as an autologous TP.
Additional barriers to the use of both hESCs and hiPSCs to produce TPs include not only safety concerns (the phenotypic instability of these cell lines poses some potential immunogenicity risks for their products), but also current limitations on large-scale hematopoietic cell expansion. HSCs derived from hESCs and iPSCs have poor erythroid expansion potential compared to that of CB (500 versus 104–105 erythroblasts per hESC/iPSC-derived or CB-derived CD34pos cell, respectively). Thus, production of TPs from hESCs and hiPSCs requires enormous amplification of the hESCs and hiPSCs themselves. However, the life span in culture of iPSCs is much longer than that of HSCs from primary sources, making iPSCs better suited to the selection procedures associated with genetic manipulation (by genomic safe-harbor targeting [Papapetrou et al., 2011] or homologous recombination [Zou et al., 2011] technology) to generate in vitro, genetically corrected HSCs for autologous transplantation in hematopoietic disorders.
Several investigators are exploring the feasibility of reprogramming somatic cells directly into RBCs bypassing a pluripotent state and/or generating stem cells with unlimited expansion potential by epigenetic or genetic in vitro treatments. Treatment with chromatin modifying agents increases the expansion potential of CB erythroid progenitor cells (Chaurasia et al., 2011). Nakamura and colleagues have obtained immortal erythroid precursor lines from murine ESCs (Hiroyama et al., 2011). In both cases, the modified cells generated erythroblasts that matured into circulating RBCs when injected into immunodeficient NOD/SCID/γcnull mice. These in vivo analyses suggest that in addition to being used to make TPs in vitro, these cells might potentially be directly infused into patients in a manner that is conceptually similar to the use of in-vitro-expanded myeloid progenitor cells to reduce the period of neutropenia following HSC transplantation. The generation of erythroid cells by reprogramming somatic cells directly into erythroblasts by overexpression of suitable gene combinations is also under investigation. A successful example of this approach is represented by the recent demonstration that overexpression of p45NF-E2/Maf turns human fibroblasts into megakaryocytes (Wang et al., 2011).
Economic and Logistical Challenges
Production of sufficient RBCs in vitro for transfusion (2.5×1012) is currently a costly and technically challenging proposition. One major technical limitation is the fact that erythroid cells do not proliferate at a concentration >106 cells/ml, so production of 2×1012 RBCs would require at least 2.5×103 liters of culture media. The only equipment currently available to manage such large volumes is the bioreactors used by the biotechnology industry. Whether bioengineering technology validated for production of cell therapy products (e.g., the WAWE System or perfusion hollow fibers) (Timmins et al., 2011; Housler et al., 2011) will allow growth of erythroid cells at greater concentrations, decreasing production costs, is currently unknown. A better understandin of the mechanisms regulating the terminal stages of erythroid differentiation and the identification of soluble factors (hormones, inhibitors of cell adhesion and/or of the death pathway, etc.) and/or cell-mediated factors, which may increase the density limit at which erythroid cells may grow, could facilitate in vitro production of TPs by reducing the volume of media required (Kim and Baek, 2011). An additional challenge is that in vitro enucleation, the last step of erythroid maturation, requires the presence of stromal cells, often of animal origin, limiting the development of GMP production processes (Kim and Baek, 2011). Methods to promote enucleation by using chemical compounds that favor membrane trafficking (Keerthivasan et al., 2010) also need to be developed. Overall, therefore, considerable progress in areas such as formulation of humanized culture media using clinical grade reagents, overcoming hurdles to cell derivation, and development of bioengineering processes and facilities to produce large numbers of cells will be required.
Intermediate Therapeutic Goals
In the interim, there are some realistic intermediate therapeutic goals that could be achieved with current technology, in the hope that, with adequate financial support, facilities for cost-effective production of large numbers of RBCs will be available by the time these intermediate benchmarks are reached. There are at least three applications for in-vitro-generated RBCs that are feasible with current technologies: reagent RBCs for antibody identification, drug discovery, and drug delivery. In-vitro-generated cells are suited for these applications because their immediate cell sources and precursors can be cryopreserved and stored long-term for repeated study (Migliaccio et al., 2009).
Reagent RBCs
In vitro tests using RBCs from donors with common and rare blood types are used to identify suitable transfusion matches (Zimring et al., 2011). These antibody detection and identification assays require low RBC numbers (~2 × 108 RBCs/100 assays), but blood from rare donors is available in limited quantities because guidelines restrict the frequency of donations to prevent anemia. The need for additional donations could be overcome by generating reagent RBCs in vitro from mononuclear cells usually discarded during the leukoreduction process (or from iPSCs derived from these cells). Thorough comparison of blood group antigen expression in RBCs generated in vitro and in vivo would be important for validation of this application, and such studies would also provide immunological/functional information on their use as a TP.
Drug Discovery
Preclinical toxicology and efficacy drug evaluation guidelines recommend reduction and eventually elimination of animal experimentation through development of robust and sensitive cell-based assays. Primary cells reflecting age, sex, and genetic polymorphisms of the human population are more suitable than cell lines for screening drugs that target diseases with variability in clinical responses. Sensitive, fluorimetrically based miniaturized assays have been developed that measure proliferation/ maturation rates of erythroblasts generated by several ml of blood (Migliaccio et al., 2009). These assays have been proposed as an inexpensive way to screen for inducers of hemoglobin F production for Thalassemia and Sickle Cell Anemia, inhibitors of 11 kDa-mediated caspase 10 activation to prevent B19 parvovirus’ infection; cellular-based antimalarial therapies (e.g., lentiviral-induced miRNA overexpression to inhibit intracellular proliferation of the parasite); and erythropoiesis-stimulating agents for myelodysplatic syndrome. In addition, they could be used for risk stratification in hematological malignancies and/or optimization of therapy in cancer patients (Migliaccio et al., 2009).
Drug Delivery
Proof-of-principle for the use of erythroid cells for systemic drug delivery has come from a mouse model of hemophilia. This disease is an X-linked recessive congenital disorder of coagulation due to Factor VIII or IX deficiency and is currently treated by prophylactic factor infusion, which is expensive and requires high patient compliance (biweekly administration). Transplantation with genetically engineered stem cells infected with a retrovirus that enabled high-level Factor IX production by RBCs (600–800 ng/ml human Factor IX/vector copy) cured hemophilia B mice (Chang et al., 2006a). Although this type of transplantation would not be considered for patients, it does suggest that transfusion of RBCs expanded in vitro from genetically modified autologous CD34pos cells might be a feasible way to supply therapeutic levels of Factor IX. The normal serum concentration of human Factor IX is 5,000 ng/ml but clinical symptoms are substantially reduced with levels as low as 250 ng/ml. This level was obtained in the animal model with ~30% genetically modified RBCs. Humans make 2×1011 reticulocytes/day, so one can anticipate that a single transfusion of 30% of 2×1011 (i.e., 6×109) Factor IX-producing RBCs would be sufficient to maintain therapeutic levels of Factor IX for one day and 4.2×1011 RBCs would be sufficient for at least 1 week. GMP facilities to produce 4.2×1011 RBCs already exist and the cost to produce these cells may be affordable.
Safety Concerns
Before embarking on a phase 1 safety study, RBCs would need to undergo further evaluation, including in vivo functional studies in animal models (Giarratana et al., 2011). One caveat of these analyses is that mice are poor models for human erythropoiesis. However, a recent study showed that chemical ablation of the macrophage pool greatly improves readouts of human erythroid cells in NOD/SCID/γcnull mice (Hu et al., 2011). In addition to establishing a reliable animal model for safety studies, this improved functional assay may finally enable evaluation of whether erythroblasts could in fact represent an innovative TP. Preliminary support for this idea comes from the clinical observation that 40–80 ml of matched CB, which contains 4–8×1010 RBCs plus 4–8×107 erythroblasts, is sometimes used successfully for transfusions in developing countries (Migliaccio et al., 2009). The major advantage of using erythroblasts as a TP is that each one could generate 4–64 RBCs in vivo, reducing the cell numbers required for transfusion and the costs and technical challenges of production. Erythroblast transfusions would be also advantageous for some forms of chronic anemia because, unlike transfused RBCs, erythroblasts may reduce, rather than increase, iron overload. In addition, RBCs generated in vivo by transfused erythroblasts would survive longer in vivo, reducing the frequency of transfusion.
In-vitro-produced TPs must be subjected to the same safety controls developed over time for donated blood. Donors should be selected using the same rigorous procedures developed by blood banks to minimize risks of infectious and immunological diseases following transfusion (Zimring et al., 2011). Concerns related to transmission of known and unknown adventitious agents acquired during manufacturing could be addressed using the same sterility tests developed by the pharmaceutical industry and blood banks as part of their GMP procedures (Giarratana et al., 2011). Concerns about transmission of cells potentially transformed by extensive expansion stimuli or other manipulations in culture could be reduced by terminal filtration to eliminate nucleated cells, possibly in association with irradiation, which also reduces the immunological risks of graft versus host disease following transfusion (Zimring et al., 2011). However, these techniques are not applicable to TPs containing erythroblasts and/or immortalized erythroid precursors (that are expected to be able to proliferate in vivo). Before being considered for clinical use, these alternative TPs would need to be evaluated thoroughly for risks associated with the accumulation of genetic mutations. The long-term effects of administration of in-vitro-expanded, autologous RBCs are unknown. Even minor alterations in the expression of blood group antigens and/or acquisition of neoantigens in culture would be of significant concern because multiple administrations of large cell numbers could lead to sensitization in both autologous and allogenic settings.
Concluding Remarks
In-vitro-generated erythroid cells including RBCs are needed to address specific needs in transfusion medicine. In addition, they may have broader uses as drug delivery vehicles and in other specific circumstances. Although the generation of enucleated, adult-like RBCs from hESCs and iPSCs still presents a number of challenges, the goal of generating reagent red cells and TPs is increasingly within reach.
References
- Chang AH, Stephan MT, Sadelain M. Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol. 2006a;24:1017–1021. doi: 10.1038/nbt1227. [DOI] [PubMed] [Google Scholar]
- Chang KH, Nelson AM, Cao H, Wang L, Nakamoto B, Ware CB, Papayannopoulou T. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006b;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Mitra K, Koya M, Velho M, Desprat R, Lenz J, Bouhassira EE. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS ONE. 2011;6:e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia P, Berenzon D, Hoffman R. Chromatin-modifying agents promote the ex vivo production of functional human erythroid progenitor cells. Blood. 2011;117:4632–4641. doi: 10.1182/blood-2010-10-314567. [DOI] [PubMed] [Google Scholar]
- Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec PY, François S, Trugnan G, Peyrard T, Marie T, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroyama T, Miharada K, Sudo K, Danjo I, Aoki N, Nakamura Y. Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS One. 2008;3:e1544. doi: 10.1371/journal.pone.0001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroyama T, Miharada K, Kurita R, Nakamura Y. Plasticity of cells and ex vivo production of red blood cells. Stem Cells Int. 2011;2011:195780. doi: 10.4061/2011/195780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housler GJ, et al. Compartmental Hollow Fiber Capillary Membrane Based Bioreactor Technology for in vitro Studies on Red Blood Cell Lineage Direction of Hematopoietic Stem Cells. Tissue Eng Part C Methods. 2011 doi: 10.1089/ten.tec.2011.0305. in press. Published online December 28, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Van Rooijen N, Yang YG. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthivasan G, Small S, Liu H, Wickrema A, Crispino JD. Vesicle trafficking plays a novel role in erythroblast enucleation. Blood. 2010;116:3331–3340. doi: 10.1182/blood-2010-03-277426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Baek EJ. Red Blood Cell Engineering in Stroma and Serum/Plasma-Free Conditions and Long Term Storage. Tissue Eng Part A. 2011;18:117–126. doi: 10.1089/ten.tea.2010.0711. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Feng Q, Park JS, Vida L, Lee BS, Strausbauch M, Wettstein PJ, Honig GR, Lanza R. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AR, Whitsett C, Migliaccio G. Erythroid cells in vitro: from developmental biology to blood transfusion products. Curr Opin Hema-tol. 2009;16:259–268. doi: 10.1097/MOH.0b013e32832bcaa2. [DOI] [PubMed] [Google Scholar]
- Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LM, Kadota K, Roth SL, Giardina P, Viale A, et al. Genomic safe harbors permit high β-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol. 2011;29:73–78. doi: 10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrard T, Bardiaux L, Krause C, Kobari L, Lapillonne H, Andreu G, Douay L. Banking of pluripotent adult stem cells as an unlimited source for red blood cell production: potential applications for alloimmunized patients and rare blood challenges. Transfus Med Rev. 2011;25:206–216. doi: 10.1016/j.tmrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Timmins NE, Athanasas S, Günther M, Buntine P, Nielsen LK. Ultra-high-yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng Part C Methods. 2011;17:1131–1137. doi: 10.1089/ten.TEC.2011.0207. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ono Y, Ikeda Y, Okamoto S, Murata M, Poncz M, Matsu-bara Y. Induction of Megakaryocytes From Fibroblasts by p45NF-E2/ Maf. Blood. 2011;11:908a. doi: 10.1182/blood-2012-02-413617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimring JC, Welniak L, Semple JW, Ness PM, Slichter SJ, Spital-nik SL NHLBI Alloimmunization Working Group. Current problems and future directions of transfusion-induced alloimmunization: summary of an NHLBI working group. Transfusion. 2011;51:435–441. doi: 10.1111/j.1537-2995.2010.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Mali P, Huang X, Dowey SN, Cheng L. Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood. 2011;118:4599–4608. doi: 10.1182/blood-2011-02-335554. [DOI] [PMC free article] [PubMed] [Google Scholar]
WEB RESOURCES
- American Society of Hematology. Press release: Researchers Successfully Perform First Injection of Cultured Red Blood Cells in Human Donor. 2011 Sep 1; 2011. ( http://www.hematology.org/News/2011/6995.aspx)
- European Medicines Agency. 2011 http://www.ema.europa.eu.
- LifeShare Blood Centers. Blood Facts Report. 2011 ( http://www.lifeshare.org/facts/raretraits.htm)
- US Department of Health and Human Services. Clinical Trials. Gov: Cultured Red Blood Cells: Life Span in-vivo Studies (GRc2008) 2008 ( http://www.clinicaltrials.gov/ct2/show/NCT00929266)
- US Department of Health and Human Services. American Association of Blood Banks 2009 National Blood Collection and Utilization Report. 2009 ( http://www.aabb.org/programs/biovigilance/nbcus/Documents/09-nbcus-report.pdf)
- US Food and Drug Administration. 2011 http://www.fda.gov/BiologicsBloodVaccines.
- US National Health Institute. Stem Cell Registry. 2011 ( http://stemcells.nih.gov/research/registry)
- World Health Organization. Data on blood safety and availability. 2011 ( http://www.who.int/mediacentre/factsheets/fs279/en)