SUMMARY
Mutations in human cationic trypsinogen cause hereditary pancreatitis by altering its proteolytic regulation of activation and degradation by chymotrypsin C (CTRC). CTRC stimulates trypsinogen autoactivation by processing the activation peptide to a shorter form but also promotes degradation by cleaving the calcium binding loop in trypsinogen. Mutations render trypsinogen resistant to CTRC-mediated degradation and/or increase processing of the activation peptide by CTRC. Here we demonstrate that activation peptide mutations D19A, D22G, K23R and K23_I24insIDK robustly increased the rate of trypsinogen autoactivation, both in the presence and absence of CTRC. Degradation of the mutants by CTRC was unchanged and processing of the activation peptide was increased only in the D19A mutant by 4-fold. Surprisingly, however, this increased processing had only a minimal effect on autoactivation. The tetra-aspartate motif in the trypsinogen activation peptide binds calcium (KD ~1.6 mM), which stimulates autoactivation. Unexpectedly, calcium binding was not compromised by any of the activation peptide mutations. Despite normal binding, autoactivation of mutants D22G and K23_I24insIDK was not stimulated by calcium. Finally, the activation peptide mutants exhibited reduced secretion from transfected cells, and secreted trypsinogen levels were inversely proportional with autoactivation rates. We conclude that D19A, D22G, K23R and K23_I24insIDK form a mechanistically distinct subset of hereditary pancreatitis associated mutations, which exert their effect primarily through direct stimulation of autoactivation, independently of CTRC. The potentially severe clinical impact of the markedly increased autoactivation is offset by diminished secretion, resulting in a clinical phenotype indistinguishable from typical hereditary pancreatitis.
Keywords: trypsinogen, trypsin, chymotrypsin C, autoactivation, calcium binding, hereditary pancreatitis
Mutations in the serine protease 1 (PRSS1) gene that encodes human cationic trypsinogen cause hereditary pancreatitis [1, 2]. The mechanism of action for the most frequently found mutations have been recently elucidated and involves increased resistance against chymotrypsin C (CTRC)-mediated degradation and/or increased sensitivity to CTRC-dependent stimulation of autoactivation [3]. CTRC is a pancreatic serine protease which controls autoactivation of human cationic trypsinogen by selectively cleaving regulatory sites within the trypsinogen activation peptide and the calcium binding loop. The dominant effect of CTRC is trypsinogen degradation, which is triggered by cleavage of the Leu81-Glu82 peptide bond in the calcium binding loop and is facilitated by a trypsin-mediated autolytic cleavage of the Arg122-Val123 peptide bond [3, 4]. CTRC also degrades active trypsin by the same mechanism but at a slower rate. Hereditary pancreatitis-associated mutations N29I, N29T, V39A, R122C and R122H decrease or block cleavages at these sites and thereby increase trypsin levels generated during autoactivation [3].
A secondary, less prominent effect of CTRC on autoactivation is mediated by cleavage of the activation peptide of cationic trypsinogen at the Phe18-Asp19 peptide bond (Figure 1) [5]. The activation peptide is an 8 amino-acid N-terminal extension on trypsinogen, which becomes cleaved at the Lys23-Ile24 peptide bond during activation to trypsin (Figure 1). The activating cleavage may be catalyzed by the brush-border serine protease enteropeptidase (enterokinase) in the duodenum or by trypsin (i.e. autoactivation) in the pancreas. A characteristic feature of the activation peptide is the conserved tetra-Asp sequence, which is presumed to serve as an enteropeptidase recognition motif [6]. We found, however, that individual or combined mutations of these Asp residues had minimal effect on activation of human cationic trypsinogen by human enteropeptidase [7]. On the other hand, the tetra-Asp sequence was shown to inhibit autoactivation of trypsinogen, which is partly relieved by millimolar concentrations of calcium [7]. In human cationic trypsinogen, inhibition of autoactivation is also dependent on Asp218, which participates in a repulsive electrostatic interaction with the tetra-Asp motif [7]. CTRC cleavage at the Phe18-Asp19 peptide bond results in a shortened activation peptide, causing partial liberation of the inhibitory interaction with Asp218 and increased autoactivation [5] (Figure 1). Pancreatitis-associated mutations A16V and, to a lesser extent, N29I increase N-terminal processing of the activation peptide by CTRC and thereby stimulate trypsinogen autoactivation [3, 5].
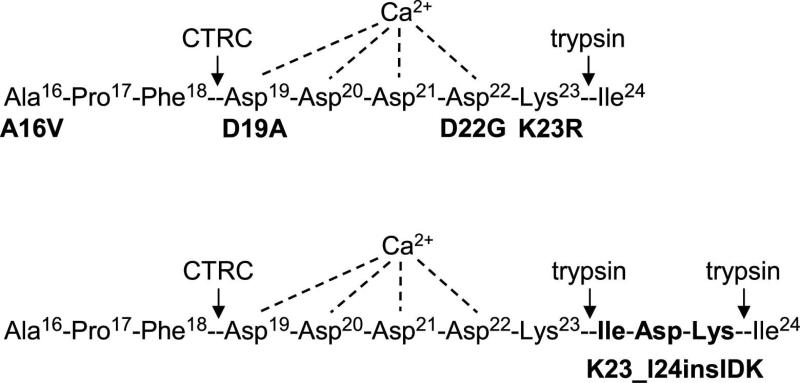
Figure 1.
Pancreatitis-associated mutations in the activation peptide of human cationic trypsinogen. Proteolytic cleavage sites for trypsin and chymotrypsin C (CTRC) and the putative Ca2+ binding site are also indicated. See text for details. Note that the N-terminal amino-acid of mature trypsinogen is Ala16, as the 15 amino-acid long secretory signal peptide is removed in the endoplasmic reticulum. Properties of the A16V mutant were published recently and it was not included in the present study [3, 5].
In addition to the relatively common A16V mutation [8], there were four other mutations, D19A, D22G, K23R and K23_I24insIDK, in the activation peptide of human cationic trypsinogen found in association with hereditary pancreatitis (Figure 1) [9-12]. The K23_I24insIDK mutation results in the insertion of the Ile-Asp-Lys sequence between Lys23 and Ile24, which normally form the activating peptide bond (Figure 1). This insertion changes the tetra-Asp motif preceding Lys23 to Asp-Lys-Ile-Asp; effectively eliminating two of the inhibitory Asp residues. A number of previous studies demonstrated that mutations D19A, D22G, K23R and K23_I24insIDK markedly increased autoactivation of cationic trypsinogen [7, 9, 12, 13]. In fact, these mutations offered the first convincing evidence that increased autoactivation was a pathologically relevant mechanism in hereditary pancreatitis.
In light of the recently discovered CTRC-dependent unifying pathomechanism for hereditary-pancreatitis, the present study was aimed at clarifying the role of CTRC in the mechanism of action of the activation peptide mutations. Because of their location, the mutations are unlikely to affect trypsinogen degradation but may have profound effects on CTRC-mediated processing of the activation peptide. Furthermore, we sought to elucidate how the biochemical phenotype of strikingly increased autoactivation can be reconciled with the clinical phenotype of these mutations which is typical of hereditary pancreatitis and does not indicate increased severity or penetrance. Our results demonstrate that D19A, D22G, K23R and K23_I24insIDK form a special subset of hereditary pancreatitis-associated mutations characterized by robust autoactivation that is largely independent of CTRC and decreased cellular secretion, which is inversely proportional to their ability to autoactivate. The combination of these unique properties adequately explains the observed clinical effect of these mutations.
RESULTS
Autoactivation of activation peptide mutants in absence and presence of CTRC
We studied four mutations which cause alterations in the conserved region of the activation peptide of human cationic trypsinogen, D19A, D22G, K23R and K23_I24insIDK (Figure 1). The A16V mutation which affects the N-terminal residue of the activation peptide was characterized in a recent study [3].
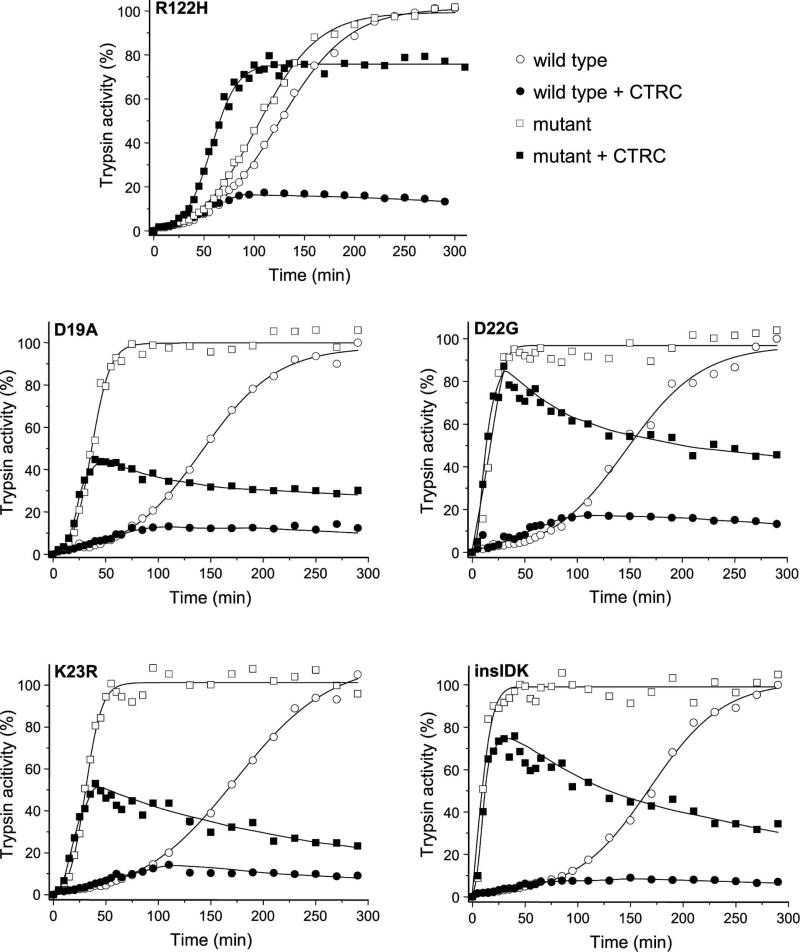
Autoactivation of human cationic trypsinogen in the presence of 25 nM CTRC resulted in a slight increase in the rate accompanied by a marked reduction in final trypsin levels attained (Figure 2). The increased rate is due to N-terminal processing of the activation peptide by CTRC, whereas the reduced trypsin levels are a consequence of CTRC-dependent trypsinogen degradation [3]. Recently, we demonstrated that mutations commonly associated with hereditary pancreatitis exert their effect primarily in the presence of CTRC [3]. A typical case is shown for the archetypal R122H mutation in Figure 2, which in the absence of CTRC increased autoactivation only slightly (1.2-fold). In the presence of CTRC, however, autoactivation of wild-type trypsinogen was drastically suppressed, while mutant R122H autoactivated at an increased rate and reached high trypsin levels.
Figure 2.
Autoactivation of human cationic trypsinogen and activation peptide mutants in the absence and presence of chymotrypsin C (CTRC). For comparison, mutant R122H was also included. Wild-type (circles) and mutant (squares) trypsinogen were incubated at 1 μM with 10 nM initial trypsin in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2, 100 mM NaCl and 0.05% Tween 20, at 37 °C, in the absence (empty symbols) or presence (solid symbols) of 25 nM CTRC (final concentrations). Aliquots (2 μL) were withdrawn at the indicated times and trypsin activity was determined as described in Experimental Procedures. Trypsin activity was expressed as percentage of the maximal activity in the absence of CTRC. Representative experiments from two or three replicates are shown. Mutant K23_I24insIDK is denoted as insIDK.
When mutants D19A, D22G, K23R and K23_I24insIDK were tested under similar conditions, a markedly different phenotype became apparent. All four mutations increased the rate of trypsinogen autoactivation robustly (3.9-fold, 9.2-fold, 5.7-fold and 17.5-fold, respectively) even in the absence of CTRC, in agreement with previous observations [9, 12]. Similarly, in the presence of CTRC, all four mutants autoactivated markedly faster than wild-type trypsinogen and reached much higher trypsin levels, which then slowly decreased due to CTRC-mediated trypsin degradation. Peak trypsin levels correlated with the rate of autoactivation and were higher in mutants D22G and K23_I24insIDK versus D19A and K23R. Surprisingly, even in the presence of CTRC, activation peptide mutants autoactivated much faster than the reference mutant R122H. Thus, the estimated rates of autoactivation were approximately 3-fold higher for mutants D19A and K23R and 10-fold higher for mutants D22G and K23_I24insIDK, relative to mutant R122H.
Cleavage of the Leu81-Glu82 peptide bond by CTRC in activation peptide mutants
Because the activation peptide is not in the proximity of the calcium binding loop, mutations D19A, D22G, K23R and K23_I24insIDK are unlikely to affect CTRC-mediated trypsinogen degradation. We tested this assumption experimentally and found that cleavage of the Leu81-Glu82 peptide bond by CTRC was unchanged in the activation peptide mutants compared to wild-type cationic trypsinogen (Figure 3).
Figure 3.
Cleavage of the Leu81-Glu82 peptide bond in human cationic trypsinogen and activation peptide mutants by chymotrypsin C (CTRC). Wild-type and mutant trypsinogen were incubated at 2 μM with 20 nM CTRC in 0.1 M Tris-HCl (pH 8.0) (final concentrations), at 37 °C. Trypsinogens contained the S200A mutation to prevent autoactivation. A, At the indicated times reactions were terminated by precipitation of 75 μL aliquots (~3.8 μg protein) with 10% trichloroacetic acid (final concentration) and analyzed by 15% reducing SDS-PAGE and Coomassie Blue staining. Representative gels of two or three experiments are shown. B, Densitometric analysis of stained gels showing the changes in the intensity of the unprocessed, intact trypsinogen band. Error bars were omitted for clarity, the error was within 10% of the mean. Mutant K23_I24insIDK is denoted as insIDK.
N-terminal processing of activation peptide mutants by CTRC
CTRC cleaves the Phe18-Asp19 peptide bond in the trypsinogen activation peptide and removes three amino acids from the N terminus (Figure 1) [5]. This, in turn, results in increased autoactivation of cationic trypsinogen. The N-terminal truncation of the activation peptide is readily detectable by non-reducing SDS-PAGE as a small mobility shift. To assess whether the activation peptide mutations altered CTRC-mediated N-terminal processing, we incubated wild-type and mutant trypsinogens with 50 nM CTRC at pH 8.0, in 1 mM CaCl2, to minimize cleavage after Leu81. To prevent autoactivation during the incubation, we used an inactive trypsinogen background in which the catalytic Ser200 was changed to Ala (S200A). Figure 4A and B demonstrate that mutant D19A exhibited 4-fold increased N-terminal processing, whereas mutants D22G, K23R and K23_I24insIDK were processed at rates comparable with wild type. Inspection of the early time points of the autoactivation curves in the presence of CTRC (see Figure 2, compare black with white symbols) reveals that N-terminal processing by CTRC increased the rate of autoactivation for mutants D19A, D22G and K23R. Surprisingly, the enhanced processing of mutant D19A did not translate to a more robust autoactivation increase than seen in mutants D22G or K23R (see Figure 2). Similarly, even though mutant K23_I24insIDK was processed normally, this modification had no impact on autoactivation.
Figure 4.
N-terminal processing of human cationic trypsinogen and activation peptide mutants by chymotrypsin C (CTRC). A, Wild-type and mutant trypsinogen were incubated at 2 μM with 50 nM CTRC in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2 and 100 mM NaCl (final concentrations), at 37 °C. Trypsinogens contained the S200A mutation to prevent autoactivation. At the indicated times reactions were terminated by precipitation of 75 μL aliquots (~3.8 μg protein) with 10% trichloroacetic acid (final concentration) and samples were analyzed by 15% non-reducing SDS-PAGE and Coomassie Blue staining. Relevant segments of representative gels demonstrate the small mobility shift of the trypsinogen band caused by CTRC-mediated cleavage of the activation peptide (see Figure 1). B, Densitometric analysis of stained gels showing the changes in the intensity of the unprocessed, intact trypsinogen band as percent of the total intensity of the processed and unprocessed bands. C, N-terminal processing of the activation peptide is specific for CTRC. Wild-type and mutant trypsinogen were incubated with 50 nM of the indicated human pancreatic chymotrypsins and elastases and reactions were analyzed as described above. For clarity, only the wild-type dataset is shown; none of the mutants was cleaved by any of the proteases tested. Mutant K23_I24insIDK is denoted as insIDK.
CTRC-mediated cleavage of the Phe18-Asp19 peptide bond in the trypsinogen activation peptide is highly specific and other human chymotrypsins (CTRB1, CTRB2, CTRL1) and elastases (ELA2A, ELA3A, ELA3B) do not catalyze this reaction (Figure 4C). We considered the possibility that activation peptide mutations might allow for cleavages by proteases other than CTRC, however, this was not the case. None of the mutant activation peptides was cleaved by any of the chymotrypsins or elastases tested in Figure 4C (data not shown).
Effect of activation peptide mutations on calcium binding
To determine the effect of the activation peptide mutations on the calcium binding affinity of the activation peptide, we measured the rate of N-terminal processing by CTRC as a function of increasing calcium concentrations. For these experiments we used trypsinogen constructs carrying the L81A and S200A mutations to prevent unwanted cleavage at Leu81 by CTRC and to avoid trypsinogen autoactivation during incubations. As shown in Figure 5, calcium inhibited cleavage of the Phe18-Asp19 peptide bond by CTRC in wild-type cationic trypsinogen with a KD value around 1.9 mM. Calcium dependence of N-terminal processing by CTRC was comparable for the activation peptide mutants (Figure 5B). The calculated KD values for mutants D19A, D22G, K23R and K23_I24insIDK were 1.1 mM, 1.3 mM, 2.6 mM and 1.3 mM, respectively. Inspection of Figure 5B suggests that these values fall within the experimental error of the method. When the entire dataset including wild-type and mutants was fitted with a single curve a KD of 1.6±0.2 mM was obtained. We conclude that calcium binding to the activation peptide is not affected by the activation peptide mutations.
Figure 5.
Effect of calcium on the N-terminal processing of the trypsinogen activation peptide by chymotrypsin C (CTRC). A, Wild-type and mutant trypsinogen were incubated at 2 μM with 50 nM CTRC in 0.1 M Tris-HCl (pH 8.0) and 100 mM NaCl in the absence or presence of CaCl2 (Ca2+) at the indicated concentrations, at 37 °C. Trypsinogens contained the S200A mutation to prevent autoactivation and the L81A mutation to prevent CTRC cleavage after Leu81 in the absence of calcium. At the indicated times reactions were terminated and analyzed as described in Figure 4A. As an example, the relevant gel segments for wild type are shown. Similar gel sets were generated for all mutants and used to determine reaction rates, as described below. B, Densitometric analysis was performed as given in Figure 4B. Rates of processing were calculated from linear fits to semilogarithmic graphs and plotted as a function of the calcium concentration with errors of the fits shown. The equilibrium binding constants (KD) for calcium were calculated from fits to the y = y(min)+[y(max)-y(min)] / 1+[Ca2+]/KD equation where y is the measured reaction rate, y(max) is the maximal reaction rate in the absence of calcium and y(min) is the residual reaction rate under fully saturating calcium concentrations. The error of the fits is also indicated. Mutant K23_I24insIDK is denoted as insIDK.
Effect of calcium on autoactivation of activation peptide mutants
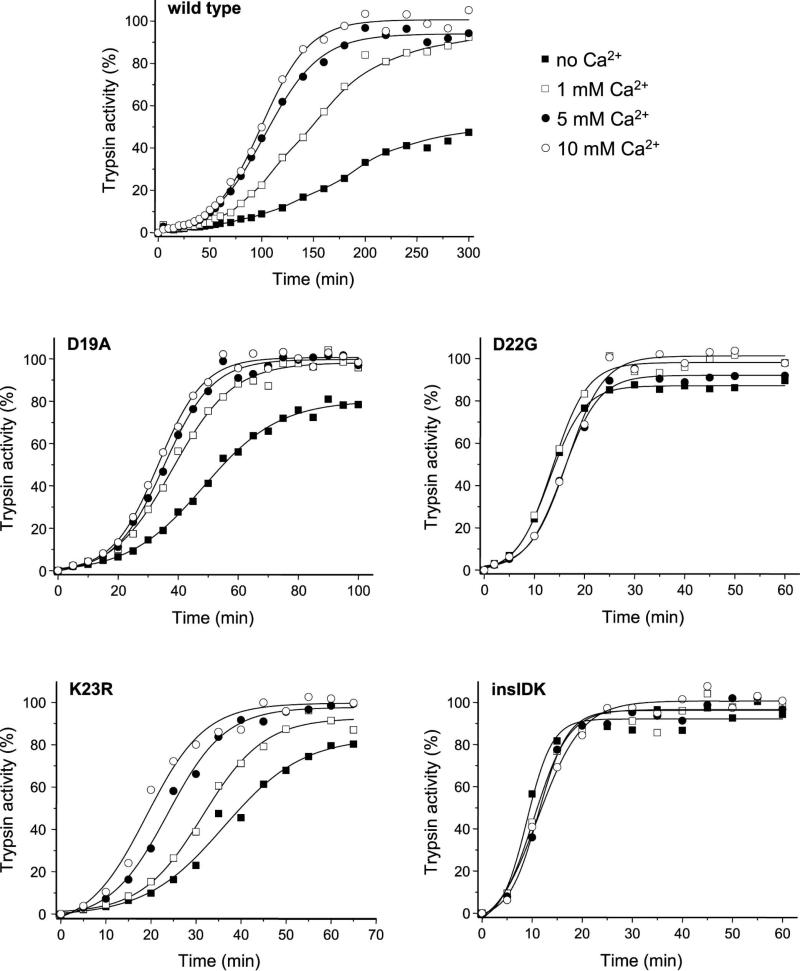
Calcium binding to the tetra-aspartate motif in the trypsinogen activation peptide stimulates autoactivation [14, 15]. Even though the activation peptide mutants appear to bind calcium normally, the effect of calcium on autoactivation may be altered. When autoactivation of wild-type and mutant trypsinogens was measured in the presence of 0, 1, 5 and 10 mM calcium, wild-type trypsinogen and mutants D19A and K23R were stimulated in a concentration dependent manner, whereas autoactivation of mutants D22G and K23_I24insIDK was insensitive to calcium (Figure 6). Although the half-maximal stimulatory calcium concentration was difficult to determine due to the confounding effect of degradation in the absence of calcium, it appeared that calcium dependence of autoactivation of wild-type, D19A and K23R trypsinogens was consistent with the KD values obtained for binding of calcium to the activation peptide. The effect of calcium on the autoactivation of mutant D19A seemed to saturate at a lower concentration when compared to mutant K23R, which might reflect a true but small difference in binding affinity (see KD values above). The observations indicate that activation peptide mutations do not affect binding of calcium to the activation peptide but may diminish the functional effect of calcium binding.
Figure 6.
Effect of calcium on the autoactivation of human cationic trypsinogen and activation peptide mutants. Wild-type and mutant trypsinogen were incubated at 1 μM with 10 nM initial trypsin in 0.1 M Tris-HCl (pH 8.0), 100 mM NaCl, and 0.05% Tween 20, at 37 °C, in the absence or presence of CaCl2 (Ca2+) at the indicated concentrations. At given times 2 μL aliquots were removed and trypsin activity was determined as described in Experimental Procedures. Trypsin activity was expressed as percentage of the maximal activity in the presence of 10 mM CaCl2. Representative experiments from two or three replicates are shown. Mutant K23_I24insIDK is denoted as insIDK.
Secretion of activation peptide mutants from transfected 293T cells
The biochemical properties of the activation peptide mutants described so far suggest that these mutations should be associated with a more severe clinical phenotype than mutation R122H. However, this is not the case. Previously, we found that secretion of activation peptide mutants from transfected cells was reduced, although the four mutants have never been studied in a comparative manner within the same experiment [12, 13]. We also demonstrated that the secretion loss was related to intracellular autoactivation and degradation [13]. To establish whether this secretion defect might be a mechanism that partly offsets the drastically increased autoactivation, we quantified secretion of wild-type and mutant trypsinogens from transiently transfected human embryonic kidney (HEK) 293T cells. The use of HEK 293T cells for cellular secretion studies is a compromise, as efficient transfection of pancreatic acinar cells is not feasible. For these experiments we used a short time course (12 h) to prevent autoactivation in the medium and consequent trypsinization of the cells. We measured trypsin activity in the conditioned media after enteropeptidase-mediated activation (Figure 7A) and trypsinogen levels by western blotting (Figure 7B). We found that the activation peptide mutants were secreted at significantly lower levels than wild-type trypsinogen, in agreement with previous observations [12, 13]. Importantly, secretion rates for the mutants were inversely proportional with their ability to autoactivate (D19A > K23R > D22G ≈ K23_I24insIDK).
Figure 7.
Secretion of human cationic trypsinogen and activation peptide mutants from transiently transfected HEK 293T cells. Trypsinogen expression constructs contained the K237D and N241D mutations which improve secretion from transfected cells [22]. A, At 4, 8 and 12 hours after transfection conditioned media were collected and trypsin activity was measured as described under Experimental Procedures. Trypsin activity was expressed as percent of the 12 h wild-type activity. The average of three independent transfection experiments with standard deviation is shown. B, Aliquots (20 μL) of conditioned media collected at 12 h were electrophoresed on 15% SDS-polyacrylamide gels and analyzed by western blotting, as described in Experimental Procedures. A representative blot of three is shown. Mutant K23_I24insIDK is denoted as insIDK.
DISCUSSION
The primary objective of the present study was to investigate the effect of CTRC on the autoactivation of trypsinogen activation peptide mutants D19A, D22G, K23R and K23_I24insIDK found in hereditary pancreatitis. Previously, we demonstrated that wild-type cationic trypsinogen is largely degraded by CTRC during autoactivation, whereas hereditary pancreatitis associated mutants N29I, N29T, V39A, R122C and R122H exhibited resistance to CTRC-mediated degradation and autoactivated to higher trypsin levels [3]. In addition, we found that mutations A16V and N29I increased CTRC-mediated cleavage of the activation peptide, and thereby accelerated autoactivation (see Figure 1) [3, 5]. We speculated that activation peptide mutants might also affect processing of the activation peptide by CTRC and/or alter the effect of this cleavage, perhaps even resulting in decreased autoactivation. This would then offer an explanation for the puzzling observation that the robust autoactivation of activation peptide mutants is not accompanied by a more severe clinical picture.
Our results confirmed that even in the absence of CTRC activation peptide mutants autoactivated at markedly increased rates (~4-18-fold) [9, 12]. In the presence of CTRC, activation rates were further increased slightly (D19A, D22G, K23R) or remained unchanged (K23_I24insIDK), indicating that the robust autoactivation of the activation peptide mutants is mostly independent of CTRC. Peak trypsin levels attained during autoactivation positively correlated with the rate of autoactivation, indicating that faster conversion of trypsinogen to trypsin results in higher trypsin levels, as trypsin is less sensitive to CTRC-mediated degradation than trypsinogen. As expected, the mutations had no effect on CTRC cleavage of the Leu81-Glu82 peptide bond in the calcium binding loop and consequent trypsinogen degradation. Importantly, when compared to the most common pancreatitis-associated mutant R122H, in the presence of CTRC the activation peptide mutants still exhibited much higher rates of autoactivation (~3-10-fold).
N-terminal processing of the activation peptide at Phe18 by CTRC was unchanged in mutants D22G, K23R and K23_I24insIDK, whereas mutant D19A was processed at 4-fold increased rate. This finding is consistent with recent mutagenesis studies in the calcium binding loop of cationic trypsinogen, which demonstrated that mutation of Glu82 to Ala increased cleavage after Leu81 about 3-fold, indicating that acidic residues at the P1' position hinder cleavage by CTRC [16]. Surprisingly, however, the increased processing of mutant D19A was paralleled only with a slight increase in the rate of autoactivation, suggesting that Asp19 is important for mediating the functional effect of N-terminal processing. Thus, shortening the activation peptide by CTRC may increase autoactivation by partly relieving the inhibitory interaction between Asp218 and Asp19 [5, 7]. This effect is probably due to neutralization of the negative charge on Asp19 by the newly created proximity of the positively charged amino terminus. Similarly, mutation D19A would increase autoactivation by neutralizing Asp19 but at the same time it would diminish the effect of N-terminal processing by CTRC. This notion is supported by the observations that the extent of the autoactivation increase either by CTRC processing or by mutation D19A is comparable, approximately 3-4-fold [5, 9]. The stimulatory effect of CTRC cleavage on autoactivation was also abolished in mutant K23_I24insIDK, which may be readily explained by sterical uncoupling of the original tetra-Asp motif from the activation site by the three-amino-acid insertion (see Figure 1). Furthermore, previous experiments indicated that trypsin-mediated activation (i.e. autoactivation) of the K23_I24insIDK mutant most likely proceeds by sequential cleavage of the two lysyl peptide bonds found in the mutated activation peptide [12]. In this case, cleavage of the N-terminal Lys-Ile peptide bond would eliminate the N-terminal eight amino acids together with any effect of CTRC-dependent processing (see Figure 1).
Calcium in millimolar concentrations stimulates autoactivation presumably by binding to the activation peptide and shielding the inhibitory negative charges of the tetra-Asp motif [14, 15]. Using the CTRC-dependent processing of the activation peptide as readout, we determined the calcium binding affinity of wild-type and mutant activation peptides (see Figure 5). Unexpectedly, mutant and wild-type trypsinogens bound calcium at the activation peptide with comparable affinities, 1.6 mM on average. This observation suggests that calcium probably engages only two or three Asp side chains and elimination of one of the four Asp residues either by mutation D19A or D22G is tolerated. When the functional effect of calcium binding was investigated, however, we found that autoactivation of mutants D22G and K23_I24insIDK were insensitive to calcium. The results seem to lend some credence to the speculation that the stimulatory effect calcium on autoactivation is mediated through binding to Asp22 and neutralizing its negative charge. During autoactivation, Asp22 in the activation peptide binds to the S2 subsite on trypsin, a conserved hydrophobic pocket formed by His63, Leu104 and Trp216, and this unfavorable interaction may be alleviated by calcium. In the K23_I24insIDK mutant, calcium likely binds only to the original tetra-Asp motif, which is now further removed from the activation peptide bond. The Asp-Lys-Ile-Asp sequence preceding the activation site does not seem competent to bind calcium. Furthermore, as pointed out above, during autoactivation the N-terminal eight amino acids are removed before cleavage at the activation peptide bond takes place (see Figure 1) [12]. Alternatively, another plausible interpretation for the data may be that for wild-type trypsinogen and mutants D19A and K23R the rate limiting step in the autoactivation reaction is calcium dependent, whereas for the rapidly autoactivating mutants D22G and K23_I24insIDK a calcium-insensitive step becomes rate determining.
The biochemical properties of the trypsinogen activation peptide mutants do not explain why the markedly increased autoactivation is not associated with a more severe clinical presentation of hereditary pancreatitis; i.e. with complete penetrance and earlier onset. Typical penetrance of hereditary pancreatitis in families with the R122H mutation is 70-90% and the median age of onset is 12 years with wide individual variability [1, 17]. Although clinical data for the rare activation peptide mutations is relatively scant, mutation D22G was also identified in the unaffected 20 year old sister of the index patient, indicating incomplete penetrance [10]. Published ages of diagnosis indicated both early onset (D22G 8 y, K23_I24insIDK 2 y) and late onset cases (D19A 17 y, K23_I24insIDK 21 y), consistent with typical hereditary pancreatitis [9, 10, 12]. Previously, we observed that secretion of activation peptide mutants was compromised from transfected cells due to intracellular autoactivation and ensuing degradation [12, 13]. We extended these studies here and quantitatively compared, for the first time, cellular secretion of all four activation peptide mutants to determine whether reduced secretion could offset the effect of increased autoactivation. Indeed, we confirmed not only that all four activation peptide mutants were secreted to lower levels than wild-type trypsinogen, but also that secretion rates inversely correlated with the rates of autoactivation. Our observations indicate that nature carefully titrated trypsinogen secretion against autoactivation propensity, to curb the risk for excessive pathological intra-pancreatic trypsinogen activation in carriers of the activation peptide mutations.
In summary, our results define the trypsinogen activation peptide mutations D19A, D22G, K23R and K23_I24insIDK as a special subset of hereditary-pancreatitis associated mutations which stimulate autoactivation largely independently of CTRC and this robust effect is compensated by their reduced secretion. Taken together with previous studies, the observations indicate that human cationic trypsinogen mutations may increase autoactivation by several independent but not mutually exclusive mechanisms in hereditary pancreatitis (Figure 8): (i) increased secretion, as suggested for copy number mutations [18, 19]; (ii) resistance to CTRC-mediated degradation [3, 4]; (iii) increased processing of the activation peptide by CTRC [3, 5] and (iv) direct stimulation of autoactivation, as demonstrated here for the activation peptide mutations.
Figure 8.
Mechanisms of increased autoactivation in hereditary pancreatitis associated with human cationic trypsinogen mutations. Copy number mutations (CNM) increase trypsinogen expression. Mutations N29I, N29T, V39A, R122C and R122H inhibit CTRC-dependent trypsinogen degradation. Mutations A16V and N29I stimulate N-terminal processing of the trypsinogen activation peptide by CTRC. Activation peptide mutations D19A, D22G, K23R and K23_I24insIDK directly stimulate autoactivation. A prominent example for each mechanism is indicated. See text for details.
EXPERIMENTAL PROCEDURES
Nomenclature
Amino-acid residues in human cationic trypsinogen were numbered starting with the initiator methionine of the primary translation product, in accordance with the recommendations of the Human Genome Variation Society.
Plasmid construction and mutagenesis
The pTrapT7 intein-PRSS1, pcDNA3.1(-) PRSS1 and other expression plasmids harboring the coding DNA for human pancreatic digestive proteases have been described previously. [4, 5, 20, 21]. To increase expression levels in transfected HEK 293T cells, the trypsinogen activation peptide mutations were transferred to the pcDNA3.1(-) K237D/N241D background [22]. Mutations in human cationic trypsinogen were generated by PCR mutagenesis, cloned into the expression plasmids and verified by DNA sequencing.
Expression and purification of protease zymogens
Wild-type and mutant trypsinogens were expressed in the aminopeptidase P deficient LG-3 E. coli strain as fusions with a self-splicing mini-intein, as described in [20, 23]. This expression system produces recombinant trypsinogen with uniform, authentic N termini. Refolding and purification of trypsinogen by ecotin affinity chromatography was carried out as reported previously [23] with the following modification. To stabilize trypsinogens against autoactivation, the elution solution contained 50 mM HCl and 100 mM NaCl. Concentrations of trypsinogen preparations were determined from the UV absorbance at 280 nm using the extinction coefficient 37,525 M-1 cm-1.
Expression in E. coli, in vitro refolding and purification of human proelastase ELA2A was performed as described previously [21]. Histidine-tagged forms of human chymotrypsinogens CTRB1, CTRB2, CTRC and CTRL1 and proelastases ELA3A and ELA3B were expressed in HEK 293T cells and purified from the conditioned medium using nickel-affinity chromatography, as reported previously [3, 16]. ELA2A was activated using 10 nM human anionic trypsin [21] and other proteases were activated with immobilized bovine trypsin (Pierce/Thermo Fisher Scientific, Rockford, IL) in 0.1 M Tris-HCl (pH 8.0) and 0.05% Tween 20 (final concentrations) and the trypsin beads were removed by centrifugation. Active protease concentrations were determined by active site titration with ecotin, as described [24].
Trypsinogen autoactivation
Trypsinogen at 1 μM concentration was incubated in the absence or presence of 25 nM human CTRC, as indicated, and 10 nM cationic trypsin in 0.1 M Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM CaCl2 and 0.05% Tween 20 (final concentrations) at 37 °C. At given times, 2 μL aliquots were withdrawn and mixed with 48 μL assay buffer containing 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2, and 0.05% Tween 20. Trypsin activity was measured by adding 150 μL 200 μM N-CBZ-Gly-Pro-Arg-p-nitroanilide substrate (dissolved in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2 and 0.05% Tween 20) and following the release of the yellow p-nitroanilin at 405 nm in a SpectraMax plus 384 microplate reader (Molecular Devices, Sunnyvale, CA) for 1 min. Reaction rates were calculated from fits to the initial linear portions of the curves. Note that in the present study all experiments were performed in the presence of 100 mM NaCl, which resulted in slower autoactivation, compared to previous studies.
Cell culture and transfection
HEK 293T cells were cultured and transfected as described previously [25]. Transfections were performed using 2 μg expression plasmid and 5 μL Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in 2 mL Dulbecco's Modified Eagle Medium (Invitrogen). After overnight incubation, cells were washed and the transfection medium was replaced with 1 mL OPTI-MEM I Reduced Serum Medium (Invitrogen). Time courses of expression were measured starting from this medium change and were followed for 12 hours. Trypsin activity in the conditioned media was measured after enteropeptidase activation, as described in [26].
Gel electrophoresis and densitometry
Trypsinogen samples (75 μL of a 2 μM solution, corresponding to approximately 3.8 μg protein) were precipitated with trichloroacetic acid (10% final concentration), and the precipitate was recovered by centrifugation, dissolved in 20 μL Laemmli sample buffer with 100 mM dithiothreitol (final concentration), and heat-denatured at 95 °C for 5 min. Where non-reducing conditions are indicated, dithiothreitol was omitted from the sample buffer. Electrophoretic separation was performed on 15% SDS-polyacrylamide mini gels in standard Tris glycine buffer. Gels were stained with Brilliant Blue R-250 and destained as described earlier [15]. Quantitation of bands was carried out with the Quantity One 4.6.9 software (Bio-Rad, Hercules, CA).
Western blot analysis
Aliquots (20 μL) of conditioned media were mixed with sample buffer, heat-denatured, electrophoresed on Tris-glycine minigels and transferred onto an Immobilon-P membrane (Millipore Corporation, Bedford, MA) at 300 mA for 1.5 h. The membrane was blocked with 5% milk powder dissolved in phosphate-buffered saline supplemented with 0.1% Tween 20 (final concentration), at 4 °C overnight. Trypsinogen was detected with a sheep polyclonal antibody (#AF3848, R&D Systems, Minneapolis, MN) used at a dilution of 1:2000 followed by horse-radish peroxidase (HRP)-conjugated donkey polyclonal anti-sheep IgG (#HAF016, R&D Systems) used at 1:2000 dilution, as described previously [26].
ACKNOWLEDGMENTS
This work was supported by NIH grants R01DK058088, R01DK082412, R01DK082412-S2 and R01DK095753 (to M.S-T). A.G. was also supported by a mini-sabbatical grant from the American Pancreatic Association. The authors thank András Szabó for help with plasmid construction and trypsinogen degradation assays; and Éva Kereszturi and Béla Ózsvári for performing preliminary experiments on calcium binding to the activation peptide.
REFERENCES
- 1.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 2.Szmola R, Sahin-Tóth M. Uncertainties in the classification of human cationic trypsinogen (PRSS1) variants as hereditary pancreatitis-associated mutations. J Med Genet. 2010;47:348–350. doi: 10.1136/jmg.2009.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabó A, Sahin-Tóth M. Increased activation of hereditary pancreatitis-associated human cationic trypsinogen mutants in presence of chymotrypsin C. J Biol Chem. 2012;287:20701–20710. doi: 10.1074/jbc.M112.360065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szmola R, Sahin-Tóth M. Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht's enzyme Y. Proc Natl Acad Sci USA. 2007;104:11227–11232. doi: 10.1073/pnas.0703714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem. 2006;281:11879–11886. doi: 10.1074/jbc.M600124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu D, Fütterer K, Korolev S, Zheng X, Tan K, Waksman G, Sadler JE. Crystal structure of enteropeptidase light chain complexed with an analog of the trypsinogen activation peptide. J Mol Biol. 1999;292:361–373. doi: 10.1006/jmbi.1999.3089. [DOI] [PubMed] [Google Scholar]
- 7.Nemoda Z, Sahin-Tóth M. The tetra-aspartate motif in the activation peptide of human cationic trypsinogen is essential for autoactivation control but not for enteropeptidase recognition. J Biol Chem. 2005;280:29645–29652. doi: 10.1074/jbc.M505661200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witt H, Luck W, Becker M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology. 1999;117:7–10. doi: 10.1016/s0016-5085(99)70543-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen JM, Kukor Z, Le Maréchal C, Tóth M, Tsakiris L, Raguénès O, Férec C, Sahin-Tóth M. Evolution of trypsinogen activation peptides. Mol Biol Evol. 2003;20:1767–1777. doi: 10.1093/molbev/msg183. [DOI] [PubMed] [Google Scholar]
- 10.Teich N, Ockenga J, Hoffmeister A, Manns M, Mössner J, Keim V. Chronic pancreatitis associated with an activation peptide mutation that facilitates trypsin activation. Gastroenterology. 2000;119:461–465. doi: 10.1053/gast.2000.9312. [DOI] [PubMed] [Google Scholar]
- 11.Férec C, Raguénès O, Salomon R, Roche C, Bernard JP, Guillot M, Quéré I, Faure C, Mercier B, Audrézet MP, Guillausseau PJ, Dupont C, Munnich A, Bignon JD, Le Bodic L. Mutations in the cationic trypsinogen gene and evidence for genetic heterogeneity in hereditary pancreatitis. J Med Genet. 1999;36:228–232. [PMC free article] [PubMed] [Google Scholar]
- 12.Joergensen MT, Geisz A, Brusgaard K, Schaffalitzky de Muckadell OB, Hegyi P, Gerdes AM, Sahin-Tóth M. Intragenic duplication: a novel mutational mechanism in hereditary pancreatitis. Pancreas. 2011;40:540–546. doi: 10.1097/MPA.0b013e3182152fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kereszturi E, Sahin-Tóth M. Intracellular autoactivation of human cationic trypsinogen mutants causes reduced trypsinogen secretion and acinar cell death. J Biol Chem. 2009;284:33392–33399. doi: 10.1074/jbc.M109.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abita JP, Delaage M, Lazdunski M. The mechanism of activation of trypsinogen. The role of the four N-terminal aspartyl residues. Eur J Biochem. 1969;8:314–324. doi: 10.1111/j.1432-1033.1969.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 15.Kukor Z, Tóth M, Sahin-Tóth M. Human anionic trypsinogen: properties of autocatalytic activation and degradation and implications in pancreatic diseases. Eur J Biochem. 2003;270:2047–2058. doi: 10.1046/j.1432-1033.2003.03581.x. [DOI] [PubMed] [Google Scholar]
- 16.Szabó A, Sahin-Tóth M. Determinants of chymotrypsin C cleavage specificity in the calcium-binding loop of human cationic trypsinogen. FEBS J. 2012;279:4283–4292. doi: 10.1111/febs.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM, Uomo G, Delhaye M, Spicak J, Drumm B, Jansen J, Mountford R, Whitcomb DC, Neoptolemos JP, European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC) Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 18.Le Maréchal C, Masson E, Chen JM, Morel F, Ruszniewski P, Levy P, Férec C. Hereditary pancreatitis caused by triplication of the trypsinogen locus. Nat Genet. 2006;38:1372–1374. doi: 10.1038/ng1904. [DOI] [PubMed] [Google Scholar]
- 19.Masson E, Le Maréchal C, Chandak GR, Lamoril J, Bezieau S, Mahurkar S, Bhaskar S, Reddy DN, Chen JM, Férec C. Trypsinogen copy number mutations in patients with idiopathic chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6:82–88. doi: 10.1016/j.cgh.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Király O, Guan L, Szepessy E, Tóth M, Kukor Z, Sahin-Tóth M. Expression of human cationic trypsinogen with an authentic N terminus using intein-mediated splicing in aminopeptidase P deficient Escherichia coli. Protein Expr Purif. 2006;48:104–111. doi: 10.1016/j.pep.2006.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szepessy E, Sahin-Tóth M. Inactivity of recombinant ELA2B provides a new example of evolutionary elastase silencing in humans. Pancreatology. 2006;6:117–122. doi: 10.1159/000090031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rónai Z, Witt H, Rickards O, Destro-Bisol G, Bradbury AR, Sahin-Tóth M. A common African polymorphism abolishes tyrosine sulfation of human anionic trypsinogen (PRSS2). Biochem J. 2009;418:155–161. doi: 10.1042/BJ20081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Király O, Guan L, Sahin-Tóth M. Expression of recombinant proteins with uniform N-termini. Methods Mol Biol. 2011;705:175–194. doi: 10.1007/978-1-61737-967-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabó A, Héja D, Szakács D, Zboray K, Kékesi KA, Radisky ES, Sahin-Tóth M, Pál G. High affinity small protein inhibitors of human chymotrypsin C (CTRC) selected by phage display reveal unusual preference for P4' acidic residues. J Biol Chem. 2011;286:22535–22545. doi: 10.1074/jbc.M111.235754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beer S, Zhou J, Szabó A, Keiles S, Chandak GR, Witt H, Sahin-Tóth M. Comprehensive functional analysis of chymotrypsin C (CTRC) variants reveals distinct loss-of-function mechanisms associated with pancreatitis risk. Gut. 2012 doi: 10.1136/gutjnl-2012-303090. Published Online First: 1 September 2012, doi:10.1136/gutjnl-2012-303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnúr A, Beer S, Witt H, Hegyi P, Sahin-Tóth M. Functional effects of 13 rare PRSS1 variants presumed to cause chronic pancreatitis. Gut. 2013 doi: 10.1136/gutjnl-2012-304331. Published Online First: 1 March 2013, doi:10.1136/gutjnl-2012-304331. [DOI] [PMC free article] [PubMed] [Google Scholar]