Abstract
The proinflammatory cytokine interleukin-1β (IL-1β), whose levels are elevated in the brain in Alzheimer’s and other neurodegenerative diseases, has been shown to have both detrimental and beneficial effects on disease progression. In this paper, we demonstrate that incubation of mouse primary cortical neurons (mPCNs) with IL-1β increases the expression of the P2Y2 nucleotide receptor (P2Y2R) and that activation of the upregulated receptor with UTP, a relatively selective agonist of the P2Y2R, increases neurite outgrowth. Consistent with the accepted role of cofilin in the regulation of neurite extension, results indicate that incubation of IL-1β-treated mPCNs with UTP increases the phosphorylation of cofilin, a response absent in PCNs isolated from P2Y2R−/− mice. Other findings indicate that function blocking anti-αvβ3/5 integrin antibodies prevent UTP-induced cofilin activation in IL-1β-treated mPCNs, suggesting that established P2Y2R/αvβ3/5 interactions that promote G12-dependent Rho activation lead to cofilin phosphorylation involved in neurite extension. Cofilin phosphorylation induced by UTP in IL-1β-treated mPCNs is also decreased by inhibitors of Ca2+/calmodulin-dependent protein kinase II (CaMKII), suggesting a role for P2Y2R-mediated and Gq-dependent calcium mobilization in neurite outgrowth. Taken together, these studies indicate that upregulation of P2Y2Rs in mPCNs under proinflammatory conditions can promote cofilin-dependent neurite outgrowth, a neuroprotective response that may be a novel pharmacological target in the treatment of neurodegenerative diseases.
Keywords: cofilin, extracellular nucleotides, IL-1β, neurite outgrowth, neuroinflammation, neuroprotection, P2 receptors
Introduction
Neurodegenerative diseases are a serious cause of mortality in the United States with more than 5 million people currently afflicted (Duncan 2011, Thies & Bleiler 2011). Alzheimer’s disease (AD) is the most prevalent of these conditions and it is predicted that AD will affect 80 million people worldwide within 30 years (Blennow et al. 2006, Thies & Bleiler 2011). There are currently no effective treatments to prevent the onset or delay the progression of neurological deficits that degrade the quality of life of AD patients for many years prior to death (Thies & Bleiler 2011). It is now widely accepted that chronic inflammation plays a role in the progression of neurological changes observed in the AD brain, including neuronal loss and degeneration of neurological functions (Zilka et al. 2006, Lee et al. 2010, Obulesu et al. 2011, Wyss-Coray & Rogers 2012). Nevertheless, the initiating factors in AD remain obscure and whether neuroinflammation is primarily a neurodegenerative or a neuroprotective response in AD is an area of intense investigation (Zilka et al. 2006, Lee et al. 2010, Broussard et al. 2012).
Chronic inflammation in the central nervous system (CNS) is a conspicuous feature of many neurodegenerative diseases, including AD, Parkinson’s disease and multiple sclerosis (Akiyama et al. 2000, Rothwell & Luheshi 2000, Broussard et al. 2012). A key cytokine associated with the neuroinflammatory phenotype is IL-1β, a proinflammatory cytokine produced by microglial cells and macrophages that regulates the production of other proinflammatory cytokines (e.g., TNF-α, IL-6 and interferons) and chemokines (e.g., CXCL1 and CXCL2) (Rothwell & Luheshi 2000, Shaftel et al. 2008). Although studies have investigated the neurodegenerative roles of IL-1β in AD progression (Rothwell & Luheshi 2000, Shaftel et al. 2008), observations in a mouse model of AD indicate that overexpression of IL-1β in the hippocampus can promote phagocyte recruitment and the clearance of β-amyloid plaques (Shaftel et al. 2007b). This suggests that IL-1β can also serve a neuroprotective role in the CNS that requires further investigation.
Recently, we demonstrated that the P2Y2 nucleotide receptor (P2Y2R), a G protein-coupled receptor that is activated equally well by ATP and UTP, is upregulated in rat primary cortical neurons in response to IL-1β (Kong et al. 2009). Subsequent activation of the P2Y2R by extracellular nucleotides promotes the non-amyloidogenic processing of amyloid precursor protein (APP) (Camden et al. 2005, Kong et al. 2009). In mouse primary microglial cells, the P2Y2R is upregulated by the neurotoxic β-amyloid (Aβ1-42) peptide associated with AD pathogenesis, whereupon activation of the microglial P2Y2R enhances Aβ phagocytosis and degradation (Kim et al. 2012), suggesting that P2Y2R upregulation and P2Y2R-mediated non-amyloidogenic APP processing are neuroprotective responses that prevent excessive neurotoxic Aβ1-42 accumulation. Other studies have found that activation of ionotropic P2X7 receptors in microglial cells by extracellular ATP, a pathway that induces cell apoptosis, increases both IL-1β and ATP release from microglia (Takenouchi et al. 2009, Takenouchi et al. 2011), thereby providing the agonists for both P2Y2R upregulation and activation. Other potential neuroprotective responses to P2Y2R activation include the induction of intracellular calcium waves (Halassa et al. 2009), the upregulation of anti-apoptotic protein expression in astrocytes (Chorna et al. 2004) and the enhancement of neuronal differentiation and survival (Arthur et al. 2005, Pooler et al. 2005, Arthur et al. 2006a, Arthur et al. 2006b). Thus, P2Y2Rs in neurons, microglial cells and astrocytes likely coordinately regulate neuroprotective responses to elevated levels of extracellular nucleotides that occur under proinflammatory, proapoptotic and necrotic conditions (Peterson et al. 2010, Weisman et al. 2012a, Weisman et al. 2012b) and may prevent or delay neurodegeneration. Therefore, P2Y2Rs represent promising pharmacological targets in the treatment of AD and other diseases of the CNS.
This study was undertaken to further evaluate the role of P2Y2Rs in mPCNs, in particular the mechanism underlying the effect of extracellular nucleotides on neurite extension, a neuroprotective pathway that has not been characterized. It has been established that neurite outgrowth in response to activation of other G protein-coupled receptors requires the sequential activation of Rho, ROCK, LIMK and cofilin (Meng et al. 2002, Bamburg et al. 2010, Bernstein & Bamburg 2010). Furthermore, we have previously demonstrated that activation of the P2Y2R can increase Rho and ROCK activities due to the presence of an Arg-Gly-Asp (RGD) sequence in the first extracellular loop of the P2Y2R that promotes its direct binding to αvβ3/5 integrins, an interaction required for extracellular nucleotides to activate heterotrimeric G12 protein and subsequently the small G protein Rho (Erb et al. 2001, Bagchi et al. 2005, Liao et al. 2007). In the present study, we utilized mPCNs to demonstrate that upregulation of the P2Y2R due to the proinflammatory cytokine IL-1β followed by P2Y2R activation with UTP increases both cofilin phosphorylation and neurite outgrowth. Furthermore, UTP-induced cofilin phosphorylation and neurite outgrowth was found to be absent in mPCNs from P2Y2R−/− mice and occurred via a pathway involving αvβ3/5 and CaMKII. These results strongly suggest that by virtue of P2Y2R interactions with αvβ3/5 integrins, nucleotides can activate Rho-dependent cofilin phosphorylation to regulate cytoskeletal rearrangements required for neurite extension and stabilization, which is critical for neuronal survival.
Methods
Reagents
Fetal bovine serum was obtained from Hyclone (Logan, UT). High glucose Dulbecco’s modified Eagle’s medium (HG-DMEM), Neurobasal medium, penicillin (100 units/ml), streptomycin (100 units/ml) and B27-AO were obtained from Gibco-BRL (Carlsbad, CA). The Dual Color Protein Standards and nitrocellulose membranes (0.45 µm) were obtained from Bio-Rad (Hercules, CA). LumiGLO chemiluminescent substrates were obtained from New England Biolabs (Beverly, MA). The RNeasy Plus Mini Kit was obtained from Qiagen (Chatsworth, CA). The First Strand cDNA Synthesis Kit was obtained from Roche (Indianapolis, IN). Real-time PCR was performed on an Applied Biosystems 7500 Real-Time PCR machine with TaqMan Gene Expression assay probes (P2Y2R, NM_017255.1 and GAPDH, NM_017008.3) and TaqMan Universal PCR Master Mix (2X) from Applied Biosystems (Foster City, CA). Function blocking studies with the αV integrin utilized anti-integrin αVβ3 antibody (ab78289) produced by AbCam (Cambridge, MA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless stated otherwise.
Mice
C57BL/6 (wild type), C57BL/6-Tg(UBC-GFP)30Scha/J (GFP-wild type) and P2Y2R−/− mice on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the Christopher S. Bond Life Sciences Center Animal Facility of the University of Missouri, Columbia, MO. P2Y2R−/− mice were bred to GFP-wild type mice until a P2Y2R−/− was established with the ubiquitous expression of GFP (GFP-P2Y2R−/−). Animals were housed in vented cages with 12 h light/dark cycles and received food and water ad libitum. All animals were handled using protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Missouri (protocol no. 6728).
Primary cell culture of cortical neurons
Preparation of 95% pure mouse primary cortical neurons (mPCNs) was carried out as previously described with minor modifications (Kong et al. 2009). Briefly, cerebral cortices from 17-day-old embryos of wild type, GFP-wild type, P2Y2R−/− or GFP- P2Y2R−/− mice were removed and the meninges discarded. The tissue was mechanically dissociated in HG-DMEM comprised of 10% (v/v) fetal bovine serum (FBS), 2 mM glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin and 7.5 mg/ml fungizone. The tissue clumps were dispersed with a 10 ml pipette and suspended in 6 ml of 0.25% (w/v) trypsin at 37°C for 30 min. Then, 2 ml of heat-inactivated horse serum were added to neutralize trypsin activity and the suspension was triturated with a fire-polished Pasteur pipette. The cell suspension was centrifuged at 900 × g for 2 min and the cell pellet was suspended in HG-DMEM. The resulting cell suspension was filtered through a sterilized 75 µm cell strainer (Becton Dickinson, Franklin Lakes, NJ) and the cells were seeded at 500 cells/mm2 in plastic culture plates pre-coated with poly-D-lysine or poly-L-lysine (0.1 µg/ml). After 16–18 h and every 3 days thereafter, half the medium was replaced with B27-AO Neurobasal medium (2 mM glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin, 7.5 mg/ml fungizone, 10 ml of B27-AO and Neurobasal medium to 500 ml). After 6 days in vitro (DIV6) at 37°C in a humidified atmosphere of 95% air and 5% CO2, neurons were pretreated for 24 h at 37°C in HG-DMEM containing 5% (v/v) FBS with or without IL-1β at the indicated concentration and used for experiments on DIV7.
Real-Time and Reverse Transcription-PCR analysis of P2Y2R mRNA expression
Total RNA was isolated from mPCNs using the RNeasy Plus Mini Kit (Qiagen). cDNA was synthesized from 500 ng of purified RNA using the First Strand cDNA Synthesis Kit for RT-PCR (AMV; Roche). Ten percent of the synthesized cDNA was used as a template in 50 μl real-time PCR reactions. For TaqMan quantitative real-time PCR analysis, the probes were labeled at the 5’ end with 6-carboxy-fluorescein phosphoramidite (FAM) (for P2Y2R ) or VIC® (for GAPDH ; stable endogenous control; Applied Biosystems, Foster City, CA) and at the 3’ end with minor groove binder (MGB) dye as the quencher. The samples were run in quadruplicate for the P2Y2R target and the endogenous GAPDH control. The relative levels of P2Y2R and GAPDH in each sample were determined and expressed as a ratio of P2Y2R to GAPDH (normalized to 1) using Applied Biosystems software.
Western analysis
As described previously, mPCNs were cultured on 6-well plates and grown for 6 days in vitro (Kong et al. 2009). Cells were serum-starved and incubated in 1 ml of B27-AO Neurobasal medium with or without indicated compounds for specified time periods, as described in the figure legends. Samples were lysed in an equal volume of 2X Laemmli buffer (20 mM sodium phosphate, pH 7.0, 20% (v/v) glycerol, 4% (w/v) SDS, 0.01% (w/v) bromophenol blue and 100 mM DTT) and analyzed by Western blot analysis. Total cofilin was used as a loading control. Cell lysates were heated for 5 min at 95°C, subjected to 7.5% (w/v) SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes for protein immunoblotting. After overnight blocking at 4°C with 5% (w/v) fat-free milk in TBS-T (10 mM Tris-HCl, pH 7.4, 120 mM NaCl and 0.1% (v/v) Tween-20), membranes were incubated with either 1:1000 dilution of rabbit anti-cofilin (#3312) or rabbit anti-phospho-cofilin (Ser3; 77G2; #3313) antibody (Cell Signaling, Danvers, MA) overnight at 4°C followed by incubation with HRP-conjugated anti-rabbit IgG antibody (1:1000 dilution in TBS-T containing 5% (w/v) fat-free milk) for 1 h at room temperature. Protein immunoreactivity was visualized on autoradiographic film using chemiluminescence. The protein bands detected on X-ray film were quantified using a computer-driven scanner and Quantity One software (Bio-Rad, Hercules, CA). The level of phospho-cofilin in each sample was expressed as a percentage of total cofilin.
Image analysis
Primary cortical neurons from GFP-wild type or GFP-P2Y2R−/− mice were pretreated with IL-1β (100 ng/ml) or vehicle for 24 h in serum-free media. Cells were imaged in real time on a Zeiss LSM 510 META NLO inverted microscope equipped with an incubation chamber maintained at 37°C with 95% air and 5% CO2. UTP (100 00B5M) or vehicle was added and cells were imaged at 0, 30, 60 and 120 min. Neurite outgrowth was quantified at these time points by measuring the percent increase in neuron perimeter, using 4–8 images per condition from 3–6 independent experiments. Perimeters were calculated using image analysis software (Adobe Photoshop CS4 or ImageJ v. 1.46r). Perimeter distances in pixels or microns were used to calculate percent increases in the cell perimeter, as compared to the initial perimeter. Videos were captured on a Nikon Eclipse Ti inverted microscope with an incubation chamber maintained at 37°C with 95% air and 5% CO2.
Statistical analysis
Results are expressed as the means ± S.E.M. of data obtained from at least 3 experiments. Statistical analysis of data was performed using Graph Pad Prism version 5.0. Statistical significance was determined by a one tailed Student’s t-test between groups. Differences were considered to be statistically significant when P < 0.05.
Results
IL-1β increases the expression of P2Y2R mRNA in mPCNs
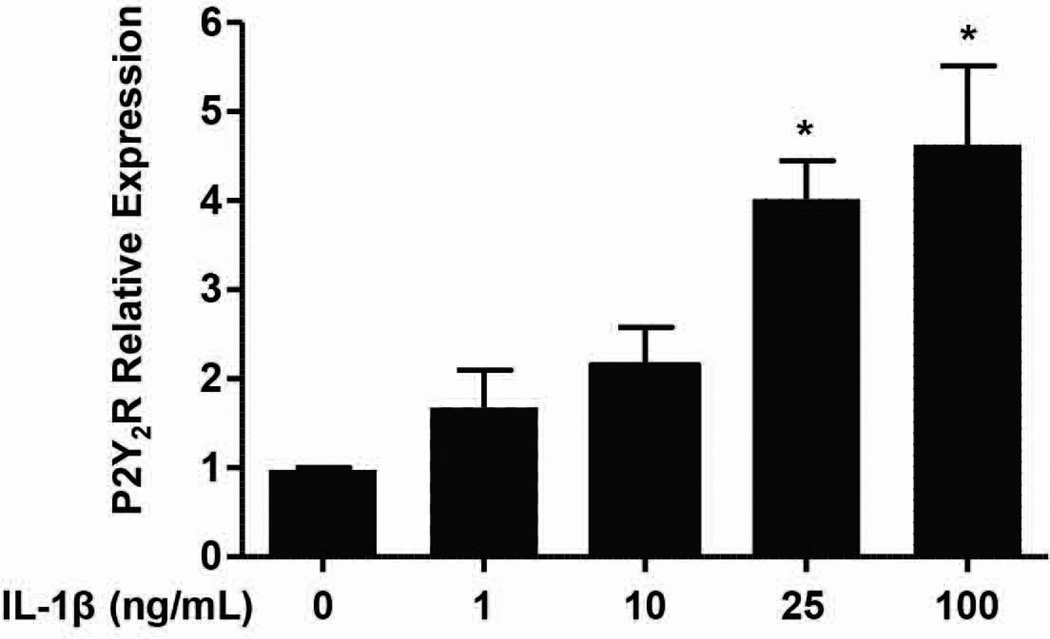
Upregulation of P2Y2R mRNA expression has been observed under a variety of pathophysiolgical conditions associated with inflammation and/or tissue damage (Turner et al. 1997, Seye et al. 2002, Schrader et al. 2005, Kong et al. 2009). P2Y2R expression is upregulated by NF-κB binding to the P2Y2R promoter (Degagne et al. 2009), consistent with the established role of NF-κB in the induction of proinflammatory gene expression (Wullaert et al. 2011). Similar to our previous studies with rat primary neurons (Kong et al. 2009), we determined that P2Y2R mRNA expression increased in mPCNs treated for 24 h with IL-1β, and a maximal response was obtained at 100 ng/ml IL-1β (Fig. 1). Thus, subsequent experiments use mPCNs pretreated with100 ng/ml IL-1β for 24 h to upregulate P2Y2R expression.
Fig. 1.
IL-1β induces upregulation of P2Y2R mRNA in mPCNs. Primary cortical neurons from wild type mice were treated at DIV6 with 0, 1, 10, 25 or 100 ng/ml IL-1β in serum-free media, as described in Methods. After 24 h, cDNA was prepared from RNA isolated from the neurons and RT-PCR was performed using specific primers for P2Y2R and GAPDH, as a stable endogenous control. Data represent means ± S.E.M. (n = 4) where *P < 0.05 indicates a significant increase in mRNA expression, as compared to neurons without IL-1β treatment.
UTP increases neurite outgrowth in mPCNs
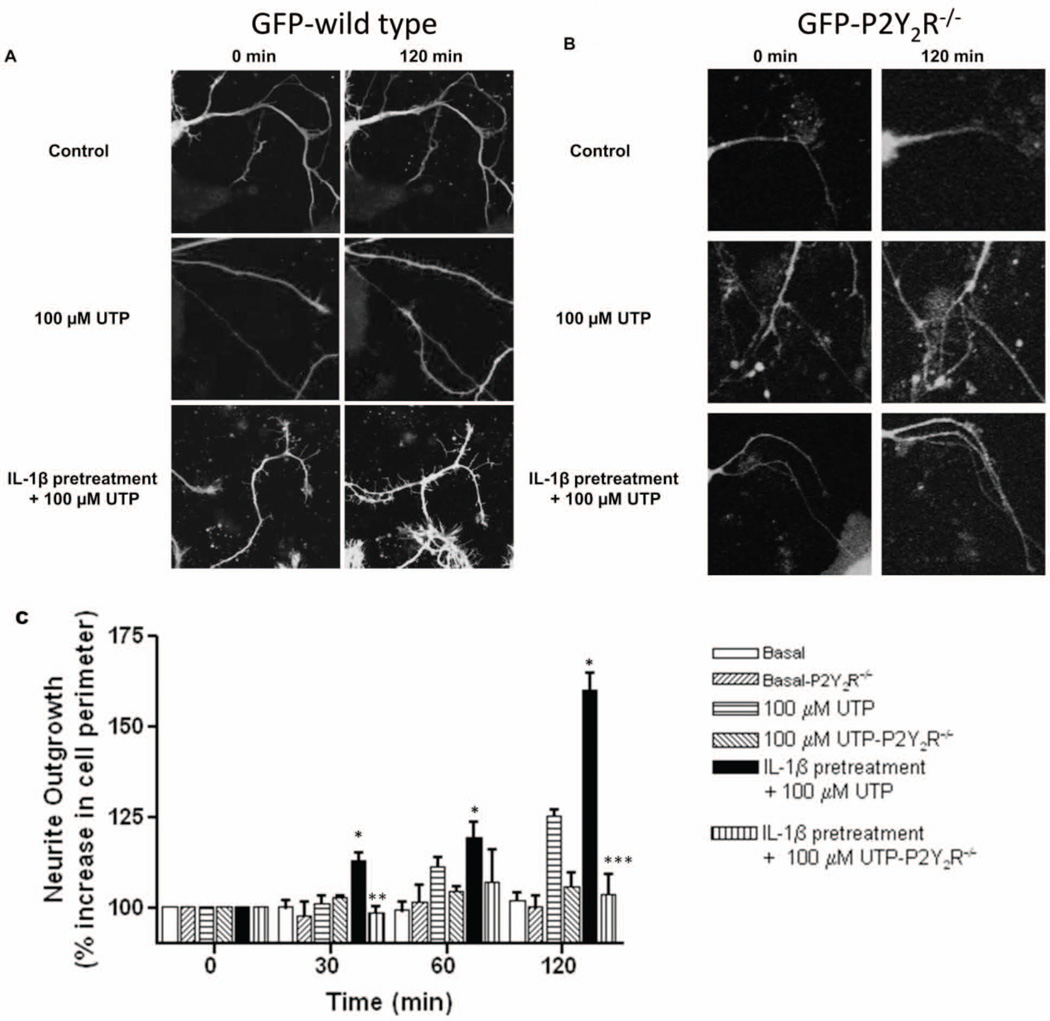
Using real time microscopy, we determined that P2Y2R activation in IL-1β-pretreated primary PCNs isolated from GFP-wild type mice caused extensive neurite outgrowth after a 120 min incubation with 100 µM UTP, as compared to GFP-wild type mPCNs treated with UTP for 0–120 min without IL-1β pretreatment (Figs. 2A and 2C). Addition of the P2Y2R agonist UTP to mPCNs from GFP-P2Y2R−/− mice with or without IL-1β pretreatment did not cause significant neurite outgrowth, as compared to GFP-wild type mice (Figs. 2B and 2C). Additionally, we utilized live imaging of mPCNs isolated from GFP-wild type mice to generate time-lapse videos of neurite outgrowth induced by 100 µM UTP alone (Video 1) or 100 µM UTP following IL-1β pretreatment (Video 2). Representative videos provide further evidence of increased UTP-induced neurite outgrowth following IL-1β pretreatment, as compared to UTP treatment alone. These data are consistent with the conclusion that P2Y2R upregulation induced by IL-1β is required to manifest the effect of UTP on neurite outgrowth in mPCNs.
Fig. 2. UTP induces neurite outgrowth in IL-1β-pretreated GFP-wild type mPCNs but not in GFP-P2Y2R−/− mPCNs.
Primary cortical neurons from (A) GFP-wild type or (B) GFP-P2Y2R−/− mice were pretreated with IL-1β (100 ng/ml) or vehicle for 24 h in serum-free media. Then, cells were incubated with or without (control) 100 µM UTP and imaged after 0 and 120 min (A and B) or 0, 30, 60 and 120 min (C), as described in Methods. In (C), neurite outgrowth including axons and dendrites was quantified by measuring the percent increase in neuron perimeter in 4 to 8 images per condition from 3 to 6 independent experiments, as described in Methods. Data represent means ± S.E.M. (n = 6) where * P < 0.05 indicates a significant increase in neurite outgrowth, as compared to GFP-wild type mPCNs without IL-1β pretreatment, and **P < 0.005 and ***P < 0.0001 indicate insignificant UTP-induced neurite outgrowth in IL-1β-treated GFP-P2Y2R−/− mPCNs, as compared to GFP-wild type mPCNs.
P2Y2Rs mediate cofilin phosphorylation in IL-1β-pretreated mPCNs
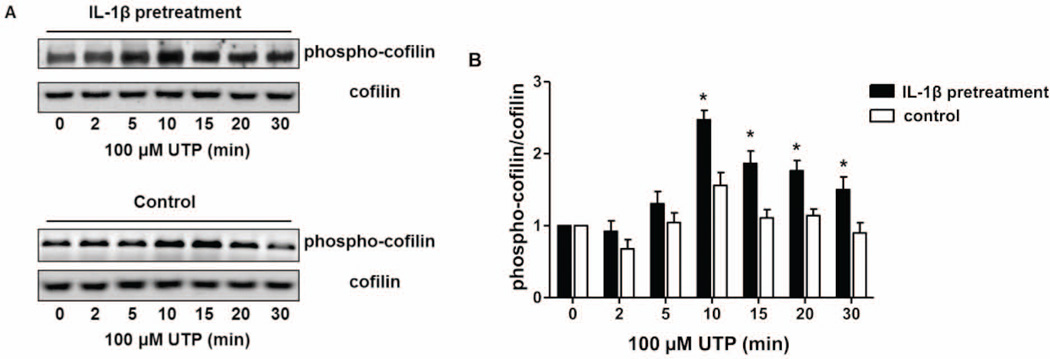
The regulation of neurite outgrowth and stabilization is a dynamic process that is necessary for the induction of long-term potentiation and requires a number of molecules that bind, sever, polymerize or depolymerize actin filaments (Malenka & Nicoll 1999, Cingolani & Goda 2008, Okamoto et al. 2009). Neurite outgrowth is dependent on the phosphorylation/dephosphorylation of the actin-depolymerizing factor cofilin (Meberg et al. 1998, Meberg & Bamburg 2000, Gungabissoon & Bamburg 2003, Bamburg et al. 2010), a 19 kDa protein that regulates both G- and F-actin polymerization during cytoskeletal remodeling (Maciver & Hussey 2002). Therefore, we determined whether P2Y2R activation by UTP modifies the phosphorylation of cofilin required for neurite extension in IL-1β-pretreated wild type mPCNs. Western blot analysis using an anti-phospho-cofilin-specific antibody from extracts of mPCNs pretreated for 24 h with IL-1β (100 ng/ml) indicate that UTP causes significant time-dependent enhancement of cofilin phosphorylation, as compared to mPCNs not pretreated with IL-1β (Figs. 3A and B). Cofilin phosphorylation was maximal within 10 min of UTP treatment and was sustained for up to 30 min. To determine unequivocally whether UTP induces cofilin phosphorylation via P2Y2R activation, mPCNs isolated from P2Y2R−/− mice were pretreated with 100 ng/ml of IL-1β for 24 h, then stimulated with 100 µM UTP for up to 30 min, and levels of cofilin phosphorylation were compared to similarly-treated mPCNs from wild type mice. In contrast to wild type mPCNs, the level of UTP-induced cofilin phosphorylation in IL-1β-pretreated PCNs from P2Y2R−/− mice did not significantly increase over the basal level (Fig. 4). Taken together, these data indicate that P2Y2R expression and activation in mPCNs under proinflammatory conditions enhances cofilin phosphorylation that inactivates actin depolymerization, a pathway required for neurite outgrowth (Van Troys et al. 2008).
Fig. 3. UTP induces cofilin phosphorylation in IL-1β-pretreated mPCNs.
(A) Primary cortical neurons from wild type mice were pretreated with IL-1β (100 ng/ml) or vehicle (control) in serum-free media for 24 h followed by UTP (100 µM). Cell lysates were prepared at the indicated time points and analyzed by immunoblot analysis using anti-cofilin and anti-phospho-cofilin antibodies, as described in Methods. Blots are representative of 6 independent experiments with similar results. (B) Quantification of protein bands was performed using Bio-Rad Quantity One software and data are presented as the ratio of phospho-cofilin expression to total cofilin expression after subtracting the basal level (untreated) of cofilin phosphorylation. Data represent means ± S.E.M. (n = 6) where * P < 0.05 indicates a significant increase in the ratio of phospho-cofilin to total cofilin, as compared to the control at the same time point.
Fig. 4. UTP induces cofilin phosphorylation in IL-1β-pretreated PCNs from wild type but not P2Y2R−/− mice.
Primary cortical neurons from wild type (●) or P2Y2R−/− (■) mice were pretreated with IL-1β (100 ng/ml) in serum-free media for 24 h followed by UTP (100 µM) treatment (↓) for 0–30 min. Immunoblot analysis and protein quantification were performed, as described for Fig. 3. Blots are representatives of 3 individual experiments showing similar results. Data represent means ± S.E.M. (n = 3) where * P < 0.05 indicates a significant increase in the ratio of phospho-cofilin to total cofilin, as compared to the 0 min time point.
Mechanisms underlying P2Y2R-mediated cofilin phosphorylation
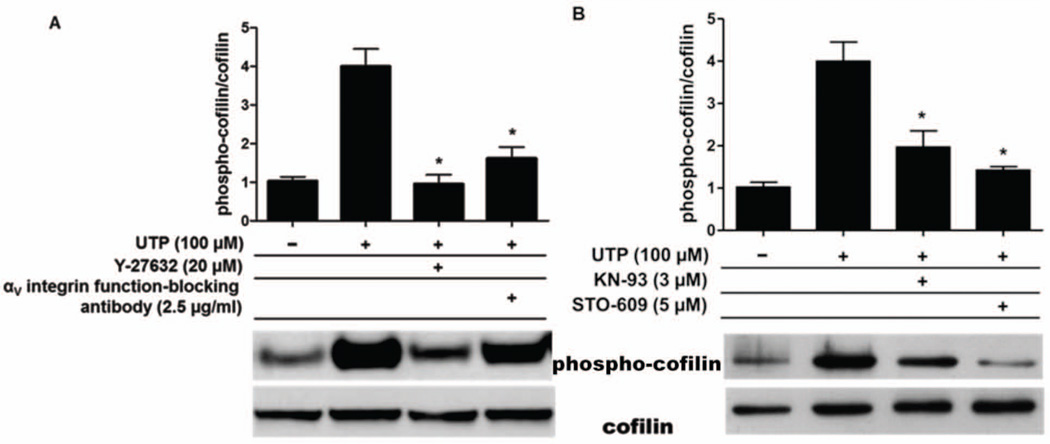
The Gq-coupled P2Y2R is unique among GPCRs in that it contains a RGD-sequence in its first extracellular loop that interacts directly with αvβ3/5 integrins and enables UTP to stimulate integrin-dependent activation of heterotrimeric Go and G12 proteins (Erb et al. 2001, Wang et al. 2005, Bagchi et al. 2005, Liao et al. 2007), which respectively activate Rac and Rho, monomeric GTPases known to regulate cytoskeletal rearrangements (Symons & Settleman 2000). It is well established that cofilin phosphorylation is mediated by Rho and Rac (Gungabissoon & Bamburg 2003, Van Troys et al. 2008); therefore, we investigated whether P2Y2R activation by UTP requires αvβ3/5 integrin to increase cofilin phosphorylation in mPCNs. P2Y2R-mediated activation of the αvβ3/5 integrin signaling pathway in IL-1β–pretreated wild type mPCNs was inhibited, as in our previous studies (Liao et al. 2007), with function-blocking anti-αv integrin antibody. Western analysis of extracts from IL-1β–pretreated mPCNs indicate that UTP-induced cofilin phosphorylation was reduced to near basal levels by anti-αv antibody (Fig. 5A), suggesting that interactions between the P2Y2R and αv integrin are required for UTP to increase phospho-cofilin levels. UTP-induced cofilin phosphorylation also was significantly attenuated by Y-27632 (20 µM) (Fig. 5A), an inhibitor of ROCK, the downstream target of Rho that regulates LIMK and cofilin phosphorylation (Meng et al. 2002, Bamburg et al. 2010). The function-blocking anti-αv antibody or Y-27632 alone had no significant effect on the phospho-cofilin/cofilin level in the absence of UTP (data not shown). These results strongly suggest a role for αvβ3/5 integrin-dependent Rho activation in P2Y2R-mediated increases in cofilin phosphorylation that enhance neurite outgrowth in IL-1β-pretreated mPCNs.
Fig. 5. UTP-induced cofilin phosphorylation is attenuated by inhibition of αV integrin or CaMKII signaling pathways.
Primary cortical neurons from wild type mice were pretreated with IL-1β (100 ng/ml) in serum-free media for 24 h and selective inhibitors were applied 30 min prior to UTP (100 µM) treatment. After 10 min, cell lysates were prepared and analyzed by immunoblot analysis using anti-cofilin and anti-phospho-cofilin antibodies, as described in Methods. Quantification of protein bands was performed, as described for Fig. 3. Blots are representative of 3 individual experiments showing similar results. (A) Pretreatment with Y-27632 (20 µM), a selective inhibitor of ROCK, or a function-blocking anti-αv integrin antibody (2.5 µg/ml) attenuated UTP-induced cofilin phosphorylation. (B) Pretreatment with KN-93 (3 µM), a selective inhibitor of CaMKII, or STO-609 (5 µM), a selective CaMKK inhibitor, significantly attenuated UTP-induced cofilin phosphorylation. Inhibitors alone had no significant effect on the phospho-cofilin/cofilin level in the absence of UTP (data not shown). Data represent means ± S.E.M. (n = 3) where *P < 0.05 indicates a significant decrease in the ratio of phospho-cofilin to total cofilin, as compared to UTP treatment in the absence of inhibitor.
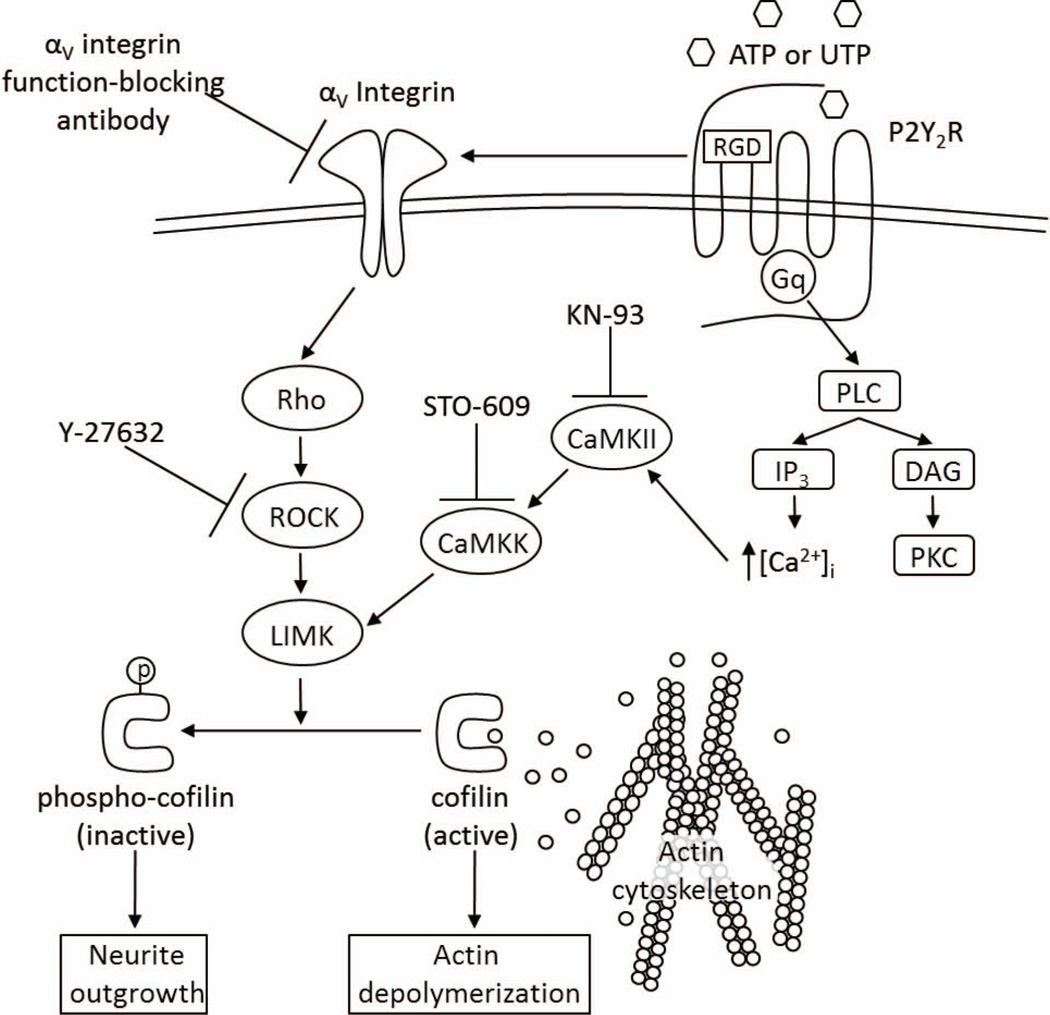
CaMKII is a well-documented modulator of actin dynamics and dendritic spine growth (Malenka & Nicoll 1999, Matsuzaki et al. 2004, Takemura et al. 2009, Pi et al. 2010). In vitro studies have shown that active CaMKII induces neurite outgrowth (Jourdain et al. 2003, Pi et al. 2010) and the phosphorylation of cofilin (Takemura et al. 2009). The activation of CaMKII by calmodulin is dependent on rises in the intracellular [Ca2+] (Koulakov et al. 2002, Fink & Meyer 2002) and previous studies in several laboratories, including ours, have shown that P2Y2R activation increases the intracellular free calcium concentration, [Ca2+]i, through Gq-dependent activation of PLC leading to the generation of IP3 and the subsequent release of Ca2+ from intracellular stores (Lustig et al. 1992, Viana et al. 1998, Weisman et al. 1999). Once activated, CaMKII phosphorylates multiple downstream targets, including kalirin-7 (Xie et al. 2007) and CaMKK (Saneyoshi et al. 2008), ultimately leading to the activation of LIMK (Cingolani & Goda 2008, Okamoto et al. 2009) and the phosphorylation of cofilin (Endo et al. 2007). To investigate whether CaMKII regulates UTP-induced cofilin phosphorylation, IL-1β-pretreated wild type mPCNs were treated with selective inhibitors of CaMKII (KN-93, 3 µM) or CaMKK (STO-609, 5 µM) for 30 min prior to UTP (100 µM) treatment. Cell lysates were subjected to immunoblot analysis to evaluate cofilin and phospho-cofilin levels. We found that KN-93 or STO-609 significantly attenuates cofilin phosphorylation induced by UTP in IL-1β-pretreated mPCNs (Fig. 5B). KN-93 or STP-609 alone had no significant effect on the phospho-cofilin/cofilin level in the absence of UTP (data not shown). These results indicate that increases in CaMKII/CaMKK activities regulate P2Y2R-mediated cofilin phosphorylation, along with the αV integrin/Rho/ROCK pathway. The data presented in this study suggest a signaling pathway whereby the P2Y2R mediates cofilin-dependent neurite outgrowth in mPCNs under proinflammatory conditions (Fig. 6).
Fig. 6.
A schematic describing the proposed P2Y2R-mediated signaling pathways leading to cofilin phosphorylation and neurite outgrowth in mPCNs.
Discussion
In the current study, we demonstrate that IL-1β upregulates the P2Y2R in mouse primary cortical neurons (mPCNs), whereupon activation of the P2Y2R promotes neurite extension through inactivation (phosphorylation) of the actin depolymerizing protein cofilin, a known regulator of neurite extension (Meberg et al. 1998, Meberg & Bamburg 2000, Aizawa et al. 2001, Endo et al. 2003, Endo et al. 2007, Figge et al. 2012)). Using an antibody that blocks αv integrin function and selective inhibitors, we also provide evidence that the P2Y2R mediates cofilin inactivation required for neurite outgrowth in mPCNs by a mechanism involving αv integrin, Rho/ROCK/LIMK and CaMKII/CaMKK/LIMK. The P2Y2R also is known to modulate ADAM activity in neurons (Kong et al. 2009); however, we determined that TAPI-2, a matrix metalloprotease inhibitor, had no effect on UTP-induced cofilin phosphorylation in IL-1β-treated mPCNs (data not shown). Based on these findings, a model of the mechanisms underlying P2Y2R-mediated neurite extension is provided in Fig. 6.
IL-1β is thought to have both detrimental and beneficial effects on the pathogenesis of AD (Mrak & Griffin 2005, Shaftel et al. 2007b). Studies in humans have shown that the IL-1β gene (Colangelo et al. 2002) and IL-1β protein expression (Griffin et al. 1989) are elevated in AD brain tissue and experimental models demonstrate that IL-1β is involved in neuronal injury, degeneration and loss (Rothwell & Luheshi 2000). Other studies, however, demonstrate that IL-1β promotes remyelination of neurons in the mouse CNS (Mason et al. 2001) and that chronic IL-1β expression causes an increase in blood-brain barrier permeability to leukocytes in mouse brain without causing neurodegeneration (Shaftel et al. 2007a). We show here that a 24 h pretreatment of mPCNs with IL-1β causes an ~4-fold increase in P2Y2R mRNA expression (Fig. 1), suggesting that signaling cues from the P2Y2R in neurons are intensified under inflammatory conditions. Since P2Y2R activation in IL-1β-treated wild type mPCNs promotes neurite extension, in contrast to P2Y2R−/− mPCNs (Fig. 2), we postulate that P2Y2R upregulation in the CNS may delay the progression of neurodegeneration that occurs with chronic inflammation. P2Y2R expression levels were found to be lower in post-mortem human AD brain tissue compared to normal tissue (Lai et al. 2008). This may be due to brain atrophy and the degeneration of P2Y2R-expressing cells in the CNS or the downregulation of the P2Y2R in specific subsets of cells in the CNS during end stage AD, possibilities that we are currently evaluating in a mouse model of AD. A better understanding of the mechanisms of neurite extension may provide insights into the role of P2Y2Rs in the regulation of the transition from acute to chronic inflammation and neurodegeneration and the apparent neuroprotective role of P2Y2Rs during inflammation.
P2Y2R gene expression induced by IL-1β in mPCNs (Figure 1) and rat cortical neurons (Kong et al. 2009), is inhibited by pretreatment with Bay 11-7082, an inhibitor of IκB-α phosphorylation (Pierce et al. 1997). This suggests that the IκB/NF-κB signaling pathway, known to be enhanced by proinflammatory cytokines (DiDonato et al. 1997, Hacker & Karin 2006), regulates P2Y2R transcription in neurons. Consistent with this hypothesis, the P2Y2R promoter contains a NF-κB binding domain that regulates increased P2Y2R transcription in response to inflammatory agents (Degagne et al. 2009). In vascular smooth muscle cells, IL-1β was shown to upregulate P2Y2R gene expression by a mechanism involving cyclooxygenase and protein kinase C (PKC), although NF-κB activation was not examined (Hou et al. 2000).
We found that activation of the P2Y2R in wild type mPCNs increases neurite extension, as indicated by an increase in the neurite perimeter of UTP-treated cells (Fig. 2), which is critical for establishing synaptic connections and for neuronal survival (Cline & Haas 2008). Neurite outgrowth in cultured neurons is considered to be an indication of neuroregenerative potential (Mitchell et al. 2007). Therefore, development of strategies to activate the P2Y2R in vivo may help retard neurodegeneration that occurs in AD and other neuroinflammatory diseases by promoting neurite extension and neuronal survival.
Neurite extension requires molecular signals that promote remodeling of the actin cytoskeleton within the growing neurite. The actin-binding protein cofilin regulates actin dynamics in essentially every type of eukaryotic cell (Maciver & Hussey 2002, Van Troys et al. 2008), and numerous studies indicate that cofilin is important for neurite extension (Meberg et al. 1998, Meberg & Bamburg 2000, Aizawa et al. 2001, Endo et al. 2003, Endo et al. 2007, Figge et al. 2012). Recent studies using Aplysia kurodai neurons found that microinjection of dephosphorylated cofilin led to rod formation, synapse loss (e.g., a decrease in the number of pre-synaptic varicosities) and, distal to the rod, impairment of synaptic plasticity measured by electrophysiological methods (Jang et al. 2005). In addition, phospho-cofilin administration impaired basal synaptic transmission, long-term potentiation (LTP), and the structure and dynamics of postsynaptic dendritic spines (Jang et al. 2005). Other studies indicate that blockade of calcineurin with FK506 or expression of a phosphomimetic mutant of cofilin (cof-S3D) prevented Aβ-induced spine loss in a hippocampal slice model of AD (Shankar et al. 2007). These findings are consistent with previous reports that cofilin signaling is perturbed in AD brain tissue and in neurons treated with synthetic Aβ (Maloney et al. 2005, Zhou et al. 2006). Thus, the ability of the P2Y2R to regulate cofilin phosphorylation in neurons (Figs. 3 and 4) likely has relevance to neurodegenerative disorders, such as AD, where cell damage or apoptosis would be anticipated to increase levels of extracellular nucleotides that activate the P2Y2R.
The activity of cofilin in the disassembly of actin filaments (F-actin) is regulated by several mechanisms, including phosphorylation of cofilin at serine3 (Ser3) (van Rheenen et al. 2009). Ser3 phosphorylation of cofilin prevents F-actin binding and severing of actin filaments by cofilin (van Rheenen et al. 2009), thus it is thought that cofilin phosphorylation/dephosphorylation at Ser3 acts as a switch between actin assembly and disassembly (Huang et al. 2006). Several kinases and phosphatases have been identified that phosphorylate and dephosphorylate cofilin at Ser3, respectively; these include the actin-binding LIM kinases (LIMK1, LIMK2) (Arber et al. 1998, Yang et al. 1998), testicular protein kinases (Toshima et al. 2001a, Toshima et al. 2001b), slingshot (SSH) phosphatases (Niwa et al., 2002) and chronophin phosphatase (Niwa et al. 2002, Gohla et al. 2005). Here, we show that activation of the P2Y2R in IL-1β-pretreated mPCNs causes phosphorylation of cofilin at Ser3 (Figs. 3 and 4) and that the Rho/ROCK/LIMK pathway is involved in this process, since inhibition of ROCK, which is activated by RhoA and controls the activation of LIMK2 (Gungabissoon & Bamburg 2003, Bernstein & Bamburg 2010), prevents P2Y2R-mediated cofilin phosphorylation at Ser3 (Fig. 5A). We also show that P2Y2R-mediated cofilin phosphorylation requires the activities of CaMKII and CaMKK (Fig. 5B). Furthermore, CaMKII controls cofilin phosphorylation and regulates F-actin dynamics through pathways that mainly converge on the Rho family of monomeric GTPases, such as RhoA and Rac1 (Okamoto et al. 2009). The activities of these GTPases are controlled by guanine-nucleotide-exchange factors (GEFs) and GTPase activating proteins (GAPs) (Symons & Settleman 2000, Newey et al. 2005). Although we did not explore which GEFs are activated by the P2Y2R in mPCNs, a likely candidate is kalirin-7, whose activity is reported to be essential for spine enlargement (Penzes & Jones 2008). Phosphorylation of kalirin-7 by CaMKII increases its GEF activity and leads to cofilin phosphorylation through the Rac1/PAK1/LIMK1 pathway (Penzes & Jones 2008). Other GEF candidates that mediate cofilin phosphorylation in dendritic spines and are controlled by CaMKII include βPIX, which signals through the Rac1 pathway, and Lcf, which binds to the actin-binding protein spinophilin and signals through the RhoA/ROCK/LIMK2 pathway (Okamoto et al. 2009).
Ionomycin-induced cofilin phosphorylation and neurite outgrowth are also blocked by KN-93, an inhibitor of Ca2+/calmodulin-dependent protein kinases, and STO-609, an inhibitor of CaMKK (Takemura et al. 2009). Other investigators have previously shown that ROCK and PAK activate LIMK1 by phosphorylation at Thr-508, which is in the activation loop of the kinase domain in LIMK1 (Edwards et al. 1999, Maekawa et al. 1999, Ohashi et al. 2000). Various signaling pathways, including Rac/PAK1 and CaMKIV/CaMKK activate Thr-508 phosphorylation in LIMK1 (Edwards et al. 1999, Maekawa et al. 1999, Ohashi et al. 2000; Takemura et al., 2009), suggesting a similar target for the Rho/ROCK and CaMKII/CaMKK pathways activated by the P2Y2R. This also suggests that LIMK1 activation is a point of convergence that links multiple signaling pathways to the regulation of actin cytoskeletal reorganization in cells (Takemura et al. 2009). Further studies are needed to evaluate crosstalk between the CaMKII/CaMKK, RhoA/ROCK and other signaling pathways in the regulation of P2Y2R-mediated and LIMK-dependent cofilin phosphorylation and neurite extension.
Supplementary Material
Acknowledgements
This work was supported by NIH grant AG018357. The authors declare no conflict of interest. We are grateful to the Molecular Cytology Core of the Bond Life Sciences Center at the University of Missouri for their assistance in acquiring images.
Abbreviations
- AD
Alzheimer’s Disease
- ADAM
a disintegrin and metalloprotease
- APP
amyloid precursor protein
- B27-AO
B27 without cortical antioxidants
- CaMKK
calmodulin-dependent protein kinase kinase
- CNS
central nervous system
- DAG
diacylglycerol
- DIV
days in vitro
- DTT
dithiothreitol
- FAM
6-carboxy-fluorescein phosphoramidite
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- HG-DMEM
High glucose Dulbecco’s modified Eagle’s medium
- HRP
horseradish peroxidase
- IL-1β
interleukin 1β
- IP3
inositol 1,4,5-trisphosphate
- LIMK
LIM kinase
- mPCN
mouse primary cortical neuron
- P2Y2R
P2Y2 receptor
- PKC
protein kinase C
- PLC
phospholipase C
- qRT-PCR
quantitative real-time polymerase chain reaction
- RGD
arginine/glycine/aspartic acid
- ROCK
Rho associated kinase
- SDS
sodium dodecyl sulfate
- TBS-T
Tris buffered saline with Tween-20
Literature Cited
- Aizawa H, Wakatsuki S, Ishii A, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci U S A. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 and TrkA receptors interact with Src family kinase for neuronal differentiation. Biochem Biophys Res Commun. 2006a;347:678–682. doi: 10.1016/j.bbrc.2006.06.141. [DOI] [PubMed] [Google Scholar]
- Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006b;26:3798–3804. doi: 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with αv integrins to activate Go and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW, Davis RC, et al. ADF/Cofilin-actin rods in neurodegenerative diseases. Curr Alzheimer Res. 2010;7:241–250. doi: 10.2174/156720510791050902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Broussard GJ, Mytar J, Li RC, Klapstein GJ. The role of inflammatory processes in Alzheimer's disease. Inflammopharmacology. 2012;20:109–126. doi: 10.1007/s10787-012-0130-z. [DOI] [PubMed] [Google Scholar]
- Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- Degagne E, Grbic DM, Dupuis AA, Lavoie EG, Langlois C, Jain N, Weisman GA, Sevigny J, Gendron FP. P2Y2 receptor transcription is increased by NF-κB and stimulates cyclooxygenase-2 expression and PGE2 released by intestinal epithelial cells. J Immunol. 2009;183:4521–4529. doi: 10.4049/jimmunol.0803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Duncan GW. The aging brain and neurodegenerative diseases. Clinics in geriatric medicine. 2011;27:629–644. doi: 10.1016/j.cger.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Mizuno K. LIM kinase and slingshot are critical for neurite extension. J Biol Chem. 2007;282:13692–13702. doi: 10.1074/jbc.M610873200. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, Uemura T, Mizuno K. Control of growth cone motility and morphology by LIM kinase and Slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci. 2003;23:2527–2537. doi: 10.1523/JNEUROSCI.23-07-02527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L, Liu J, Ockerhausen J, et al. An RGD sequence in the P2Y2 receptor interacts with αVβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol. 2001;153:491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figge C, Loers G, Schachner M, Tilling T. Neurite outgrowth triggered by the cell adhesion molecule L1 requires activation and inactivation of the cytoskeletal protein cofilin. Mol Cell Neurosci. 2012;49:196–204. doi: 10.1016/j.mcn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungabissoon RA, Bamburg JR. Regulation of growth cone actin dynamics by ADF/cofilin. J Histochem Cytochem. 2003;51:411–420. doi: 10.1177/002215540305100402. [DOI] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Moller S, Edvinsson L, Erlinge D. Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000;20:2064–2069. doi: 10.1161/01.atv.20.9.2064. [DOI] [PubMed] [Google Scholar]
- Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Jang DH, Han JH, Lee SH, Lee YS, Park H, Kim H, Kaang BK. Cofilin expression induces cofilin-actin rod formation and disrupts synaptic structure and function in Aplysia synapses. Proc Natl Acad Sci U S A. 2005;102:16072–16077. doi: 10.1073/pnas.0507675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ajit D, Peterson TS, et al. Nucleotides released from Aβ1-42-treated microglial cells increase cell migration and Aβ1-42 uptake through P2Y2 receptor activation. J Neurochem. 2012;121:228–238. doi: 10.1111/j.1471-4159.2012.07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Peterson TS, Baker O, et al. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J Neurochem. 2009;109:1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakov AA, Raghavachari S, Kepecs A, Lisman JE. Model for a robust neural integrator. Nat Neurosci. 2002;5:775–782. doi: 10.1038/nn893. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tan MG, Kirvell S, Hobbs C, Lee J, Esiri MM, Chen CP, Francis PT. Selective loss of P2Y2 nucleotide receptor immunoreactivity is associated with Alzheimer's disease neuropathology. J Neural Transm. 2008;115:1165–1172. doi: 10.1007/s00702-008-0067-y. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer's disease. Arch Pharm Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig KD, Sportiello MG, Erb L, Weisman GA. A nucleotide receptor in vascular endothelial cells is specifically activated by the fully ionized forms of ATP and UTP. Biochem J. 1992;284(Pt 3):733–739. doi: 10.1042/bj2840733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver SK, Hussey PJ. The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 2002;3:reviews3007. doi: 10.1186/gb-2002-3-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Maloney MT, Minamide LS, Kinley AW, Boyle JA, Bamburg JR. β-secretase-cleaved amyloid precursor protein accumulates at actin inclusions induced in neurons by stress or amyloid β: a feedforward mechanism for Alzheimer's disease. J Neurosci. 2005;25:11313–11321. doi: 10.1523/JNEUROSCI.3711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1β promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Bamburg JR. Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J Neurosci. 2000;20:2459–2469. doi: 10.1523/JNEUROSCI.20-07-02459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Hanson JC, Quets-Nguyen AT, Bergeron M, Smith RC. A quantitative method for analysis of in vitro neurite outgrowth. J Neurosci Methods. 2007;164:350–362. doi: 10.1016/j.jneumeth.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer's disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Obulesu M, Venu R, Somashekhar R. Tau Mediated Neurodegeneration: An Insight into Alzheimer's Disease Pathology. Neurochem Res. 2011 doi: 10.1007/s11064-011-0475-5. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. Dendritic spine dynamics--a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol. 2010;41:356–366. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, El Gaamouch F, Lemelin D, De Koninck P, Lisman J. CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proc Natl Acad Sci U S A. 2010;107:14437–14442. doi: 10.1073/pnas.1009268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in nerve growth factor-differentiated PC12 [corrected] Neuroscience. 2005;134:207–214. doi: 10.1016/j.neuroscience.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/βPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader AM, Camden JM, Weisman GA. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD.B10 mouse model of Sjogren's syndrome. Arch Oral Biol. 2005;50:533–540. doi: 10.1016/j.archoralbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Erb L, et al. Functional P2Y2 nucleotide receptors mediate uridine 5'-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1β expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007a;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007b;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000;10:415–419. doi: 10.1016/s0962-8924(00)01832-8. [DOI] [PubMed] [Google Scholar]
- Takemura M, Mishima T, Wang Y, Kasahara J, Fukunaga K, Ohashi K, Mizuno K. Ca2+/calmodulin-dependent protein kinase IV-mediated LIM kinase activation is critical for calcium signal-induced neurite outgrowth. J Biol Chem. 2009;284:28554–28562. doi: 10.1074/jbc.M109.006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Sugama S, et al. The activation of P2X7 receptor induces cathepsin D-dependent production of a 20-kDa form of IL-1β under acidic extracellular pH in LPS-primed microglial cells. J Neurochem. 2011;117:712–723. doi: 10.1111/j.1471-4159.2011.07240.x. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1β in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. Alzheimer's Association Report 2011 Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Toshima J, Toshima JY, Amano T, Yang N, Narumiya S, Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol Biol Cell. 2001a;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J, Toshima JY, Takeuchi K, Mori R, Mizuno K. Cofilin phosphorylation and actin reorganization activities of testicular protein kinase 2 and its predominant expression in testicular Sertoli cells. J Biol Chem. 2001b;276:31449–31458. doi: 10.1074/jbc.M102988200. [DOI] [PubMed] [Google Scholar]
- Turner JT, Weisman GA, Camden JM. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am J Physiol. 1997;273:C1100–C1107. doi: 10.1152/ajpcell.1997.273.3.C1100. [DOI] [PubMed] [Google Scholar]
- van Rheenen J, Condeelis J, Glogauer M. A common cofilin activity cycle in invasive tumor cells and inflammatory cells. J Cell Sci. 2009;122:305–311. doi: 10.1242/jcs.031146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Viana F, de Smedt H, Droogmans G, Nilius B. Calcium signalling through nucleotide receptor P2Y2 in cultured human vascular endothelium. Cell Calcium. 1998;24:117–127. doi: 10.1016/s0143-4160(98)90079-3. [DOI] [PubMed] [Google Scholar]
- Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y2 nucleotide receptor interaction with αv integrin mediates astrocyte migration. J Neurochem. 2005;95:630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Ajit D, Garrad R, Peterson TS, Woods LT, Thebeau C, Camden JM, Erb L. Neuroprotective roles of the P2Y2 receptor. Purinergic Signal. 2012a doi: 10.1007/s11302-012-9307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman GA, Camden JM, Peterson TS, Ajit D, Woods LT, Erb L. P2 Receptors for Extracellular Nucleotides in the Central Nervous System: Role of P2X7 and P2Y2 Receptor Interactions in Neuroinflammation. Mol Neurobiol. 2012b doi: 10.1007/s12035-012-8263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman GA, Garrad RC, Erb LJ, Santos-Berrios C, Gonzalez FA. P2Y receptors in the nervous system: molecular studies of a P2Y2 receptor subtype from NG108-15 neuroblastoma×glioma hybrid cells. Prog Brain Res. 1999;120:33–43. doi: 10.1016/s0079-6123(08)63544-x. [DOI] [PubMed] [Google Scholar]
- Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Zhou XW, Li X, Bjorkdahl C, et al. Assessments of the accumulation severities of amyloid beta-protein and hyperphosphorylated tau in the medial temporal cortex of control and Alzheimer's brains. Neurobiol Dis. 2006;22:657–668. doi: 10.1016/j.nbd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Zilka N, Ferencik M, Hulin I. Neuroinflammation in Alzheimer's disease: protector or promoter? Bratislavske lekarske listy. 2006;107:374–383. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.