Abstract
Cardiovascular disease (CVD) remains the leading cause of death worldwide, despite significant advances in medicine. Autophagy, a process of self-cannibalization employed by mammalian cells for the recycling of cellular contents, is altered not only in a number of CVDs, but in other diseases, as well. Many FDA-approved drugs are known to induce autophagy-mediated side effects in the cardiovascular system. In some cases, such drug-induced autophagy could be harnessed and used for treating CVD, greatly reducing the duration and cost of CVD treatments. However, because induction of autophagy in cardiovascular targets can be both adaptive and maladaptive under specific settings, the challenge is to determine whether the changes stimulated by drug-induced autophagy are, in fact, beneficial. In this review, we surveyed a number of CVD in which autophagy is known to occur, and we also address the role of FDA-approved drugs for which autophagy-mediated side effects occur within the cardiovascular system. The therapeutic potential of using small molecule modulators of autophagy in the management of CVD progression is discussed.
Keywords: autophagy, cardiovascular disease, drug-eluting stents, LC3, rapamycin, verapamil
II. Introduction
Despite advances in modern medicine, cardiovascular disease (CVD) remains the leading cause of mortality in both the United States and worldwide. According to the CDC, the total CVD-related healthcare costs exceeded $450 billion in 2008 and are expected to increase in the future [1]. Thus, there is an urgent need to identify disease mechanisms and to develop novel therapies that could be used to treat and curb the burden of CVD. Of course, along with new therapeutic modalities comes the potential risk of drug-induced cardiovascular toxicity, which must also be identified, quantified and mitigated at the clinical level.
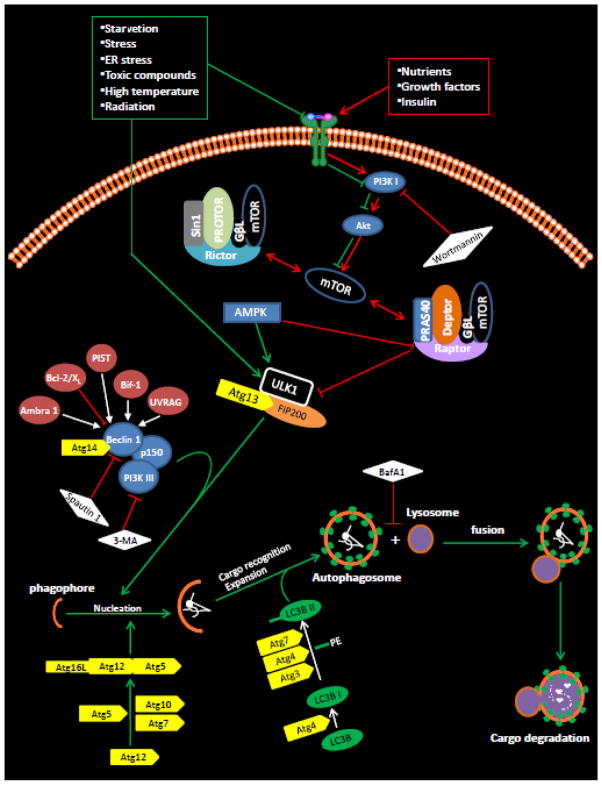
Autophagy is a process of self-cannibalization employed by mammalian cells for the recycling of cellular contents. Systematic studies in yeast have identified a number of autophagy-related (ATG) genes (and their proteins) that are evolutionarily conserved and have distinct roles in the autophagic program. Autophagy is generally activated under stress conditions, such as during starvation (i.e., increased AMP/ATP ratio) or during oxidative stress (Figure 1). Under such stress conditions, phosphorylation of ULK1 (ATG1), the first ATG protein in the autophagic pathway, results in ATG1 activation and dissociation from the mammalian target of rapamycin complex (i.e., mTOR complex). ATG1 subsequently triggers downstream events, ultimately leading to the assembly of a semi-circular double membrane vesicle called the phagophore. The precise link between ATG1 and phagophore formation, however, has yet to be defined. Phagophore formation and maturation into the autophagosome (i.e., the characteristic double membrane vesicle) depends on the interaction of the beclin 1/PI3KIII complex and other ATG proteins. During the first step of this maturation, ATG7 and ATG10 catalyze the conjugation of ATG12 to ATG5 [2, 3]. ATG16L stabilizes the ATG12–ATG5 complex, forming ATG12–ATG5-ATG16L. The stabilized ATG12–ATG5 complex then stimulates a second conjugation, which involves two steps: 1) ATG4 first primes LC3 (ATG8) by exposing a glycine residue at the -COOH terminal [4]; and, 2) this primed LC3 or LC3-I is then modified by phosphatidylethanolamine (PE) to form LC3-PE or LC3-II. The reaction is catalyzed by ATG7 and ATG3 [3, 5]. LC3-II is subsequently recruited to both outer and inner faces of the growing autophagosome and stabilizes it. This autophagosome then fuses with the lysosome leading to the lytic degradation of autophagosomal contents. Protein degradation yields amino acids and other building blocks that can be re-utilized by the cell for the biosynthesis of essential macromolecules or energy production.
Figure 1. Autophagy in mammalian cells.
In the presence of amino acids, growth factors and ample energy, phosphorylated Ser-2448 of the mammalian target of rapamycin (mTOR) by PKB (Akt) [68, 69] inhibits autophagy via Ser-757 phosphorylation in ULK1 [70]. The phosphorylation and activation of mTOR simultaneously induce growth and proliferative responses. In contrast, in the absence of amino acids and growth factors or during starvation (i.e., increase in the AMP-to-ATP ratio) or during oxidative stress, phosphorylation of ULK1 at Ser-317, -467, -555, -637, -777 or Thr-574 is associated with ULK1 activation and dissociation of the raptor complex from the ULK1 complex leading to autophagy activation [70, 71]. In addition to phosphorylating ULK1, AMPK directly phosphorylates the mTOR binding partner raptor on two well-conserved serine residues, Ser-722/Ser-792, and this phosphorylation induces 14-3-3 binding with raptor. The phosphorylation of raptor by AMPK is required for the inhibition of mTORC1 and cell-cycle arrest induced by energy deprivation [72]. The phosphorylation of ULK1 and raptor on multiple residues ensures tight control of autophagy activation, and thus, prevents inapprorpiate activation of autophagy. It is suggested that ULK1 acts as a focal point for multiple signals that control autophagy [73], and it can bind to other autophagy-related (Atg) proteins. However, the mechanism that links the ULK1 complex to downstream complexes such as the PI3KIII complex during autophagy induction is not well known. Beclin 1 in the PI3KIII complex functions as a scaffold molecule that recruits other molecules, such as PIST, UVRAG and Bif-1, important for the nucleation or maturation of the phagophore into the autophagosome. The formation of the autophagosome relies on 2 ubiquitin-like conjugation systems. In the first, ATG7 and ATG10 catalyze the conjugation of ATG12 to ATG5 [2, 3]. ATG16L stabilizes the ATG12-ATG5 complex, which is required for the stimulation of the second conjugation reaction. At this time, cellular components primed for degradation are starting to be enclosed. The second conjugation involves 2 steps: first, ATG4 primes LC3 (ATG8) by exposing a glycine residue at the COOH-terminus [4], and, second, primed LC3 (or LC3-I) is then conjugated with the polar head of phosphatidylethanolamine (PE) to form LC3-PE (or LC3-II). This reaction is catalyzed by ATG7 and ATG3, and LC3-II is recruited to both the outer and inner faces of the growing autophagosome. Formation of LC3-II is used as a measure of autophagy induction because LC3-I is converted to LC3-II only during autophagy [74, 75]. The mature autophagosome then fuses with the lysosome leading to degradation of the contents of the autophagosome by lysosomal enzymes.
Autophagy has also been shown to play an important role in the pathogenesis of several diseases such as cancer, Alzheimer’s, and ischemic heart disease [6]. It is also known to have a positive effect on longevity in animals [7–9]. The importance of autophagy and the signaling cascades that modulate it have been eloquently reviewed elsewhere [7–15]. However, the role of autophagy in specific cardiac diseases and the potential to harness drug-induced autophagy side effects in the cardiovascular system have only been modestly addressed. As such, this review is focused on autophagy in the cardiovascular system, and its role not only in CVD, but in drug-induced cardiovascular toxicity, as well. For example, even non-CVD drug treatments are known to induce autophagy (and have autophagy-mediated side effects) in the cardiovascular system. Finally, this review examines some small molecule modulators of autophagy as these could be potential drugs for treating cardiovascular diseases.
III. Autophagy in the Cardiovascular System
III.A. The heart
Basal cardiomyocyte autophagy is a recurring process required for proper functioning of the heart. Inhibition or over-activation of autophagy, however, is also associated with various cardiac diseases. A survey of specific cardiac pathologies thus reveals the importance of autophagy in various cardiomyopathies.
III.A.1. Cardiomyocyte hypertrophy and hypertensive heart disease
Hypertrophy is an increase in cardiomyocyte size that is chronically accompanied by extensive cardiac remodeling and increased protein expression. Several studies show evidence of autophagy in hypertrophy. For example a study by Nakai et al, shows that induction of hypertrophy by trans-aortic constriction (TAC) in mice led to suppression of the autophagic response [16]. In this same study, the team also suppressed the expression of key autophagic genes, including Atg5 and Atg7, and found that the loss of functional autophagic targets induced cardiomyocyte hypertrophy. Other recent studies in both experimental animals and humans have also reached similar conclusions to those of Nakai et al [17–19]. McMullen et al [20], for example, experimentally-induced autophagy using the mTOR inhibitor and found that rapamycin promotes regression of cardiac hypertrophy. In their study, 12-week-old male FVB/N mice were subjected to pressure overload (ascending aortic constriction; AAC) for 1 week, and then treated with rapamycin (2 mg·kg−1·day−1) for 1 week. Rapamycin decreased the heart weight/body weight (HW/BW) ratio in AAC mice with either compensated or decompensated hypertrophy by 68% and 41%, respectively [20]. In mice with decompensated cardiac hypertrophy, improved cardiac function – as measured by fractional shortening and ejection fraction – was observed. In 2012, Garcia et al, induced chronic hypertrophy (5 weeks of TAC) using the same FVB/N mice strain used by McMullen et al, and found that rapamycin had similar beneficial outcomes. Thus, supposing that animals are pre-treated before pressure overload, these studies suggest that the beneficial effects of rapamycin-induced autophagy could prevent and combat cardiac hypertrophy [21].
III.A.2. Cardiac arrhythmias
Cardiac arrhythmias, in general, occur when the normal pattern of depolarization, which begins at the SA node and ends in the ventricular myocardium, is disrupted. This disruption could lead to a slow, fast or irregular depolarization, and can be due to direct effects on nodal (pacemaker) and/or indirectly via damaged myocardial tissue. Cardiac arrhythmias can indicate not only potential alterations in cardiac remodeling, but the presence of both ischemia and heart block on an ECG. While there is limited literature to support the involvement of autophagy in arrhythmias, a recent study by Garcia et al., [22] did find defective autophagy in patients with post-operative atrial fibrillation (POAF). Samples of the right atrial appendages in patients with POAF showed accumulation of autophagic vacuoles and decreased expression levels of the autophagy marker, microtubule-associated protein light chain 3 (LC3), suggesting impaired cardiac autophagy in the development of POAF. In a separate study, Chen et al. [23] showed that induction of autophagy occurs in atrial cardiomyocytes of most cases with severe mitral and tricuspid regurgitation. In their study of 20 patients, LC3 positive myocytes were detected in approximately 90% of patients with fibrillation in the right atria. Interestingly, the majority of LC3 positive myocytes had a moderate-to-severe myolosis (cardiomyocyte degenration), which suggests that, in patients with mitral regurgitation, autophagy could play a significant role in the cardiomyocyte myolosis. In the context of cardiac arrhythmias, however, whether or not autophagy is protective has yet to be elucidated.
III.A.3. Ischemia reperfusion and heart failure
Evidence for the involvement of autophagy in ischemia-reperfusion (I-R) injury and heart failure abound. In the mid-1970s, Sybers et al, reported the up-regulation of autophagy in fetal mouse hearts when exposed to glucose deprivation and hypoxia [24]. Since then, controversial findings have evolved regarding the importance of autophagy in I-R injury, as well as in heart failure. For example, Matsui et al, [25] as well as Valentim et al, [26] showed that ablation of the proautophagic gene, beclin 1, or the inhibition of PI3KIII using 3-methyl adenine (3-MA) treatment, respectively, prevented cardiomyocyte death induced by I/R injury in mice. These studies suggest that autophagy activated during I-R may be maladaptive. On the contrary, Hamacher-Brady et al., [27] demonstrated that enhancing autophagy through overexpression of beclin 1 actually protects cardiomyocytes against I-R injury. They also showed that the overexpression of dominant-negative ATG5 led to increased cellular injury, and thus, demonstrated that autophagic flux constitutes a powerful mechanism against I-R injury. Similarly, activation of autophagy with rapamycin [28] or chloramphenicol succinate [29] treatment leads to a cardioprotective phenotype in mice and swine, respectively. Although these results oppose those of Matsui et al., [25] and Valentim et al., [26] the discrepancy is likely due to the use of different treatment protocols and models of I-R injury. Nevertheless, these findings not only highlight the intrinsic importance of autophagy during ischemic heart disease, but also suggest that whether the activation or inhibition of autophagy will be adaptive or maladaptive ultimately depends on the conditions.
Likewise, the role of autophagy in heart failure is can be observed to be adaptive or maladaptive as well. A study by Shimomura et al., supports the potential for dual autophagic outcomes during heart failure. This team found that cardiac autophagy in patients with end-stage heart failure is not only responsible for the removal of damaged organelles but is also involved in the progressive destruction of cardiomyocytes [30]. In a follow-up study of patients suffering from dilated cardiomyopathy, Kostin et al., confirmed a similarly maladaptive role of autophagy. They found that while cardiomyocytes in the failing heart die by multiple mechanisms, autophagy was a major instigator [31]. Interestingly, Kostin and colleagues identified autophagic myocytes that stained positive for monodansylcadaverine, which is a specific marker of autophagic vacuoles[32]. Yet, other studies have suggested a more adaptive role of autophagy during I-R injury and progressive heart failure. In a study of 9 patients with idiopathic dilated cardiomyopathy, Kassiotis et al., showed that the implantation of a left ventricular assist device (LVAD) led to a decreased expression of most autophagic markers [33]. They reasoned that, because this decrease occurs upon LVAD implantation, the heightened autophagy observed beforehand is likely an adaptive mechanism used to generate more energy for the failing heart – a main function of autophagy. It is not clear whether this difference in findings is due to the small sample size or perhaps the peculiar form of heart failure. Thus, additional human studies, using appropriate case controls, are needed to truly understand the contribution of autophagy during heart failure. If autophagy could in fact be adaptive within the context of heart failure, then the pharmacological regulation or modulation of autophagy may be a clinically relevant target.
III.B. Arterial Media and Smooth Muscle Cells
Arteries are efficient and flexible blood delivery tubes, and in the adult human body, these span >60,000 miles. Blood vessels can be both conduits and resistors because of their highly specialized, but relatively simple, 3 layer structure. The single cell inner lining, known as the endothelium, allows for gas and nutrients exchange between blood and the underlying cells. Around the endothelium is a medial layer composed of vascular smooth muscle cells (VSMCs) that are interspersed with layers or lamellae made of extracellular matrix (ECM), including collagen and elastin. Its principal function is the regulation of blood vessel tone (contraction and relaxation), which determines vascular resistance, the primary determinant of blood flow. Surrounding the media is the adventitial layer composed mainly of fibroblasts and ECM, and, in aorta elements, of the vasa vasorum and perivascular nerves. The adventitia functions on a structural and secretory level. In the diseased artery, one or more of these layers is affected, and autophagy can play a role in vascular remodeling during physiological adaptation or pathogenesis.
III.B.1. Atherosclerosis and restenosis
While atherosclerosis is an inflammatory disease of the aorta and great vessels, it can also affect the peripheral microvasculature. Atherosclerosis is typically characterized by a buildup of cholesterol in the sub-intimal space, wherein engulfment of cholesterol by monocytes/macrophages and vascular smooth muscle cells (VSMC) eventually leads to the production of foam cells, oxidative stress, and inflammation, as well as VSMC proliferation and neointima formation. Decades of such buildup can lead to plaque formation, cellular necrosis, necrotic core and plaque rupture [34]. Interestingly, both VSMCs and macrophage autophagy are known to be critical during the process of atherogenesis, and current research has focused on the modulation of autophagy in one or both of these cell types [35–37]. However, because the role of autophagy during atherosclerosis could be, in general, either protective or detrimental, there is a need for caution when exploring potential therapeutic application.
The beneficial effects of autophagy have been well-documented. It has recently been reported, for example, that inhibiting autophagy in macrophages by silencing ATG 5 or other autophagy mediators led to both increased apoptosis and plaque instability in advanced lesions [38], which increases the risk of both MI and stroke following plaque rupture. It is possible that ablation of autophagy in these macrophages led to the accumulation of damaged materials (e.g., misfolded proteins, damaged mitochondria) that triggered apoptosis. Such a finding indicates a beneficial role of autophagy in atherosclerosis. However, despite the potential benefit autophagy offers during atherosclerosis, excessive induction of autophagy might eventually trigger autophagy-induced cell death or type 2 cell death, i.e., resulting from prolonged activation of autophagy, and lead to plaque destabilization [39].
In the event of plaque rupture and artery occlusion, angioplasty is commonly used to restore blood flow. Previously opened (restored) arteries, however, often re-occlude (termed restenosis) with the excessive proliferation of underlying VSMCs that migrate luminally and form the neointima [40]. For example, in a study of 1,012 patients randomly assigned to treatment with either Sirolimus- (n = 503) or Paclitaxel-eluting (n = 509) stents, approximately 28% of subjects needed revascularization at a later date [41]. Although the outcomes of clinical studies such as this one ultimately depend on demographics (e.g., diabetic patients), it does highlight that restenosis remains a significant, ongoing health concern. The majority of drug-eluting stents (DES) designed to prevent restenosis are rapamycin-based (e.g., Sirolimus, Everolimus) and these drugs are known not only as inhibitors of the mTOR pathway but as inducers of autophagy as well [42]. It is not clear what role autophagy plays in endogenous restenosis, but clearly DES induces autophagy to both benefit and detriment as discussed below.
III.B.2. Aortic Aneurysm
The involvement of autophagy in aneurysm formation has also been documented. Zheng et al., have shown that the protein levels of key autophagic genes, such as LC3, Atg4b, Beclin1/Atg6, Bnip3 and Vps34, are markedly upregulated in abdominal aortic aneurysm (AAA) tissues [43]. mRNA microarray analysis revealed that osteopontin, a proinflammatory cytokine, was found to be the gene most often induced in these tissues.. Thus, to determine the mechanism of induced autophagy in AAA tissues, the team treated cultured smooth muscle cells with recombinant osteopontin and found a p38-MAPK-dependent induction of autophagy – a finding that implicates autophagy as a mechanism of vessel weakening in AAA. A summary of autophagy and its CVD-related roles can be found in Table 1.
Table 1.
Role of autophagy in cardiovascular complications.
| CVD Model | Autophagy in the CVS | Consequence(s) |
|---|---|---|
| Cardiac hypertrophy | Inhibited | |
| Ischemia/reperfusion | Inhibited Activated |
|
| Heart failure | Activated | |
| Arrhythmias | Inhibited | |
| Atherosclerosis | Activated | |
| Restenosis | Not determined | |
| Aortic aneurysm | Activated |
|
IV. Targeting Autophagy in CVD
IV.A. Harnessing autophagy-mediated (side) effects of cardiovascular drugs
Since it was first identified in the 1960s, the therapeutic manipulation of autophagy has been utilized for the treatment of CVD and other diseases, such as cancer. Although many clinically-approved drugs used for the treatment of CVD were not previously thought to stimulate autophagy, some drugs have recently been shown to induce autophagy independent of their conventional mechanism of action. These drugs could thus be carefully regimented for the treatment of different cardiovascular complications. Verapamil, for example, is a L-type calcium channel blocker (CCB) that has been used for over 30 years in the treatment of hypertension, angina pectoris and cardiac arrhythmia [44–47]. However, we recently showed that either individual pure Verapamil stereoisomer (as well as racemic Verapamil) alone induces autophagy and is antiproliferative in cultured VSMCs, indicating a pro-autophagic mechanism entirely independent of calcium channel blocker action [48]. Such a finding is potentially significant because it reveals a heretofore undefined mechanism of action – one that, when better understood, could be targeted for more efficacious anti-atherosclerotic and/or anti-restenosis therapy. And because the effects of Verapamil on VSMC proliferation are stereo-independent, utilization of higher dosages without calcium channel blocking activity could eliminate the risk of hypotensive crises. Moreover, study of this antiproliferative and autophagic mechanism of action could reveal a novel therapeutic target in proliferating smooth muscle cells, much like mTOR inhibitors that promote the stabilization of atherosclerotic plaque. Interestingly, Verapamil and the beta-blocker propranolol were each shown to separately induce autophagy in the rat heart in vivo, and yet, at that time, the mechanism of action was attributed to a cardiodepressive or a hypotensive effect [49].
The introduction of drug eluting stents (DES) with rapamycin also greatly reduced the frequency of restenosis after angioplasty [42, 50]. Yet the efficacy of DES therapy does suffer one major setback – DES that induce autophagy also lead to the inhibition of endothelial cell repair and re-endothelialization in the stented blood vessel lumen [51]. Thus, when weighing the beneficial effects of inhibiting VSMC proliferation vs preventing re-endothelialization, the more adverse effects must also be carefully considered, and newer generation of stents (absorbable) is being developed. Nonetheless, because the principal benefit of rapamycin is its autophagy-inducing effect, it could also potentially be used in the treatment of other CVDs. For example, studies using experimental animals have shown that rapamycin reduces infarct size after MI [52], indicating a potential for cardioprotective effects in humans.
It is known that some widely used cardiovascular drugs, such as β-receptor agonists (isoproterenol, norepinephrine) and β-receptor blockers (propranolol, salbutamol), have also been shown to alter autophagy in the cardiovascular system, and perhaps autophagy induction is a part of their beneficial effects [53–55] (see Table 2). If so, a better understanding of their autophagic inducing mechanism of action could yield new cellular targets for “old drugs.”
Table 2.
Potential applications of drug-mediated modulation of autophagy in the cardiovascular system.
| Drug | Current Use(s) | Autophagy | Potential Future Use(s) |
|---|---|---|---|
| Verapamil | Hypertension, angina, arrythmia | Activates | Atherosclerosis and restenosis [48] |
| Rapamycin | Restenosis | Inhibits | Myocardial infarction [52] preconditioning |
| Metformin | Diabetes | Activates | Heart failure and diabetic cardiomyopathy [56–58, 60, 61, 86] |
| Isoproterenol, norepinephrine, salbutamol | Brachycardia and asthma (isoproterenol) and asthma (salbutamol) | Activates | Cardiac fibrosis [53] |
| Bortezomib | Myeloma and mantle cell lymphoma | Activates | Hypertrophy? |
| Doxorubicin | Cancer chemotherapy | Activates | Hypertrophy? |
| Paclitaxel | Restenosis, ovarian, lung and breast cancers and Kaposi’s sarcomas | Inhibits | Myocardial infarction? |
| Granulocyte Colony stimulating factor | Stimulation of WBCs | Inhibits | Heart failure [87, 88] |
| Urocortin | None | Inhibits | Ischemic heart disease and chronic heart failure [26] |
| Sulfaphenazole | None | Activates | Ischemic heart disease [89] |
| 3-Methyl adenine* | None | Inhibits | Inhibition of autophagy in conditions where autophagy is hyperactivated* |
| Wortmannin* | None | Inhibits | Inhibition of autophagy in conditions where autophagy is hyperactivated* |
| Spautin-1# | None | Inhibits | Inhibition of autophagy in conditions where autophagy is hyperactivated |
| Bafilomycin A1 | None | Inhibits | Inhibition of autophagy in conditions where autophagy is hyperactivated |
| Chloroquine | Anti-malarial | Inhibits | Heart failure? [90] cardioprotection |
| Tamoxifen | Breast cancer | Activates | Useful in conditions with insufficient autophagy |
| Lithium, carbamezepine, sodium valproate | Abnormal mood changes and seizures | Activates | Useful in conditions with insufficient autophagy |
Side effects are more likely to occur with 3-MA and Wortmannin because these are inhibitors of PI3K activity. Both inhibitors inhibit VSMC proliferation after platelet-derived growth factor (PDGF) stimulation, and thus, these drugs could have anti-restenotic properties.
Spautin-1 is a recently discovered autophagy inhibitor that targets degradation of both PI3KIII and beclin 1.
IV.B. Harnessing autophagy of non-CVD targeted drugs
Although known principally for its use in type II diabetes, metformin, a glucose-lowering drug that suppresses hepatic gluconeogenesis, has beneficial effects in the cardiovascular system. Metformin is a 5′ adenosine monophosphate-activated protein kinase (AMPK) activator that induces autophagy. Experimental studies in both animals [56–58] and humans [59–61] have shown that metformin treatment plays a protective role not only in heart failure, but also in diabetic cardiomyopathy. A study of Xie et al, [56] shows that following metformin treatment, diabetic OVE26 mice had improved cardiac function as a result of enhanced cardiac autophagy. Additional beneficial cardiovascular effects of metformin could also be attributed to its eNOS-activating capabilities [57]. Despite these well-documented and beneficial effects, however, metformin is only prescribed for use in the treatment of type II diabetes and Polycystic Ovary Syndrome (PCOS). What proportion of beneficial effects in these conditions is autophagy-derived is unclear.
Some drugs have no autophagic effect on their target tissues whatsoever, but have been shown to alter autophagy in the cardiovascular system – a side effect that when better understood could be manipulated to a therapeutic advantage in the right setting. A number of such drugs are listed in Table 2. For example, the proteasome inhibitor drug, bortezomib (Velcade), and the tyrosine kinase inhibitor (TKI), imatinib (Gleevec), are used in the treatment of multiple myeloma [62, 63]. Unfortunately, cardiotoxicity is reported with both bortezomib and imatinib and, interestingly enough, autophagy is also activated during cardiotoxicity [63, 64]. Whether bortezomib- or imatinib-induced autophagy and cardiotoxicity are causally linked is not entirely clear, but a better understanding of the specific mechanisms of autophagy induction by either bortezomib or TKI is needed. This information could facilitate developing new targeted use of these drugs (or structurally-related compounds) to effectively modulate autophagy in the setting of cardiac disease, for example, and thus, potentially turn “drug toxicity into therapy.”
V. Modulation of Autophagy as an Emerging Strategy -- Targets and Toxicology
At present, there are many small molecules currently used in laboratories for the inhibition or activation of autophagy. Such molecules have great potential to be therapeutically utilized in the treatment of CVD (see Table 2). Like many of the therapies discussed in this review, however, their use could also alter autophagy in untargeted organs or tissues. Because basal physiological autophagy is necessary for normal growth and development, alteration of autophagy can also be detrimental to overall health. Moreover, some autophagy inhibitors, such as wortmannin and 3-methyl adenine (3-MA), inhibit certain upstream signaling molecules (i.e., the PI3K/Akt pathway) that are critical in the signaling of many other non-autophagy targets (e.g., eNOS). However, this limitation has not prevented the discovery of novel downstream inhibitors of autophagy. Spautin-1, for example, is an inhibitor of the autophagic pathway that could be rendered tissue-specific. Indeed, spautin-1 has recently been shown to promote beclin-1 degradation [65]. It also has one major advantage over other autophagy inhibitors; because of its specificity, spautin-1 is far less likely to inhibit other signaling processes, e.g., PI3K/Akt pathway. In recent work from our laboratory, spautin-1 was used to effectively modulate phenotype conversion in VSMCs, and thus, it could potentially be used to combat vasculoproliferative disorders such as restenosis in vivo (Salabei, unpublished data). Chloroquine and Bafilomycin A1 have also been shown to modulate autophagy [66, 67], but the effects of these compounds on cardiovascular-related complications have yet to be tested. A summary of these and other potentially useful autophagy-related compounds is in Table 2.
VI. Concluding Remarks
It is clear that autophagy is a significant physiological process, one that is altered in a number of cardiovascular diseases and drugs specifically designed to modulate autophagy under these disease conditions prove to be very promising.. Both the cost and time spent developing these new autophagy-directed therapies, however, could be greatly reduced by reutilizing existing FDA-approved drugs. Existing drugs that, as a side effect, induce autophagy in the cardiovascular system could be harnessed for additional therapeutic use. Small molecule inhibitors/activators of autophagy, such as spautin-1, show similar promise and should be further investigated. In any case, whether exploring the side effects of approved drugs or developing new chemical modulators for the treatment of CVD, great care must be taken when modulating autophagy. Perhaps a fine-tuning of autophagy could be most beneficial, such that its essential homeostatic roles are preserved, while modulating its over- or under-activation. And although such an approach involves substantial challenges, the potential therapeutic benefits of controlled autophagy should not be discounted.
Acknowledgments
This work was supported in part by NIH funds: NIGMS RR024489 and HL89380. We thank Ms. Samantha R. Clausi, M.A., for excellent editorial assistance.
Abbreviations and Acronyms
- VSMC
vascular smooth muscle cells
- Spautin 1
specific and potent inhibitor of autophagy 1
- 3-MA
3-methyl adenine
- CVD
cardiovascular disease
- mTOR
mammalian target of rapamycin
- ATG
autophagy-related gene
- TAC
trans-aortic constriction
- ECG
electrocardiogram
- POAF
post-operative atrial fibrillation
- AAA
abdominal aortic aneurysm
- DES
drug-eluting stent
- MI
myocardial infarction
- FDA
Food and Drug Administration
- LVAD
left ventricular assist device
- eNOS
endothelial nitric oxide synthase
- ULK1
UNC-like kinase 1
- ECM
extracellular matrix
- AMPK
AMP-activated protein kinase
- LC3
microtubule-associated protein light chain 3
- TKI
tyrosine kinase inhibitor
IX. References
- 1.Roger Heart Disease and Stroke Statistics-2011 Update: A Report From the American Heart Association (vol 123, pg e18, 2011) Circulation. 2011;123(6):E240–E240. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N, et al. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–8. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 3.Tanida I, et al. Apg7p/Cvt2p: A novel protein-activating enzyme essential for autophagy. Mol Biol Cell. 1999;10(5):1367–79. doi: 10.1091/mbc.10.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanida I, et al. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. Journal of Biological Chemistry. 2004;279(35):36268–76. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 5.Tanida I, et al. Human Apg3p/Aut1p homologue is an authentic E2 enzyme for multiple substrates, GATE-16, GABARAP, and MAP-LC3, and facilitates the conjugation of hApg12p to hApg5p. Journal of Biological Chemistry. 2002;277(16):13739–13744. doi: 10.1074/jbc.M200385200. [DOI] [PubMed] [Google Scholar]
- 6.Mehrpour M, et al. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. American Journal of Physiology-Cell Physiology. 2010;298(4):C776–C785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- 7.Morselli E, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nature Cell Biology. 2010;12(9):842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 9.Hansen M, et al. A role for autophagy in the extension of lifespan by dietary restriction in C-elegans. Plos Genetics. 2008;4(2) doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stromhaug PE, Klionsky DJ. Approaching the molecular mechanism of autophagy. Traffic. 2001;2(8):524–531. doi: 10.1034/j.1600-0854.2001.20802.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Molecular Medicine. 2003;9(3–4):65–76. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZF, Klionsky DJ. An Overview of the Molecular Mechanism of Autophagy. Autophagy in Infection and Immunity. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He CC, Klionsky DJ. Regulation Mechanisms and Signaling Pathways of Autophagy. Annual Review of Genetics. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death and Differentiation. 2009;16(7):956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- 15.Nakatogawa H, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nature Reviews Molecular Cell Biology. 2009;10(7):458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 16.Nakai A, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Medicine. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 17.Qipshidze N, et al. Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302(3):H688–H696. doi: 10.1152/ajpheart.00777.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hua YN, et al. Chronic akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Research in Cardiology. 2011;106(6):1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto S, et al. On the nature of cell death during remodeling of hypertrophied human myocardium. Journal of Molecular and Cellular Cardiology. 2000;32(1):161–175. doi: 10.1006/jmcc.1999.1064. [DOI] [PubMed] [Google Scholar]
- 20.McMullen JR, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109(24):3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 21.Shioi T, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107(12):1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 22.Garcia L, et al. Impaired cardiac autophagy in patients developing postoperative atrial fibrillation. Journal of Thoracic and Cardiovascular Surgery. 2012;143(2):451–U485. doi: 10.1016/j.jtcvs.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 23.Chen MC, et al. Autophagy as a mechanism for myolysis of cardiomyocytes in mitral regurgitation. European Journal of Clinical Investigation. 2011;41(3):299–307. doi: 10.1111/j.1365-2362.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- 24.Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv Stud Cardiac Struct Metab. 1976;12:453–63. [PubMed] [Google Scholar]
- 25.Matsui Y, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 26.Valentim L, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. Journal of Molecular and Cellular Cardiology. 2006;40(6):846–52. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 27.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281(40):29776–87. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 28.Khan S, et al. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41(2):256–64. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Sala-Mercado JA, et al. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation. 2010;122(11 Suppl):S179–84. doi: 10.1161/CIRCULATIONAHA.109.928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimomura H, et al. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Japanese Circulation Journal-English Edition. 2001;65(11):965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 31.Kostin S, et al. Myocytes die by multiple mechanisms in failing human hearts. Circulation Research. 2003;92(7):715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 32.Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (Mdc) Is a Specific in-Vivo Marker for Autophagic Vacuoles. European Journal of Cell Biology. 1995;66(1):3–14. [PubMed] [Google Scholar]
- 33.Kassiotis C, et al. Markers of Autophagy Are Downregulated in Failing Human Heart After Mechanical Unloading. Circulation. 2009;120(11):S191–S197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weissberg PL, Bennett MR. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(24):1928–9. [PubMed] [Google Scholar]
- 35.Liao XH, et al. Macrophage Autophagy Plays a Protective Role in Advanced Atherosclerosis. Cell Metabolism. 2012;15(4):545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razani B, et al. Autophagy Links Inflammasomes to Atherosclerotic Progression. Cell Metabolism. 2012;15(4):534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia GH, Cheng G, Agrawal DK. Autophagy of vascular smooth muscle cells in atherosclerotic lesions. Autophagy. 2007;3(1):63–64. doi: 10.4161/auto.3427. [DOI] [PubMed] [Google Scholar]
- 38.Liao X, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15(4):545–53. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1(2):66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- 40.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. Journal of Pathology. 2000;190(3):300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Billinger M, et al. Long-term clinical and angiographic outcomes of diabetic patients after revascularization with early generation drug-eluting stents. American Heart Journal. 2012;163(5):876. doi: 10.1016/j.ahj.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Grube E, Buellesfeld L. Rapamycin analogs for stent-based local drug delivery - Everolimus- and tacrolimus-eluting stents. Herz. 2004;29(2):162–166. doi: 10.1007/s00059-004-2556-6. [DOI] [PubMed] [Google Scholar]
- 43.Zheng YH, et al. Osteopontin Stimulates Autophagy Via Integrin/CD44 and p38 MAPK Signaling Pathways in Vascular Smooth Muscle Cells. Journal of Cellular Physiology. 2012;227(1):127–135. doi: 10.1002/jcp.22709. [DOI] [PubMed] [Google Scholar]
- 44.Georgiev B, et al. Use of calcium channel blockers in hypertension treatment in primary care. Journal of Hypertension. 2004;22:S198–S198. [Google Scholar]
- 45.Pepine CJ, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease - The International Verapamil-Trandolapril Study (INVEST): A randomized controlled trial. Jama-Journal of the American Medical Association. 2003;290(21):2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 46.Elliott WJ, Ram CVS. Calcium Channel Blockers. Journal of Clinical Hypertension. 2011;13(9):687–689. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stams TRG, et al. Verapamil as an antiarrhythmic agent in congestive heart failure: hopping from rabbit to human? British Journal of Pharmacology. 2012;166(2):554–556. doi: 10.1111/j.1476-5381.2011.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salabei JK, et al. Verapamil stereoisomers induce antiproliferative effects in vascular smooth muscle cells via autophagy. Toxicol Appl Pharmacol. 2012 doi: 10.1016/j.taap.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahro M, Pfeifer U. Short-term stimulation by propranolol and verapamil of cardiac cellular autophagy. J Mol Cell Cardiol. 1987;19(12):1169–78. doi: 10.1016/s0022-2828(87)80527-8. [DOI] [PubMed] [Google Scholar]
- 50.Sheiban I, et al. Drug-eluting stent: the emerging technique for the prevention of restenosis. Minerva Cardioangiol. 2002;50(5):443–53. [PubMed] [Google Scholar]
- 51.Hayashi S, et al. The Stent-Eluting Drugs Sirolimus and Paclitaxel Suppress Healing of the Endothelium by Induction of Autophagy. American Journal of Pathology. 2009;175(5):2226–2234. doi: 10.2353/ajpath.2009.090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buss SJ, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54(25):2435–46. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 53.Aranguiz-Urroz P, et al. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2011;1812(1):23–31. doi: 10.1016/j.bbadis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Pfeifer U, et al. Short-Term Inhibition of Cardiac Cellular Autophagy by Isoproterenol. Journal of Molecular and Cellular Cardiology. 1987;19(12):1179–1184. doi: 10.1016/s0022-2828(87)80528-x. [DOI] [PubMed] [Google Scholar]
- 55.Dammrich J, Pfeifer U. Acute Effects of Isoproterenol on Cellular Autophagy - Inhibition in Myocardium but Stimulation in Liver Parenchyma. Virchows Archiv B-Cell Pathology Including Molecular Pathology. 1981;38(2):209–218. doi: 10.1007/BF02892815. [DOI] [PubMed] [Google Scholar]
- 56.Xie ZL, et al. Improvement of Cardiac Functions by Chronic Metformin Treatment Is Associated With Enhanced Cardiac Autophagy in Diabetic OVE26 Mice. Diabetes. 2011;60(6):1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki H, et al. Activation of Myocardial AMP-activated Protein Kinase by Metformin Prevents Progression of Heart Failure in Dogs; Involvement of eNOS Activation. Circulation. 2008;118(18):S286–S287. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki H, et al. Metformin Prevents Progression of Heart Failure in Dogs Role of AMP-Activated Protein Kinase. Circulation. 2009;119(19):2568–U74. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 59.Burgert TS, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Diabetes. 2007;56:A471–A471. doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 60.Evia-Viscarra ML, et al. The effects of metformin on inflammatory mediators in obese adolescents with insulin resistance: controlled randomized clinical trial. J Pediatr Endocrinol Metab. 2012;25(1–2):41–9. doi: 10.1515/jpem-2011-0469. [DOI] [PubMed] [Google Scholar]
- 61.Fitch K, et al. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. Aids. 2012;26(5):587–597. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Investigation. 2004;22(2):304–11. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 63.Hu W, et al. Mechanistic investigation of imatinib-induced cardiac toxicity and the involvement of c-Abl kinase. Toxicol Sci. 2012;129(1):188–99. doi: 10.1093/toxsci/kfs192. [DOI] [PubMed] [Google Scholar]
- 64.Nowis D, et al. Cardiotoxicity of the Anticancer Therapeutic Agent Bortezomib. American Journal of Pathology. 2010;176(6):2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu JL, et al. Beclin1 Controls the Levels of p53 by Regulating the Deubiquitination Activity of USP10 and USP13. Cell. 2011;147(1):223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto A, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23(1):33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 67.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625(1–3):220–33. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 68.Nave BT, et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochemical Journal. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 69.Jung CH, et al. mTOR regulation of autophagy. Febs Letters. 2010;584(7):1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology. 2011;13(2):132–U71. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stephan JS, Herman PK. The regulation of autophagy in eukaryotic cells: do all roads pass through Atg1? Autophagy. 2006;2(2):146–8. doi: 10.4161/auto.2.2.2485. [DOI] [PubMed] [Google Scholar]
- 74.He H, et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. Journal of Biological Chemistry. 2003;278(31):29278–29287. doi: 10.1074/jbc.M303800200. [DOI] [PubMed] [Google Scholar]
- 75.Kabeya Y, et al. LC3, a mammalian homolog of yeast Apg8p, is localized in autophagosome membranes after processing (vol 19, pg 5720, 2000) Embo Journal. 2003;22(17):4577–4577. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlossarek S, et al. Defective proteolytic systems in Mybpc3-targeted mice with cardiac hypertrophy. Basic Research in Cardiology. 2012;107(1) doi: 10.1007/s00395-011-0235-3. [DOI] [PubMed] [Google Scholar]
- 77.Nemchenko A, et al. Autophagy as a therapeutic target in cardiovascular disease. Journal of Molecular and Cellular Cardiology. 2011;51(4):584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto S, et al. On the nature of cell death during remodeling of hypertrophied human myocardium. Journal of Molecular and Cellular Cardiology. 2000;32(1):161–75. doi: 10.1006/jmcc.1999.1064. [DOI] [PubMed] [Google Scholar]
- 79.McMullen JR, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109(24):3050–5. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 80.Gao XM, et al. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens. 2006;24(8):1663–70. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 81.Matsui Y, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion - Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circulation Research. 2007;100(6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 82.Valentim L, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. Journal of Molecular and Cellular Cardiology. 2006;40(6):846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 83.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. Journal of Biological Chemistry. 2006;281(40):29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 84.Martinet W, et al. Interactions between cell death induced by statins and 7-ketocholesterol in rabbit aorta smooth muscle cells. British Journal of Pharmacology. 2008;154(6):1236–1246. doi: 10.1038/bjp.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curcio A, Torella D, Indolfi C. Mechanisms of Smooth Muscle Cell Proliferation and Endothelial Regeneration After Vascular Injury and Stenting - Approach to Therapy. Circulation Journal. 2011;75(6):1287–1296. doi: 10.1253/circj.cj-11-0366. [DOI] [PubMed] [Google Scholar]
- 86.Burgert TS, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatric Diabetes. 2008;9(6):567–576. doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 87.Takemura G, et al. Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy. 2006;2(3):212–4. doi: 10.4161/auto.2608. [DOI] [PubMed] [Google Scholar]
- 88.Miyata S, et al. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. American Journal of Pathology. 2006;168(2):386–97. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, et al. Autophagy and protein kinase C are required for cardioprotection by sulfaphenazole. Am J Physiol Heart Circ Physiol. 2010;298(2):H570–9. doi: 10.1152/ajpheart.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ross TK, et al. Differential effects of chloroquine on cardiolipin biosynthesis in hepatocytes and H9c2 cardiac cells. Mol Cell Biochem. 2000;207(1–2):115–22. doi: 10.1023/a:1007066903073. [DOI] [PubMed] [Google Scholar]