Abstract
The volatile anesthetic isoflurane protects against renal ischemia and reperfusion injury by releasing renal tubular TGF-β1. Since adenosine is a powerful cytoprotective molecule, we tested whether TGF-β1 generated by isoflurane induces renal tubular ecto-5′-nucleotidase (CD73) and adenosine to protect against renal ischemia and reperfusion injury. Isoflurane induced new CD73 synthesis and increased adenosine generation in cultured kidney proximal tubule cells and in mouse kidney. Moreover, a TGF-β1 neutralizing antibody prevented isoflurane-mediated induction of CD73 activity. Mice anesthetized with isoflurane after renal ischemia and reperfusion had significantly reduced plasma creatinine and decreased renal tubular necrosis, neutrophil infiltration and apoptosis compared to pentobarbital-anesthetized mice. Isoflurane failed to protect against renal ischemia and reperfusion injury in CD73 deficient mice, in mice pretreated with a selective CD73 inhibitor or mice treated with an adenosine receptor antagonist. The TGF-β1 neutralizing antibody or the CD73 inhibitor attenuated isoflurane-mediated protection against HK-2 cell apoptosis. Thus, isoflurane causes TGF-β1-dependent induction of renal tubular CD73 and adenosine generation to protect against renal ischemia and reperfusion injury. Modulation of this pathway may have important therapeutic implications to reduce morbidity and mortality arising from ischemic acute kidney injury.
Keywords: acute kidney injury, adenosine, apoptosis, inflammation, necrosis, transforming growth factor-β1
Introduction
Acute kidney injury (AKI) results in extremely high mortality and morbidity costing more than 10 billion dollars per year in the US [1]. Renal ischemia and reperfusion (IR) injury is a frequent cause of AKI for patients subjected to kidney, liver or aortic surgery [2–4]. Unfortunately, there are no effective methods or drugs to treat or prevent AKI. [5–7]. Moreover, AKI is frequently associated with other life-threatening complications including multiorgan failure and sepsis and commonly progresses to chronic kidney disease [5,6,8]. Although incompletely understood, renal tubular cell necrosis, inflammation and apoptosis contribute to the pathogenesis of ischemic AKI [9].
The majority of patients subjected to general anesthesia receive volatile anesthetics during more than 80 million surgical procedures per year [10]. In addition to its analgesic and anesthetic properties, volatile anesthetics including isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane] protect against renal IR injury by attenuating the inflammatory response as well as necrosis [11,12]. We previously demonstrated that sevoflurane, another widely used volatile anesthetic, had direct anti-inflammatory and anti- necrotic effects in cultured human kidney proximal tubule (HK-2) cells [13,14]. We subsequently demonstrated volatile anesthetics protect against renal tubular necrosis and inflammation by direct renal tubular production of transforming growth factor-beta 1 (TGF-β1) [14–16]. However, the downstream signaling mechanisms of volatile anesthetic-mediated renal protection generated by TGF-β1 remain incompletely understood.
In this study, we aimed to identify the downstream signaling intermediates triggered by isoflurane-mediated generation of TGF-β1. Recent studies suggest that TGF-β1 induces expression of ecto-5′-nucleotidase (CD73) in leukocytes including T-lymphocytes, macrophages and dendritic cells [17,18]. CD73 is a cell surface enzyme that converts extracellular AMP to adenosine [19]. Adenosine is a powerful cytoprotective molecule that reduces all 3 components of renal tubular cell death in ischemic AKI including necrosis, inflammation and apoptosis [20]. Therefore, in this study we tested the hypothesis that isoflurane generates adenosine in the kidney via TGF-β1-mediated induction of renal tubular CD73. We also tested whether isoflurane protects against ischemic AKI by CD73-mediated adenosine generation.
Results
Isoflurane increases adenosine generated by human proximal tubule (HK-2) cells
We first determined whether isoflurane treatment increases adenosine generation in human renal proximal tubule (HK-2) cells. HK-2 cells were treated with either carrier gas or isoflurane and we collected cell culture media for adenosine analysis with HPLC (Figure 1). Representative images of adenosine HPLC tracings are shown in Figure 1A, Figure 1B and Figure 1C. Figure 1A shows adenosine standard (200 pmol) peak eluting at ~7.5 min. We determined that isoflurane treatment (2.5% for 16 hr) increased adenosine levels in HK-2 cell culture media. Figure 1D shows that average adenosine levels after isoflurane treatment increased dose-dependently when compared with carrier gas-treated (room air plus 5% CO2) cells.
Figure 1. Isoflurane increases adenosine generated by HK-2 cells.
A. HPLC tracing of 200 pmol adenosine standard. Adenosine eluted at ~7.5 min. B and C. Representative (of 4 experiments) HPLC images for adenosine generated in HK-2 cell culture media. HK-2 cells were treated with either carrier gas (B) or with 2.5% isoflurane (C) for 16 hr and cell culture media were collected for HPLC analyses to measure adenosine. D. Average adenosine levels after isoflurane treatment increased dose-dependently (0–2.5%) compared to carrier gas-treated controls (N=4). Data are presented as means ± SEM. *P<0.05 vs. carrier gas-treated controls. Error bars represent 1 SEM.
Induction CD73 activity, mRNA and protein synthesis by isoflurane in cultured human and mouse kidney proximal tubule cells
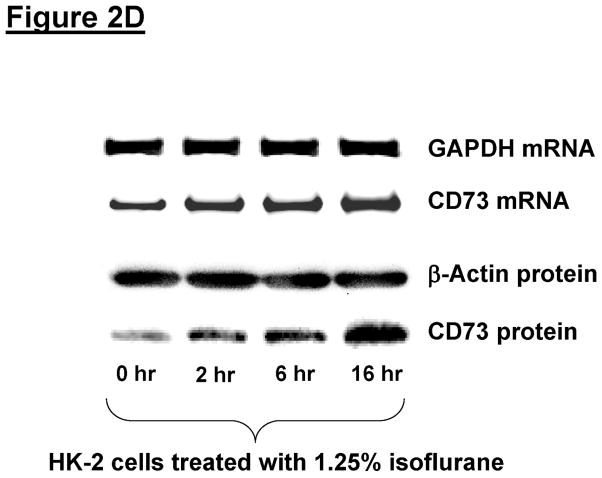
The next set of experiments determined whether isoflurane stimulates CD73 activity in immortalized human renal proximal tubule (HK-2) cells. HK-2 cells treated with isoflurane had significantly increased CD73 activity (conversion of AMP to adenosine) in a time (2.5% for 0–16 hr, Figure 2A) and dose-dependent (0–2.5% for 16 hr, Figure 2B) manner. These increases in CD73 activities were associated with dose (0–2.5%) and time (0–16 hr) dependent increases in CD73 mRNA and protein expression (Figure 2C and Figure 2D). In contrast, CD39 mRNA did not change with isoflurane treatment in HK-2 cells (data not shown). We also determined whether isoflurane treatment increases CD73 expression in primary culture of mouse proximal tubule cells. Figure 2E shows increased CD73 protein expression in primary cultures of mouse kidney proximal tubule cells treated with 2.5% isoflurane for 16 hr (representative of 4 experiments). Therefore, our studies show that isoflurane increases CD73 expression in both immortalized and primary cultures of renal proximal tubule cells.
Figure 2. Isoflurane increases CD73 activity, mRNA and protein synthesis in HK-2 cells.
A and B. HK-2 cells treated with isoflurane show time-dependent (A. N=6) and dose-dependent (B, N=4) increases in CD73 activity HK-2 cells were treated with isoflurane for 16 hr. Data are presented as means ± SEM. *P<0.05 vs. CD73 activity measured at baseline (A) or in cells treated with 0% isoflurane (B). Error bars represent 1 SEM. C and D. Representative images for CD73 mRNA (RT-PCR) and protein (immunoblotting) expression in HK-2 cells treated with isoflurane (Representative of 4 experiments). HK-2 cells were treated with 0–2.5% isoflurane for 6 hr (C) or with 1.25% isoflurane for 0–16 hr (D). Isoflurane caused dose (C) and time (D) dependent increases in CD73 mRNA and protein expression. E. Representative RTPCR images for CD73 protein (immunoblotting) expression in primary cultures of mouse kidney proximal tubule cells treated with carrier gas or with 2.5% isoflurane for 16 hr (Representative of 4 experiments).
Mechanism of isoflurane mediated CD73 activation and induction in renal proximal tubule cells
The next series of experiments determined the mechanism of isoflurane-mediated CD73 induction in renal proximal tubule cells. We previously demonstrated that volatile anesthetics released renal tubular TGF-β1 that directly contributes to the reduction in renal tubular necrosis and inflammation [15,16]. We tested the hypothesis that isoflurane-mediated generation of TGF-β1 directly induces CD73 mRNA and protein as well as activity with subsequent increase in renal tubular adenosine generation. We inhibited TGF-β1 signaling in HK-2 cells by pretreating the cells with TGF-β1 neutralizing antibody (10 μg/ml) 30 min. before isoflurane exposure (2.5% for 6 or 16 hr) as described [15,16]. HK-2 cells pretreated with control isotype antibody (mouse IgG) demonstrated significant (>2 fold) induction of CD73 expression as well as activity (Figure 3A-C). We determined that neutralization of TGF-β1 significantly attenuated the upregulation of CD73 mRNA (Figure 3A) as well as protein expression (Figure 3B) with isoflurane treatment in HK-2 cells. Furthermore, TGF-β1 neutralizing antibody treatment attenuated the induction of CD73 activity and adenosine generation in HK-2 cells (Figure 3C).
Figure 3. TGF-β1 is responsible for isoflurane-mediated induction of CD73 and adenosine generation.
A and B. CD73 mRNA (RT-PCR, A) and protein (immunoblotting, B) expression in HK-2 cells treated with 2.5% isoflurane for 6 or 16 hr (N=4–6). Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in CD73 expression over carrier gas and IgG isotype antibody treated controls. C. Adenosine levels in cell culture media (top) and CD73 activity (bottom) in HK-2 cells treated with 2.5% isoflurane for 6 hr (N=6). D and E. CD73 mRNA (RT-PCR, A) and protein (immunoblotting, B) expression in primary culture of mouse proximal tubule cells treated with 2.5% isoflurane for 6 or 16 hr (N=3). Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in CD73 expression over carrier gas and IgG isotype antibody treated controls. GAPDH mRNA and b-actin protein expression were also quantified to normalize lane loading. *P<0.05 vs. carrier gas group treated with IgG isotype antibody. #P<0.05 vs. isoflurane group treated with IgG isotype antibody. Error bars represent 1 SEM. TGF-β1 antibody (10 μg/ml) prevents isoflurane-mediated induction of CD73 mRNA and protein expression, adenosine generation as well as CD73 activity in human and mouse proximal tubule cells.
We next determined whether isoflurane-mediated generation of TGF-β1 also induces CD73 expression in mouse proximal tubule cells in culture. Figure 3D and Figure 3E show that primary cultures of mouse kidney proximal tubule cells pretreated with control isotype antibody (mouse IgG) had significant CD73 mRNA and protein induction after 6 and 16 hr treatment with 2.5% isoflurane, respectively (representative of 3 experiments). TGF-β1 neutralization significantly attenuated the upregulation of CD73 mRNA and protein in isoflurane-treated mouse proximal tubule cells. Therefore, our studies show that isoflurane-induced TGF-β1 induces CD73 in both immortalized and primary cultures of renal proximal tubule cells.
Isoflurane-mediated induction of kidney CD73 in vivo via TGF-β1 signaling
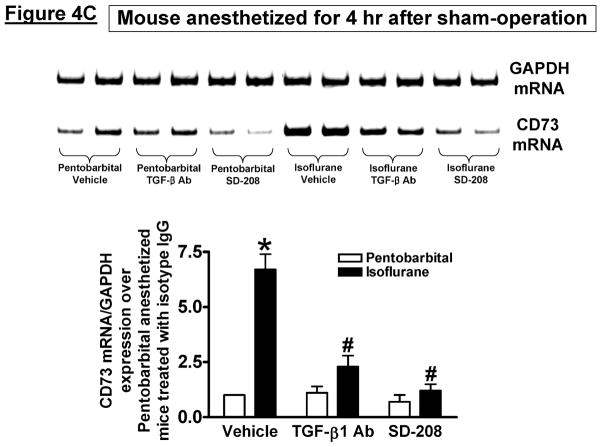
Next, we aimed to determine whether isoflurane induces CD73 expression and activity in vivo as we observed in vitro. We previously demonstrated that there were no differences in systemic arterial blood pressure, renal blood flow, or core body temperature in mice anesthetized with pentobarbital or with 1 minimum alveolar concentration (MAC) isoflurane [12,21]. We initially determined that kidney CD73 mRNA and protein expression increased in CD73 wild type mice exposed 1.2% isoflurane (ISO) for 4 hr after sham-operation or 30 min. renal IR injury (Figure 4A, representative of 3–4 experiments). We were unable to detect CD73 mRNA or protein expression in CD73 deficient mice. We also measured kidney CD73 activity in kidneys from CD73 deficient or CD73 wild type mice subjected to isoflurane (1.2%) or pentobarbital anesthesia for 4 hr (Figure 4B). We show that CD73 expression increased in isoflurane-anesthetized mice compared to mice anesthetized with equi-anesthetic doses of pentobarbital. Moreover, the CD73 activity was slightly higher in kidneys from mice subjected to renal IR compared to sham-operated mice. CD73 activity measured in CD73 deficient mice was dramatically lower (>90%) when compared to CD73 wild type mice (Figure 4B). To test the critical role of TGF-β1 signaling in isoflurane-mediated CD73 induction in vivo, some CD73 wild type mice were injected with 5 mg/kg monoclonal anti-TGF-β1 (MAB240) or control isotype antibody i.v. 10 min. before renal ischemia. We also treated some CD73 wild type mice with a specific TGF-β receptor I kinase inhibitor SD-208 (60 mg/kg p.o.) 1 hr before renal ischemia. We determined that TGF-β1 neutralization or inhibition of TGF-β1 receptor I kinase prevented the induction of kidney CD73 activity after isoflurane anesthesia in mice (Figure 4B). Consistent with this, TGF-β1 neutralization or inhibition of TGF-β1 receptor I kinase prevented CD73 mRNA induction after isoflurane anesthesia (Figure 4C, representative of 3 experiments). Therefore, from these experiments we can conclude that isoflurane induces CD73 activity and synthesis in vivo via TGF-β1 signaling.
Figure 4. Isoflurane increases mouse kidney CD73 activity and expression via TGF-β1 signaling.
A. CD73 mRNA and protein expression (representative of 3–4 experiments) in kidneys from CD73 deficient (KO) or CD73 wild type (WT) mice exposed to pentobarbital (PB) or 1.2% isoflurane (ISO) for 4 hr after sham-operation or 30 min. renal ischemia and reperfusion (IR). CD73 expression increased after isoflurane anesthesia in sham-operated mice and in mice subjected to renal IR. GAPDH and β-actin served as internal loading controls. B. Kidney CD73 activity in CD73 deficient (KO) or CD73 wild type (WT) mice exposed to pentobarbital (PB) or 1.2% isoflurane (ISO) for 4 hr after sham-operation or to 30 min. renal IR (N=3–4). To neutralize TGF-β1 in vivo, some CD73 wild type mice were injected with 5 mg/kg monoclonal anti-TGF-β1 (MAB240) antibody i.v. 10 min. before renal ischemia. To inhibit TGF-β receptor I kinase, we also treated some CD73 wild type mice with a specific inhibitor SD-208 (60 mg/kg p.o.) 1 hr before renal ischemia. Isoflurane post-conditioning significantly increased kidney CD73 activity in CD73 wild type mice subjected to sham-surgery or to renal IR. TGF-β1 neutralization or inhibition of TGF-β1 receptor I kinase prevented the induction of CD73 activity after isoflurane anesthesia. *P<0.05 vs. pentobarbital-anesthetized mice subjected to sham-operation and vehicle treatment. #P<0.05 vs. isoflurane-anesthetized mice subjected to sham-operation and vehicle treatment. Error bars represent 1 SEM. C. Representative RTPCR images (top) and band intensity quantifications (bottom, N=3) expressed as fold increases in kidney CD73 mRNA expression in kidneys of CD73 wild type mice exposed to pentobarbital (PB) or 1.2% isoflurane (ISO) for 4 hr after sham-operation or to 30 min. renal IR. Again, isoflurane post-conditioning significantly increased kidney CD73 mRNA in mice. TGF-β1 neutralization or inhibition of TGF-β1 receptor I kinase with SD-208 prevented CD73 mRNA induction after isoflurane anesthesia. GAPDH and β-actin served as internal loading controls. *P<0.05 vs. pentobarbital-anesthetized mice treated with vehicle. #P<0.05 vs. isoflurane-anesthetized mice treated with vehicle. Error bars represent 1 SEM. D. CD73 immunohistochemistry (400X) in CD73 wild type (WT) or CD73 deficient (KO) mouse kidneys anesthetized with 1.2% isoflurane or with pentobarbital for 4 hr. We observed increased CD73 staining in the kidneys of CD73 WT mice anesthetized with 1.2% isoflurane for 4 hr. CD73 was not visible in kidneys of CD73 WT or CD73 KO mice stained with negative isotype control antibody or in the kidneys from CD73 KO mice stained with CD73 antibody (representative of 4 experiments). E. Quantifications of kidney CD73 immunoreactivity in CD73 WT or CD73 KO mice anesthetized with 1.2% isoflurane or with pentobarbital for 4 hr. CD73 immunoreactivity increased in CD73 WT mice anesthetized with isoflurane. *P<0.05 vs. CD73 WT mice anesthetized with pentobarbital. Error bars represent 1 SEM.
We also performed CD73 immunohistochemistry in mouse kidneys anesthetized with 1.2% isoflurane or equi-anesthetic dose of pentobarbital for 4 hr. Figure 4D shows diffuse renal tubular CD73 staining in mice anesthetized with pentobarbital and increased CD73 staining in the kidneys of mice anesthetized with 1.2% isoflurane for 4 hr (representative of 4 experiments). CD73 was not visible in the kidneys stained with negative isotype control antibody or in the kidneys from CD73 deficient mice stained with CD73 antibody. Quantification of immunohistochemical staining confirmed significantly increase in CD73 immunoreactivity in CD73 wild type mice anesthetized with isoflurane (Figure 4E).
We next determined whether isoflurane anesthesia in mice lead to increased adenosine generation. We determined that renal cortical adenosine levels were higher in mice anesthetized with isoflurane (19.4±2.7 pmol/μg protein, N=4) compared to pentobarbital anesthetized mice (12±2 pmol/μg protein, N=4).
Critical role of CD73 induction and adenosine generation in isoflurane-mediated renal protection in vivo
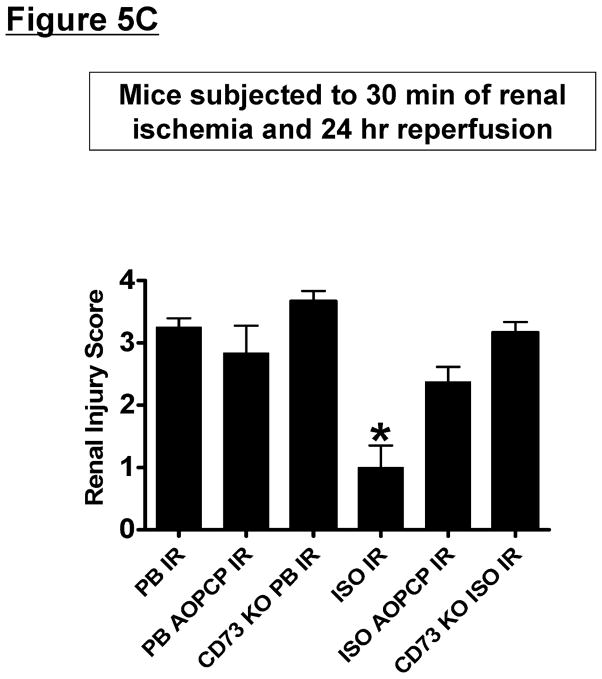
The next series of experiments tested whether direct induction of CD73 with a subsequent increase in adenosine generation is critical for isoflurane-mediated renal protection. CD73 wild type mice anesthetized with pentobarbital (Cr=0.48±0.02 mg/dL, N=4) or with 1.2% isoflurane for 4 hr (Cr=0.47±0.02 mg/dL, N=4) had similar plasma creatinine values after sham-operation. Plasma creatinine significantly increased in CD73 wild type mice subjected to 30 min. of renal ischemia and 24 hr reperfusion compared to sham-operated mice (Figure 5A). However, mice anesthetized with 1.2% isoflurane for 4 hr after renal ischemia (isoflurane post-conditioning) had significantly decreased plasma creatinine 24 hr after injury compared to mice anesthetized with pentobarbital after renal ischemic. Supporting a critical role for CD73 in isoflurane-mediated renal protection against IR, CD73 deficient mice or CD73 wild type mice pretreated with a specific CD73 inhibitor (AOPCP) before renal ischemia were not protected against renal injury with isoflurane post-conditioning. Furthermore, a non-specific but selective antagonist for adenosine receptors (8-Phenyltheophylline) pretreated also abolished renal protective effects of isoflurane post-conditioning (Figure 5A). In addition, mice pretreated with a TGF-β1 neutralizing antibody or with a specific TGF-β receptor 1 kinase inhibitor (SD-208) were not protected against renal injury with isoflurane post-conditioning (Figure 5A). In contrast, isotype antibody-treated mice were protected against ischemic AKI with isoflurane post-conditioning. Finally, reconstitution of CD73 deficient mice with soluble 5′-nucleotidase resulted in restoration of renal protection with isoflurane post-conditioning (Cr=1.89±0.28 mg/dL, N=5, P<0.01 vs. CD73 deficient mice subjected to renal IR). Plasma creatinine in mice treated with adenosine receptor or TGF-β1 signaling inhibitors did not significantly differ from control isotype antibody-treated or vehicle-treated sham-operated mice. Collectively, these studies suggest that CD73 induction as well as increased adenosine generated by isoflurane directly activate adenosine receptors to trigger in vivo renal protection.
Figure 5. CD73-mediated adenosine generation is critical for isoflurane-mediated renal protection.
A. Plasma creatinine levels from CD73 wild type or CD73 deficient (KO) mice subjected to 30 min. renal ischemia and 24 hr reperfusion (IR). After renal IR, mice were further anesthetized with 1.2% isoflurane (ISO) or with equi-anesthetic dose of pentobarbital (PB). Some mice were pretreated with AOPCP (a selective CD73 inhibitor, 2 mg/kg, i.p.) or with 8-PT (non-selective but specific adenosine receptor antagonist, 1 mg/kg, i.p.) 15 min. before sham-surgery or renal ischemia (N=5–6 per group). To neutralize TGF-β1 in vivo, some CD73 wild type mice were injected with 5 mg/kg monoclonal anti-TGF-β1 (MAB240) or control isotype antibody i.v. 10 min. before renal ischemia. To inhibit TGF-β receptor I kinase, we also treated some CD73 wild type mice with a specific inhibitor SD-208 (60 mg/kg p.o.) 1 hr before renal ischemia. Isoflurane post-conditioning significantly attenuated the increases in plasma creatinine after renal IR. CD73 genetic deficiency, CD73 inhibition, adenosine receptor blockade or inhibition of TGF-β1 signaling prevented renal protection with isoflurane post-conditioning. #P<0.05 vs. vehicle-treated pentobarbital-anesthetized mice subjected to renal IR. B. Representative photomicrographs of 5–6 experiments for hematoxylin and eosin staining (magnification 200X) of kidneys of vehicle-treated wild type mice, AOPCP-treated wild type mice or CD73 deficient (KO) mice subjected to 30 min. renal ischemia and 24 hr reperfusion (IR). C. Summary of Jablonski scale renal injury scores (N=4, graded from hematoxylin and eosin staining, scale 0–4) for mice subjected to renal IR. *P<0.05 vs. pentobarbital-anesthetized mice subjected to renal IR. Error bars represent 1 SEM. Vehicle-treated mice anesthetized with pentobarbital after renal ischemia showed severe renal tubular necrosis. Isoflurane post-conditioning significantly attenuated renal tubular necrosis and renal injury scores after renal IR. CD73 deficiency or CD73 inhibition prevented renal protection with isoflurane post-conditioning in mice.
In addition to plasma creatinine, we also examined renal histology after IR. Figure 5B demonstrates severe necrotic renal injury in vehicle-mice subjected to renal IR and anesthetized with pentobarbital. Compared to sham-operated vehicle-treated mice (not shown), the kidneys of mice subjected to renal IR showed significant tubular necrosis, proteinaceous casts with increased congestion. In contrast, consistent with the plasma creatinine data, isoflurane post-conditioning reduced renal tubular necrosis after IR 24 hr after injury. Supporting a critical role of CD73 in isoflurane-mediated renal protection against IR, isoflurane post-conditioning failed to reduce renal tubular necrosis in wild type mice pretreated with AOPCP (a specific CD73 inhibitor) or in CD73 deficient mice. The Jablonski scale [22] renal injury score was used to grade renal tubular necrosis 24 hr after renal IR (Figure 5C). Thirty min. of renal ischemia and 24 hr of reperfusion resulted in severe acute tubular necrosis in vehicle-treated mice anesthetized with pentobarbital after renal ischemic. Consistent with the renal histology data, isoflurane post-conditioning significantly reduced the renal injury score in vehicle-treated mice but not in mice pretreated with AOPCP or in CD73 deficient mice.
CD73 deletion or inhibition prevents isoflurane post-conditioning-mediated reduction in kidney neutrophil infiltration
Figure 6A shows representative images (from 4–5 experiments) of neutrophil immunohistochemitry in kidneys (magnification 200X) of mice subjected to 30 min. of renal ischemia and 24 hr reperfusion. There was heavy neutrophil infiltration in the kidneys of vehicle-treated and pentobarbital anesthetized subjected to renal IR. Stained neutrophils appear dark brown. In sham-operated mice, we were unable to detect any neutrophils in the kidney (data not shown). In contrast, mice anesthetized with isoflurane for 4 hr after renal ischemia had significantly reduced neutrophil infiltration in the kidney 24 hr after IR (Figure 6B). Again, isoflurane post-conditioning failed to reduce renal neutrophil infiltration in wild type mice pretreated with AOPCP (a specific CD73 inhibitor) or in CD73 deficient mice.
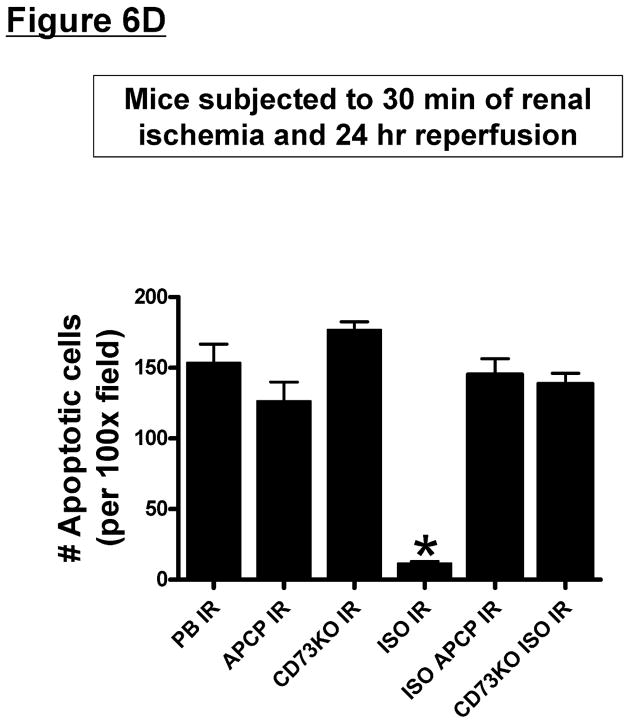
Figure 6. CD73 is critical for isoflurane post-conditioning mediated reduction in renal neutrophil infiltration and renal tubular apoptosis after IR.
A and C. Representative photomicrographs of 4–5 experiments for immunohistochemistry (brown staining) for neutrophil infiltration (A, magnification 200X) and TUNEL staining (C, representing apoptotic nuclei, magnification 100X) from kidneys of vehicle-treated wild type mice, AOPCP-treated wild type mice or CD73 deficient (KO) mice subjected to 30 min. renal ischemia and 24 hr reperfusion (IR). B and D. Quantifications of infiltrated neutrophils per 200X field (B) and apoptotic cells per 100X field (D) in the kidneys of mice after renal IR. *P<0.05 vs. vehicle-treated pentobarbital anesthetized mice subjected to renal IR. Error bars represent 1 SEM. Vehicle-treated mice anesthetized with pentobarbital after renal ischemia showed heavy neutrophil infiltration and numerous TUNEL positive cells. Isoflurane post-conditioning significantly attenuated renal tubular neutrophil infiltration and apoptosis after renal IR. CD73 deficiency or CD73 inhibition with AOPCP attenuated these reductions in renal neutrophil infiltration and apoptosis with isoflurane post-conditioning in mice.
CD73 deletion or inhibition abolishes isoflurane post-conditioning-mediated protection against renal apoptosis
TUNEL staining detected apoptotic renal cells in the kidneys of mice subjected to renal IR resulting in severe proximal tubule cell apoptosis (Figure 6C and 6D, 100X). Renal ischemia and 24 hr of reperfusion resulted in significant apoptosis in the kidneys of vehicle-treated and pentobarbital anesthetized mice. However, mice anesthetized with isoflurane for 4 hr after renal ischemia had significantly reduced number of apoptotic TUNEL-positive cells in the kidney 24 hr after IR (Figure 6D). Again, isoflurane post-conditioning failed to reduce renal tubular apoptosis in wild type mice pretreated with AOPCP (a specific CD73 inhibitor) or in CD73 deficient mice.
Isoflurane treatment reduces HK-2 cell apoptosis via CD73 activation and adenosine generation
Since renal injury after IR in vivo is orchestrated by complex interactions between renal tubule cells, endothelial cells as well as infiltrating pro-inflammatory leukocytes, we tested whether isoflurane directly modulates renal tubular apoptosis in vitro via induction of CD73 activity. HK-2 cells treated with TNF-α and cycloheximide for 16 hr died from apoptosis with robust PARP and caspase-3 fragmentation (Figure 7A) and DNA laddering (Figure 7B). In contrast, HK-2 cells treated with isoflurane showed reduced apoptotic death indicated by decreased PARP and caspase-3 fragmentation and DNA laddering (Figure 7). Supporting a critical role of TGF-β1 as well as CD73 in isoflurane-mediated in vitro protection against HK-2 cell apoptosis, cells pretreated with TGF-β1 neutralizing antibody or with AOPCP were not protected against renal tubular apoptosis with isoflurane treatment (Figure 7).
Figure 7. TGF-β1-mediated CD73 induction is critical for isoflurane-mediated reduction in HK-2 cell apoptosis.
Representative immunoblot of poly(adenosine diphosphate-ribose) polymerase (PARP) and caspase-3 fragmentation (N=3–4 for each group, A) and DNA laddering (B) as indices of HK-2 cell apoptosis induced by TNF-α (20 ng/ml) and cycloheximide (CHX; 10 μg/ml) treatment for 16 hr. HK-2 cells treated with TNF-α and cycloheximide for 16 hr showed robust PARP and caspase-3 fragmentation and DNA laddering. In contrast, HK-2 cells treated with isoflurane showed reduced apoptotic death indicated by decreased PARP and caspase-3 fragmentation and DNA laddering. Supporting a critical role of TGF-β1 as well as CD73 in isoflurane-mediated in vitro protection against HK-2 cell apoptosis, cells pretreated with TGF-β1 neutralizing antibody or with AOPCP were not protected against renal tubular apoptosis with isoflurane treatment.
Isoflurane post-conditioning improves outer medullary renal blood flow after ischemic AKI
We next tested whether isoflurane post-conditioning modulates renal blood flow after IR. Laser Doppler flow probes allowed us to measure outer medullary renal blood cell flux as an index of renal blood flow [23]. Thirty min. of renal IR resulted in significant (~30–35%) reductions in outer medullary renal blood flow in both pentobarbital-anesthetized and isoflurane-anesthetized mice (Figure 8). Although isoflurane anesthesia (1.2%) did not affect renal blood flow at baseline [12,21], isoflurane post-conditioning significantly improved the outer medullary renal blood flow after reperfusion.
Figure 8. Isoflurane improves post-ischemic renal blood flow in mice.
Mice were subjected to 30 min. of renal ischemia and 180 min. of reperfusion (IR, N=4) under pentobarbital anesthesia. Renal blood flow was measured with needle Doppler flow probe before renal ischemia, during renal ischemia and during reperfusion. Mice were kept anesthetized with 1.2% isoflurane or with pentobarbital during the reperfusion period. Changes in renal outer medullary blood compared with pre-ischemic values (% pre-ischemic medullary blood flow) are represented. Mice anesthetized with isoflurane after renal ischemia had significantly improved outer medullary renal blood flow during reperfusion. #P<0.05 vs. pre-ischemic renal blood flow by unpaired t-test. *P < 0.05 vs. mice anesthetized with pentobarbital by 2-way ANOVA.
Discussion
The major new findings of our study are that 1) clinically relevant concentrations of isoflurane (1.25–2.5%) increased adenosine generation and induces CD73 in cultured proximal tubule cells, 2) TGF-β1 released by renal proximal tubules is directly responsible for isoflurane-mediated induction of CD73 activity and synthesis, 3) isoflurane also increased CD73 activity as well as CD73 expression in mouse kidney in vivo via TGF-β1 signaling, 4) TGF-β1 mediated CD73 induction is critical for isoflurane-mediated renal protection against ischemic AKI in mice, 5) isoflurane-mediated in vitro protection against apoptosis in HK-2 cells was significantly attenuated by neutralizing TGF-β1, blocking CD73 activity or antagonizing adenosine receptors and 6) isoflurane post-conditioning accelerated the recovery of outer medullary renal blood flow after ischemic AKI. Since volatile anesthetics are one of the most widely used drugs during the perioperative period, our findings may have significant clinical implications for attenuating the peri-operative ischemic AKI.
Ischemic AKI due to surgical renal ischemia (e.g., kidney transplantation, partial nephrectomy) or renal hypo-perfusion (e.g., due to cardiogenic shock, aortic surgery or sepsis) is a leading cause of AKI [24]. Renal IR results in AKI by necrosis of renal tubules during ischemia as well as by severe inflammatory insults that occur during reperfusion from free radicals as well as infiltrating pro-inflammatory leukocytes [25,26]. We previously showed that volatile anesthetics including isoflurane protect against ischemic AKI by attenuating renal tubular necrosis and decreasing influx of pro-inflammatory neutrophils, lymphocytes and macrophages [11,12]. We also demonstrated that volatile anesthetics produce direct anti-inflammatory and anti- necrotic effects in cultured human kidney proximal tubule (HK-2) cells [13,14]. Here, we additionally demonstrated that isoflurane post-conditioning accelerated the recovery of post-ischemic renal blood flow. Therefore, volatile anesthetics-mediated renal protection is due to combination of direct reductions in ischemic renal tubular necrosis, reductions in renal inflammation and accelerated recovery of no re-flow response after ischemic AKI.
The most novel and exciting finding of our studies is that isoflurane-mediated release of TGF-β1 induced CD73 synthesis in vivo as well as in vitro leading to increased renal tubular adenosine generation. We previously showed that volatile anesthetics-mediated reduction in renal tubular necrosis and inflammation is dependent on the release of renal tubular TGF-β1 [15,16]. TGF-β1 neutralizing antibody prevented isoflurane-mediated reduction in NF-κB nuclear translocation and attenuated the anti-inflammatory effects of isoflurane [27]. Furthermore, volatile anesthetic-mediated renal protection was abolished in mice deficient in TGF-β1 or wild type mice treated with neutralizing TGF-β1 antibody. Recently, Regateiro et al. showed that TGF-β1 induces cell surface CD73 (ecto-5′-nucleotidase) expression in leukocytes including T-lymphocytes, macrophages and dendritic cells [17]. While it is well known that hypoxia or ischemia drives CD73 induction via hypoxia inducible factor-1α binding to the CD73 promoter [28,29], TGF-β1 driven induction of CD73 in renal proximal tubule cells has never been investigated previously. Our current results show that TGF-β1 stimulates new CD73 mRNA and protein synthesis leading to increased CD73 activity and increased renal tubular adenosine generation.
We show in this study that TGF-β1-mediated CD73 induction and adenosine generation is critical for isoflurane-mediated protection against ischemic AKI. Isoflurane-mediated CD73 induction and adenosine generation not only protected cultured renal proximal tubule cells against apoptosis but also reduced in vivo renal IR injury with attenuated renal tubular necrosis, neutrophil infiltration and apoptosis. We used both genetic and pharmacological approaches to test the role of CD73 in isoflurane-mediated renal protection. Cell surface CD73 catalyzes the hydrolysis of AMP to generate adenosine and is considered the rate-limiting step in extracellular adenosine generation [19]. Adenosine signaling regulates diverse physiological effects including cardiovascular control, tissue injury and inflammation in many organs [30–33]. Furthermore, CD73 activation and adenosine generation protects against renal, intestinal and cardiac IR injury and improves barrier function in these organs [34–36]. Indeed, mice deficient in CD73 have increased vascular pathology, tissue injury and inflammation after IR [35,36]. Moreover, enhanced CD73 activity is critical in protecting against intestinal, cardiac and renal IR injury [35–37] as well as ischemic preconditioning [35,36,38]. Finally, CD73 is critical in decreasing mortality and organ injury in a mouse model of sepsis [39]. Taken together, we propose that enhanced CD73 synthesis and activity with increased adenosine generation is the central mechanism of isoflurane-mediated protection against ischemic AKI.
Adenosine generated by CD73 mediates a variety of cellular effects through 4 G-protein coupled purinergic receptors [A1, A2a, A2b and A3 adenosine receptors (ARs)] [19,40]. Activation of A1, A2a or A2bARs protects against IR injury in several organs including the kidney [41–43]. In particular, activation of A1, A2a or A2bARs protects against ischemic AKI [42–45]. A1AR activation protects against ischemic AKI through mechanisms involving Extracellular signal-Regulated Kinase, Akt and HSP27 synthesis [46,47] whereas A2aARs protect the kidney via mechanisms involving cAMP and cAMP response element-binding protein pathways [48]. Our studies show that AR activation is critical for isoflurane-mediated renal protection as a specific AR antagonist 8-phenyltheophylline abolished the renal protective effects of isoflurane. Future studies will elucidate the specific AR subtype(s) involved in renal protection provided by isoflurane-mediated CD73 induction and adenosine generation.
In addition, degradation of AMP to adenosine by isoflurane-mediated CD73 activation may reduce inflammation further by decreasing the availability of extracellular ATP, a recently recognized danger signal that promotes tissue injury and cell death [49–51]. Indeed, after tissue injury, high intracellular concentrations of ATP are released from damaged or necrotic cells to the extracellular compartment [51,52]. Necrotic renal epithelial cells, injured endothelial cells as well as infiltrating leukocytes will continue to release cytotoxic ATP in the kidney after renal reperfusion. Recent studies showed that ATP released by necrotic cells after IR serves as a danger signal, recruits inflammatory leukocytes and further promotes inflammation [49,50,53]. We propose that volatile anesthetic-mediated stimulation of CD73 would serve the dual protective roles by decreasing cytotoxic extracellular ATP and generating cytoprotective adenosine.
We showed that mice exposed to a clinically relevant concentration of isoflurane (1.2%) during the reperfusion period after completion of 30 min. warm renal ischemia were significantly protected against renal IR injury (isoflurane post-conditioning). This greatly increases clinical significance as while ischemia can be predicted in many complicated surgical procedures leading to renal injury, a significant number of patients present to the clinic after renal ischemic injury has already occurred. Volatile anesthetics perhaps may be used to sedate patients in the ICU. Volatile anesthetics provide not only sedation and analgesia but may produce adenosine-mediated organ protection by reducing necrosis and inflammation as opposed to currently utilized intravenous anesthetics with no renal protective properties.
While many previous studies demonstrate tissue-protective effects of several clinically utilized volatile anesthetics [15,54,55], some studies showed detrimental and cytotoxic effects of volatile anesthetics [56–58]. In particular, volatile anesthetics have been shown to produce neuroapoptosis and reduce dendritic branching and synaptogenesis in the developing brain. However, in adult organs, volatile anesthetics in general produce tissue protective effects. It is possible that short term exposure to volatile anesthetics in adults results in tissue protection whereas relatively long exposure in a neonatal tissue may produce cytotoxicity.
To our surprise, isoflurane post-conditioning after renal ischemia significantly improved outer medullary renal blood flow after reperfusion. Based on our findings of in vitro renal tubular protection as well as isoflurane’s favorable effects against post-ischemic renal blood flow, we conclude that isoflurane-mediated induction of CD73 and increased adenosine generation protects against ischemic AKI via at least 2 mechanisms. Isoflurane-mediated adenosine generation may directly provide renal tubular protection against necrotic and apoptotic cell death via activation of renal tubular cell surface adenosine receptors. Furthermore, isoflurane-mediated adenosine generation may protect against ischemic AKI by attenuating pos-ischemic no-reflow phenomenon. Consistent with our findings, Grenz et al. [59] recently demonstrated that crosstalk between renal equilibrative nucleoside transporter 1 and A2b adenosine receptors expressed in vascular endothelia powerfully regulate post-ischemic no-reflow phenomenon. Therefore, increased adenosine generation by isoflurane post-conditioning directly improves post-ischemia no-reflow phenomenon in the kidney via activating adenosine receptors expressed in the vasculature. Future studies are required to examine whether isoflurane post-conditioning increases vascular adenosine by transcriptional regulation of equilibrative nucleoside transporters as eloquently demonstrated by Grenz et al. [59].
Previous studies have shown that ischemia or hypoxia increases extracellular adenosine by hypoxia inducible factor (HIF)-1α dependent transcriptional regulation of adenosine generating enzymes [60]. Indeed, HIF-1α signaling directly induced CD73 expression resulting in adenosine-mediated intestinal protection against IR injury [61]. Although we found induction of CD73 activity in mice subjected to renal IR and anesthetized with either pentobarbital or isoflurane (Figure 4B), we also observed a paradoxical reduction in CD73 mRNA and protein expression in mice subjected to IR (Figure 4A). In contrast, mice treated with isoflurane post-conditioning induced both CD73 expression as well as activity. Therefore, induction of CD73 activity due to renal ischemia/hypoxia may not be sufficient to trigger kidney protection in our study.
In summary, we demonstrated that a commonly utilized volatile anesthetic isoflurane protects against renal tubular necrosis and inflammation after renal IR by inducing cytoprotective adenosine generation (Figure 9). We hypothesize that isoflurane via release of TGF-β1 directly induces CD73 synthesis as well as activity. Induction of CD73 and subsequent adenosine generation results in cytoprotective effects on neighboring renal tubules, endothelial cells or leukocytes via activation of adenosine receptors. Furthermore, degradation of AMP to adenosine by CD73 activation may reduce cell death and inflammation further by decreasing the availability of extracellular ATP, a recently recognized danger signal that promotes tissue injury and cell death. Therefore, modulation of the CD73 signaling pathway and generation of adenosine by isoflurane may have important therapeutic implications to reduce morbidity and mortality arising from AKI. In addition, further studies are required whether other cell types increase CD73 expression and activity in response to isoflurane anesthesia.
Figure 9. Schematic of proposed mechanisms of isoflurane-mediated renal protection.
Abbreviations: ATP = adenosine triphopshate. ADP = adenosine diphosphate, AMP = adenosine monophosphate, CD73 = ecto-5′-nucleotidase, PS = phosphatidylserine, TGF-β1 = transforming growth factor-β1. We hypothesize that isoflurane via PS-mediated release of TGF-β1 directly induces CD73 synthesis as well as activity. Induction of CD73 and subsequent adenosine generation results in cytoprotective effects on neighboring renal tubules, endothelial cells or leukocytes via activation of adenosine receptors. Furthermore, degradation of AMP to adenosine by CD73 activation may reduce cell death and inflammation further by decreasing the availability of extracellular ATP, a recently recognized danger signal that promotes tissue injury and cell death.
Methods
Materials
Isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane] was purchased from Abbott Laboratories (North Chicago, IL). AOPCP (α,β-methylene adenosine-5′-diphosphate, a selective CD73 inhibitor) and 8-PT(8-Phenyltheophylline, an antagonist for adenosine receptors) were obtained from Sigma. Soluble CD73 from Crotalus atrox venom was obtained from Enzo Life Sciences (Farmingdale, NY). Unless otherwise specified, all other reagents were purchased from Sigma (St. Louis, MO). TGF-β1 neutralizing antibody was from R&D Systems (Minneapolis, MN) and the control isotype antibody was obtained from BD Biosciences (San Jose, CA).
Proximal tubule cell culture
Immortalized human renal proximal tubule (HK-2) cells (American Type Culture Collection, Manassas, VA) were grown and passaged with 50:50 mixture of Dulbecco’s Modified Eagle Media/F12 with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Invitrogen) at 37°C in a 100% humidified atmosphere of 5% CO2-95% air. This cell line has been characterized extensively and retains the phenotypic and functional characteristics of proximal tubule cells in culture [62]. We also confirmed our findings in cultured mouse kidney proximal tubule cells. Mouse kidneys were removed, rinsed in PBS containing 0.5% BSA and 2 mM EDTA (GIBCO), minced and digested in collagenase A (1 mg/mL, Sigma) at 37°C for 45 min. with occasional agitation. The cellular digest was filtered through a nylon mesh, centrifuged at 600g for 10 min, and washed twice. Mouse kidney proximal tubules were isolated according to the method of Vinay et al. using Percoll density gradient separation [63]. Cells were plated in 6-well plates when 80% confluent and used in the experiments described below when confluent after 24 hr serum deprivation.
Exposure of HK-2 cells to isoflurane
HK-2 cells were placed in an air tight, 37 °C, humidified modular incubator chamber (Billups-Rothenberg, Inc, Del Mar, CA) with inflow and outflow ports. The inlet port was connected to a vaporizer (Datex-Ohmeda) to deliver isoflurane mixed with 95% air and 5%CO2 (carrier gas) at 10 L/min. The outlet port was connected to a Datex-Ohmeda 5250 RGM gas analyzer that measured isoflurane concentrations. Exposure to isoflurane (0–2.5%) lasted 0–16 hrs. Control cells were exposed to carrier gas in an identical modular incubator chamber. To block the effects of TGF-β1 generated by isoflurane, some HK-2 cells were pretreated with neutralizing TGF-β1 antibody (10 μg/ml, R&D Systems, Minneapolis, MN) 30 min. before isoflurane treatment, respectively. We also used non-neutralizing control isotype antibody to test the specificity of the neutralizing TGF-β1 antibody (BD Biosciences, San Jose, CA).
Reverse transcription polymerase chain reaction and immunoblotting analysis for CD73
We measured mRNA encoding human (HK-2 cells) or mouse CD73 after isoflurane treatment as described [64]. Amplification of the CD73 cDNA was performed using the following primers: forward primer, 5′-CCA ATT CTG AGT GCA AAC AT-3′ and reverse primer, 5′-CCT CCC ACC ACG ACG TCC AC-3′ with an annealing temperature of 62°C resulting in a 315 bp product. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA amplification was performed to control for lane loading: forward primer, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse primer, 5′-CAC CAC CCT GTT GCT GTA GCC-3′ with an annealing temperature of 65°C resulting in a 450 bp product. In addition, HK-2 cell lysates or mouse kidney cortex were also collected for immunoblotting analyses of CD73 (Santa Cruz Biotechnologies) and β-actin (internal protein loading control, Sigma) as described previously after isoflurane treatment [64].
Induction of renal IR injury in mice
After Columbia University Institutional Animal Care and Use Committee approval, we subjected adult male C57BL/6 (Harlan, Indianapolis, IN) as well as CD73 deficient mice (obtained from Dr. Linda Thompson, Oklahoma Medical Research Foundation) [29] to 30 min. of renal IR as described [47,65]. These mice were backcrossed on to the C57BL/6 background for 14 generations. In our model of renal IR, mice were initially anesthetized with intraperitoneal pentobarbital (Henry Schein Veterinary Co., Indianapolis, IN; 50 mg/kg body weight, or to effect) and subjected to right nephrectomy and 30 min. of left renal ischemia or to sham-operation (laparotomy, right nephretomy without renal ischemia). After closure of the abdomen in two layers, the mice were then exposed to an additional 4 hrs of equipotent doses of either pentobarbital or isoflurane (1.2% or ~1 MAC (minimum alveolar concentration defined as the concentration of volatile anesthetic in the lungs that is needed to prevent movement in 50% of subjects in response to a painful stimulus) as described previously [66]. The mice were placed on a heating pad under a warming light to maintain body temperature ~36–38°C. Some CD73 wild type mice were pretreated with AOPCP (a selective inhibitor of CD73, 2mg/kg, i.p.) or 8-PT (a potent but non-selective adenosine receptor antagonist, 1 mg/kg, i.p.) 15 min. before renal ischemia. To neutralize TGF-β1 in vivo, CD73 wild type mice were injected with 5 mg/kg monoclonal anti-TGF-β1 (MAB240) or control isotype antibody i.v. 10 min. before renal ischemia or sham-operation. To further implicate the role of TGF-β1 in isoflurane-mediated CD73 induction and renal protection, we also treated some CD73 WT mice with a specific TGF-β receptor I kinase inhibitor SD-208 (2-(5-Chloro-2-fluorophenyl)pteridin-4-yl]pyridin-4-yl-amine, 60 mg/kg p.o.) 1 hr before renal ischemia or sham-operation [67]. To test whether reconstitution of CD73 in CD73 deficient mice restores the renal protective effects of isoflurane post-conditioning, some CD73 deficient mice were injected with soluble 5′-nucleotidase (5000U i.v.) from Crotalus atrox venom 20 min. before renal ischemia. We collected kidney (cortex and cortico-medullary junction) and plasma 24 hr after IR injury to examine the severity of renal dysfunction (plasma creatinine, renal histology, apoptosis and neutrophil infiltration).
Measurement of Renal Function
Plasma creatinine was measured as described with an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA) [68]. Unlike the Jaffe method, this method of creatinine measurement largely eliminates the interferences from mouse plasma chromagens.
Histological detection of kidney necrosis, apoptosis and neutrophil infiltration
Morphological assessment of kidney H&E staining was performed by an experienced renal pathologist (V.DA.) who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (0–4, Renal Injury Score) to the proximal tubules was used for the histopathological assessment of IR-induced damage as outlined by Jablonski et al. [22] and as described previously in our studies [42,45]. We detected apoptosis after renal IR with TUNEL staining as described elsewhere [69] using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer. Apoptotic TUNEL positive cells were quantified in 5–7 randomly chosen 100X microscope images fields in the corticomedullary junction and results were expressed as neutrophils counted per 100X field. Renal inflammation after IR injury was determined by detecting neutrophil infiltration with immunohistochemistry 24 hr after renal IR. Immunohistochemistry for neutrophils was performed as described previously [70] with a monoclonal antibody against PMN (clone 7/4). A primary antibody that recognized IgG2a (MCA1212, Serotec, Raleigh, NC) was used as a negative isotype control in all experiments. Neutrophils infiltrating the kidney were quantified in 5–7 randomly chosen 200X microscope image fields in the corticomedullary junction and results were expressed as neutrophils counted per 200X field.
Measurement of renal blood flow after IR Injury
We measured changes in renal outer medullary blood flow near the corticomedullary junction before, during and after renal ischemia as described previously [23]. We used a needle flow probe (480 μm diameter; Model TSD145) connected to a laser Doppler flow meter (Biopac Systems, Goleta, CA). After pentobarbital anesthesia, the needle flow probe was inserted directly into outer medullary regions (approximately 1.5 mm beneath the surface of the kidney) and voltage output was recorded on a Biopac data acquisition system and represented as blood perfusion unit. The flow data were represented as the percentage change compared with pre-ischemic blood perfusion unit. We confirmed zero flow after renal pedicle occlusion. After reperfusion of ischemic kidney, mice received with either 1.2% isoflurane anesthesia or with equi-anesthetic doses of pentobarbital for 3 hr at 37 °C.
Induction of HK-2 cell apoptosis
To induce apoptosis, HK-2 cells were exposed to tumor necrosis factor-alpha (TNF-α, 20 ng/ml) plus cycloheximide (10 μg/ml) for 16 hr as described previously [71]. Cycloheximide was added in addition to TNF-α to facilitate apoptosis. Some HK-2 cells were treated with 2.5% isoflurane or with carrier gas for 16 hr after induction of apoptosis. Some HK-2 cells were pretreated with TGF-β1 neutralizing antibody (10 μg/ml) or AOPCP (100 μM) 30 min. prior to isoflurane treatment. HK-2 cell apoptosis was assessed by detecting poly-(adensosine diphosphate-ribose)-polymerase (PARP) and caspase 3 fragmentations as described [47,65].
CD73 activity assay
CD73 activity was measured by tracking the conversion of AMP to adenosine with or without 100μM AOPCP using a modified protocol according to Gelain et al. [72].
High pressure liquid chromatography (HPLC) to measure adenosine
HK-2 cell culture media were collected after isoflurane treatment. Mouse kidneys were snap-frozen in liquid nitrogen at 5 min. intervals immediately after renal ischemia and 30 min. after reperfusion. Kidneys were sonicated in 0.6 M perchloric acid and neutralized by adding 0.6 M potassium phosphate tribasic. The supernatant or cell culture media was assayed for adenosine by HPLC. Adenosine was quantified on a C18 reversed-phase column with a binary low-pressure gradient elution system with a UV detector set to 254 nm as described [73]. Adenosine deaminase activity and adenosine uptake were inhibited with 10μM erythro-9-(2-hydroxy-3-nonly)adenine (EHNA) and 10μM dipyridamole, respectively.
Immunohistochemistry for CD73
CD73 protein expression in mouse kidney was detected 24 hr after isoflurane treatment by immunohistochemistry using CD73 antibody (Santa Cruz Biotechnologies). Paraffin-embedded kidney sections from mice were deparaffinized in xylene and rehydrated through a graded ethanol series to water. Antigen retrieval was performed in 95°C 10 mM sodium citrate (pH 6.0) for 20 min. Endogenous peroxidase activity for all sections was quenched with 0.3% H2O2 while nonspecific binding was reduced with a blocking with buffer containing 10% normal goat serum. The slides were stained for CD73 in sequential incubations with rabbit anti-CD73 antibody (sc-25603, 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C, horseradish peroxidase-conjugated goat anti-rabbit IgG (PI-1000, 1:100 dilution, Vector Laboratories, Burlingame, CA) for 1 hr at room temperature and diaminobenzidine reagent (Vector Laboratories) for 30 sec to 2 min. A rabbit IgG (I-1000, Vector Laboratories) was used at the same concentration as the primary antibody as a negative isotype control. We also tested CD73 immunohistochemistry in the kidneys from CD73 deficient mice anesthetized with pentobarbital or with isoflurane. Renal tubular CD73 immunohistochemistry was quantified as described by Kristina et al. with some modifications [74]. Integrated image densities of 5–7 randomly selected renal tubule areas from each slide were averaged and background measured from isotype control slides were subtracted. Renal tubular CD73 intensity was expressed as fold increase over pentobarbital anesthetized mice.
Statistical analysis
The data were analyzed with Student’s t-test when comparing means between 2 groups or with one way (e.g., plasma creatinine) or two way (e.g., renal medullary blood flow) analysis of variance plus TUKEY’s post hoc multiple comparison test to compare mean values across multiple treatment groups. The ordinal values of the renal injury scores were analyzed by the Mann–Whitney nonparametric test. In all cases, a probability statistic P<0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SEM.
Acknowledgments
This work was supported by National Institutes of Health Grant GM-067081.
We appreciate the surgical and technical assistance provided by Sang Won Park, Ph.D.
We thank Dr. Linda F. Thompson (Oklahoma Medical Research Foundation, Oklahoma City, OK) for the generous gift of CD73 deficient mice.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT. Protecting the kidney during critical illness. Curr Opin Anaesthesiol. 2007;20:106–112. doi: 10.1097/ACO.0b013e328013f83c. [DOI] [PubMed] [Google Scholar]
- 3.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth. 1998;17:117–130. doi: 10.1016/s1053-0770(98)90106-9. [DOI] [PubMed] [Google Scholar]
- 4.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 5.Okusa MD. The changing pattern of acute kidney injury: from one to multiple organ failure. Contrib Nephrol. 2010;165:153–158. doi: 10.1159/000313754. [DOI] [PubMed] [Google Scholar]
- 6.Faubel S. Acute kidney injury and multiple organ dysfunction sindrome. Minerva Urol Nefrol. 2009;61:171–188. [PubMed] [Google Scholar]
- 7.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007:326–331. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 10.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009:1–25. [PubMed] [Google Scholar]
- 11.Lee HT, Ota-Setlik A, Fu Y, et al. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–1324. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Lee HT, Kim M, Kim M, et al. Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–F722. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- 13.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–F78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 14.Lee HT, Kim M, Song JH, et al. Sevoflurane mediated TGF-beta1 signaling in renal proximal tubule cells. Am J Physiol: Renal Physiology. 2008;294:F371–F378. doi: 10.1152/ajprenal.00277.2007. [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Kim M, Kim J, et al. TGF-Beta1 Release by Volatile Anesthetics Mediates Protection against Renal Proximal Tubule Cell Necrosis. Am J Nephrol. 2007;27:416–424. doi: 10.1159/000105124. [DOI] [PubMed] [Google Scholar]
- 16.Song JH, Kim M, Park SW, et al. Isoflurane via TGF-beta1 release increases caveolae formation and organizes sphingosine kinase signaling in renal proximal tubules. Am J Physiol Renal Physiol. 2010;298:F1041–F1050. doi: 10.1152/ajprenal.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regateiro FS, Howie D, Nolan KF, et al. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 18.Chalmin F, Mignot G, Bruchard M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauerle JD, Grenz A, Kim JH, et al. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 21.Kim M, Kim M, Kim N, et al. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–F1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 22.Jablonski P, Howden BO, Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Joo JD, Kim M, D’Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006;17:3115–3123. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda M, Prachasilchai W, Burne-Taney MJ, et al. Ischemic acute tubular necrosis models and drug discovery: a focus on cellular inflammation. Drug Discov Today. 2006;11:364–370. doi: 10.1016/j.drudis.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Bonventre JV. Pathophysiology of ischemic acute renal failure. Inflammation, lung-kidney cross-talk, and biomarkers. Contrib Nephrol. 2004;144:19–30. [PubMed] [Google Scholar]
- 26.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee HT, Chen SW, Doetschman TC, et al. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295:F128–F136. doi: 10.1152/ajprenal.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson LF, Eltzschig HK, Ibla JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csoka B, Nemeth ZH, Rosenberger P, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SW, Chen SW, Kim M, et al. Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther. 2010;333:736–747. doi: 10.1124/jpet.110.166884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Kim M, Song JH, Lee HT. Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transpl. 2008;14:845–854. doi: 10.1002/lt.21432. [DOI] [PubMed] [Google Scholar]
- 33.Lappas CM, Day YJ, Marshall MA, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart ML, Jacobi B, Schittenhelm J, et al. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 35.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 36.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 37.Hart ML, Henn M, Kohler D, et al. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart ML, Much C, Gorzolla IC, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 39.Hasko G, Csoka B, Koscso B, et al. Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strohmeier GR, Lencer WI, Patapoff TW, et al. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 42.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 43.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 45.Lee HT, Xu H, Nasr SH, et al. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 46.Joo JD, Kim M, Horst P, et al. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 47.Kim M, Chen SW, Park SW, et al. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;75:809–823. doi: 10.1038/ki.2008.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia- reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–F412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 49.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 50.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 51.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 54.Bienengraeber MW, Weihrauch D, Kersten JR, et al. Cardioprotection by volatile anesthetics. Vascul Pharmacol. 2005;42:243–252. doi: 10.1016/j.vph.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 55.De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg. 2005;100:1584–1593. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- 56.Lemkuil BP, Head BP, Pearn ML, et al. Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology. 2011;114:49–57. doi: 10.1097/ALN.0b013e318201dcb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, Huang Y, Xu H, et al. Hypoxia inducible factor-1alpha is involved in the neurodegeneration induced by isoflurane in the brain of neonatal rats. J Neurochem. 2012;120:453–460. doi: 10.1111/j.1471-4159.2011.07589.x. [DOI] [PubMed] [Google Scholar]
- 58.Loepke AW, McCann JC, Kurth CD, McAuliffe JJ. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg. 2006;102:75–80. doi: 10.1213/01.ANE.0000181102.92729.B8. [DOI] [PubMed] [Google Scholar]
- 59.Grenz A, Bauerle JD, Dalton JH, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hart ML, Grenz A, Gorzolla IC, et al. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Ryan MJ, Johnson G, Kirk J, et al. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 63.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 64.Kim M, Kim M, Park SW, et al. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–362. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim M, Park SW, Kim M, et al. Selective Renal Over-Expression of Human Heat Shock Protein 27 Reduces Renal Ischemia-Reperfusion Injury in Mice. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Redel A, Stumpner J, Tischer-Zeitz T, et al. Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood) 2009;234:1186–1191. doi: 10.3181/0902-RM-58. [DOI] [PubMed] [Google Scholar]
- 67.Geng H, Lan R, Wang G, et al. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol. 2009;174:1291–1308. doi: 10.2353/ajpath.2009.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.SLOT C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 69.Park SW, Kim M, Chen SW, et al. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen SW, Kim M, Kim M, et al. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 2008;75:499–510. doi: 10.1038/ki.2008.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee HT, Kim M, Jan M, et al. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007;71(12):1249–1261. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- 72.Gelain DP, de Souza LF, Bernard EA. Extracellular purines from cells of seminiferous tubules. Mol Cell Biochem. 2003;245:1–9. doi: 10.1023/a:1022857608849. [DOI] [PubMed] [Google Scholar]
- 73.Delabar U, Kloor D, Luippold G, Muhlbauer B. Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;724:231–238. doi: 10.1016/s0378-4347(98)00580-5. [DOI] [PubMed] [Google Scholar]
- 74.Matkowskyj KA, Schonfeld D, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem. 2000;48:303–312. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]