Abstract
Oxidative stress (OS) and reactive oxygen species (ROS) play a modulatory role in synaptic plasticity and signaling pathways. Mitochondria (MT), a major source of ROS due to their involvement in energy metabolism, are important for brain function. MT-generated ROS are proposed to be responsible for a significant proportion of OS and are associated with developmental abnormalities and aspects of cellular aging. The role of ROS and MT function in cognition of healthy individuals is relatively understudied. In this study, we characterized behavioral and cognitive performance of 5–6 month mice overexpressing mitochondrial catalase (MCAT). MCAT mice showed enhancements in hippocampus-dependent spatial learning and memory in the water maze and contextual fear conditioning, and reduced measures of anxiety in the elevated zero maze. Catalase activity was elevated in MCAT mice in all brain regions examined. Measures of oxidative stress (glutathione, protein carbonyl content, lipid peroxidation, and 8-hydroxyguanine) did not significantly differ between the groups. The lack of differences in these markers of oxidative stress suggests that the differences observed in this study may be due to altered redox signaling. Catalase overexpression might be sufficient to enhance cognition and reduce measures of anxiety even in the absence of alteration in levels of OS.
Keywords: cognition, anxiety, catalase, water maze, fear conditioning, zero maze
INTRODUCTION
Reactive Oxygen Species (ROS) play an important role in cell signaling pathways and metabolic functioning; however, chronic or acute increases in ROS can be damaging to cellular function and integrity (Dröge 2002). The involvement of oxidative stress (OS) in neuropsychiatric pathology and age-related cognitive performance has been characterized (Berr et al. 2000) (Praticò et al. 2002) (Gemma et al. 2007) (Mariani et al. 2005), but is not well understood. Mitochondria (MT), a major source of ROS due to their involvement in energy metabolism, are being increasingly recognized as important in cognitive and neurobiological functioning. MT do not completely metabolize oxygen, resulting in a net production of superoxide (O2•−), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•) (Nohl and Hegner 1978), and MT-generated ROS are proposed to be responsible for a significant proportion of OS (Harman 1972). Over time this may lead to mutations in MT DNA (Ishikawa et al. 3009), perturb mitogenic processes (Dröge 2002), initiate apoptosis in otherwise healthy cells (Sastre et al. 2000), alter redox signaling pathways (Rhee 1999), produce developmental abnormalities (Blomgren and Hagberg 2006) (Rossignol and Frye 2011) and multiple aspects of cellular aging (Miguel et al. 1980). The role of ROS and MT function in cognition of healthy individuals is relatively understudied.
The role of ROS in normal cognitive function is muddled by a paucity of studies in healthy non-pathological controls, and a continuum of effects depending on a multitude of factors including the particular ROS and concentration (Knapp and Klanne 2002). Significant evidence implicates ROS in cell signaling, including the regulation of long term potentiation (LTP) in hippocampal neurons. In this regard, H2O2 is not well understood except that it variably inhibits LTP and long-term depression (LDP) at different incubation times and concentrations (Kamsler and Segal 2003). Studies in this area have focused on superoxide dismutase (SOD), which catalyzes the dismutation of O2•− into H2O2 and O2, sometimes targeted to extracellular compartments (Levin 2005) but not other enzymes such as catalase. Catalase metabolizes H2O2 (the product of SOD) into H2O and O2. Mice overexpressing catalase targeted to the mitochondria (MCAT mice) exhibit significantly prolonged maximum life spans, reduced cardiac and cataract pathology, as well as reduced OS (Shriner et al. 2005). As potential effects of overexpressing catalase in mitochondria on behavioral and cognitive functions of healthy adult mice are unknown, we characterized behavioral and cognitive performance of MCAT mice. Catalase activity in a selection of brain regions was analyzed. Due to the modulatory role of OS and ROS in synaptic plasticity (Serrano and Klann 2004) (Knapp and Klanne 2002) and signaling pathways (D’Autréaux and Toledano 2007), we also assessed whether potential behavioral and cognitive changes were associated with altered measures of oxidative stress, lipid peroxidation, and protein and DNA oxidation in the amygdala and hippocampus, brain regions strongly implicated in anxiety (Davis 1992) and long-term and spatial memory (Good 2002). These markers are useful and common measures of OS in the brain (Butterfield 2006). Additionally, MT DNA exhibits greater accumulation of 8-hydroxyguanosine than nuclear DNA, making it a prime candidate for measuring oxidative damage to DNA in this particular study (Tsutsui et al. 2006). Studies that have examined behavior in the context of antioxidant enzyme function have utilized these measures as well (Clausen et al. 2010) (Liu et al. 2003).
METHODS
Animals
MCAT mice (on a C57BL/6J background) were generated by Peter Rabnovitch at the University of Washington. WT mice were from JAX laboratories (Bar Harbor, Maine). Mice were 5–6 month-old at the start of testing. Animals were maintained on a 12 h light/dark schedule (lights on at 06:00). Laboratory chow (PicoLab Rodent diet 20, # 5,053; PMI Nutrition International, St. Louis MO, USA) and water were provided ad libitum. Behavioral testing took place during the light phase. All procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with IACUC approval at Oregon Health & Sciences University.
Behavioral Testing
Mice were behaviorally tested in the following order: exploratory behavior in the open field (days 1–2), novel object recognition (days 4–5), measures of anxiety in the elevated zero maze (day 5), spatial learning and memory in the water maze (days 8–12), sensorimotor function on the rotarod (days 15–17), and associative learning and hippocampus-dependent memory in contextual and cued fear conditioning (days 18–19). Mice were euthanized on day 19. Equipment was cleaned using 0.5% acetic acid unless otherwise noted.
Open field
Exploratory and anxiety-like behaviors were first assessed in the open-field. Mice were placed into a square arena (40.64 × 40.64 cm) and allowed to explore for 10 minutes in two sessions a day, over a period of two days. Behavioral performance was tracked and scored using an automated video system (Ethovision 7.0 XT, Noldus, Sterling VA). Exploratory behavior was analyzed using total distance moved (cm). Time spent in the more anxiety-provoking center of the open field was analyzed as well.
Elevated zero maze
Measures of anxiety were also assessed in the elevated zero-maze. The enclosure (Kinder Scientific, Poway, CA) consisted of four sections (6 cm wide), alternating between open and closed sections. Mice were placed into an open area of the maze and allowed to explore for 10 minutes. As in the open field test, mice treated with anxiety-reducing agents spend more time in the open areas (Shephard et al. 1994). An automated photobeam detection method (Kinder Motor-Monitor software, Kinder Scientific, Poway, CA) was used to track mouse movements: distance moved (cm), time spent in the open and closed areas as well as crossings between the open and closed areas were analyzed. The elevated zero maze is considered more anxiety specific than the open field test (Choleris et al. 2001).
Novel object recognition
The novel object recognition task is used to measure hippocampus-dependent recognition memory (Broadbent, et al., 2010). Rodents naturally orient their head toward a novel stimulus (Honey et al. 1998), which provides a simple and effective method for quantifying visual recognition. Visuospatial orientation toward a familiar object will attenuate with exposure time (habituation), and contrasting exploration of a novel versus a familiar object provides an index of object recognition and discrimination. Mice were habituated to the open field described above over three days, one ten-minute trial per day. On day 4, mice were exposed to the arena containing two identical objects (small plastic Playmobil® horse figurines, occupying an area of 6 cm2, 5 cm tall) placed near the center of the arena (approximately 6 cm apart). On day 5, one of the objects (henceforth “familiar”) was replaced by a novel object (a small plastic Playmobil® camel figurine) of identical dimensions. Performance of the mice was video recorded and hand scored by a researcher. Orientation to the object, within 2 cm proximity, as well as interaction with the object (climbing, sniffing, pushing) was defined as exploring the object. Novel object recognition and discrimination was calculated as the percent time spent exploring the novel object out of the total time spent exploring both objects. Rearing behavior on top of the object, as in the case of attempting to peer over the walls obscuring the exterior of the environment, was not considered exploring the object. Distance moved was analyzed using automated multiple body point video tracking (Ethovision XT 7.0, Noldus Information Technology, Wageningen, the Netherlands), using parameters previously described (Benice, et al., 2008). Because exploration of the objects relies on proximal visuospatial orientation and interaction, potential differences in locomotor or attentional processing and exploratory behavior may affect the object discrimination measure. Therefore, total distance moved, as well as time spent exploring all objects were also analyzed.
Watermaze
Hippocampus-dependent spatial learning and memory was assessed in the water maze. The maze consisted of a circular pool (diameter 140 cm), filled with opaque water (24°C), divided conceptually into four quadrants. Mice were first trained to locate an “escape” platform (plexiglass circle, 6 cm radius) submerged 2 cm below the surface of the water and made visible by the use of a cue (a colored cylinder, 2.5 cm radius, 8 cm height) during the “visible” trials (days 1 and 2). For the visible platform training days, there were two daily sessions, morning and afternoon, which were separated by an intersession interval of 2 hours. Each session consisted of three trials, with 10-min inter-trial intervals. Mice were placed into the water facing the edge of the pool in one of nine randomized locations (consistent for each mouse). A trial ended when the mouse located the platform. Mice that failed to locate the platform within 60 s were led to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they remained on the platform for a minimum of 10 s.
During the visible platform sessions, the location of the platform was moved between each of the four quadrants to avoid procedural biases in task learning. Subsequent to the visual trials, mice were trained to locate a hidden platform, requiring the mice to rely on extra maze cues for spatial reference and orientation. The platform was not rotated during the hidden platform trials and remained in the same location. One hour after the last trial on each day of hidden platform training, spatial memory retention of the mice was assessed in a “probe” trial (no platform). During the probe trials, mice were placed into the water in the quadrant opposite of the target quadrant. The time spent in the target quadrant compared to the time spent in the three non-target quadrants was analyzed.
The swimming patterns of the mice were recorded with Noldus Ethovision video tracking software (Ethovision XT, Noldus Information Technology, Wageningen, Netherlands) set at six samples/s. The time to locate the platform (latency) was used as a measure of performance for the visible and hidden platform sessions. Latency to reach the target was measured in seconds, and was calculated for each day by averaging values from the six daily trials. Because swim speeds can influence the time it takes to reach the platform, they were also analyzed.
Rotarod
Sensorimotor performance was assessed on a rotarod. Mice were placed on an elevated rotating rod (diameter: 3 cm, elevated: 45 cm, Rotamex-5, Columbus Instruments, Columbus, OH, USA), initially rotating at 5.0 rpm. The rod accelerated 1.0 rpm every 3 seconds. A line of photobeams beneath the rod recorded the latency to fall (seconds). Each mouse received three trials per day, with no delay between trials, on three consecutive days.
Fear Conditioning
Pavlovian fear conditioning is a versatile and well-understood method of assessing associative learning (Maren 2001). In this task, mice learn to associate a conditioned stimulus (CS, e.g. the environmental context, or a discrete cue) with a mild foot shock (unconditioned stimulus, US). CS-US pairings are preceded by a short habituation period, during which a baseline measure of locomotor activity is analyzed. Contextual fear conditioning is considered to involve complex polymodal processing, and to be hippocampus- and amygdala-dependent, while fear conditioning to a modality-specific cue (e.g. a tone) is considered to be hippocampus independent (Phillips and LeDoux 1992). Freezing, defined as immobility with the exception of respiration, is considered a post-exposure fear response (Fanselow 1994), and is a widely used indicator of conditioned fear.
Mice were trained and tested using a Med Associates mouse fear conditioning system and VideoFreeze automated scoring system (Med Associates, St. Albans, Vermont), previously described in detail and validated against traditional hand scoring methods (Anagnostaras et al. 2010). On day 1, the mice were placed inside a dark fear-conditioning chamber. Chamber lights (at 100 lux) turned on at zero seconds, followed by a 120-second habituation period and a subsequent 10-second (2800 hz, 80 dB) tone (cue). A 2-second 0.35 mA or 0.9 mA footshock was administered at 128 seconds, co-terminating with the tone at 130 seconds. After a 20-second inter-stimulus-interval the 10-second tone, which co-terminated with the 2-second shock, was repeated. This pattern repeated for a total of five tone-shock pairings. After the last shock, lights remained on for an additional 20 seconds, for a total trial length of four minutes and 30 seconds. On day 2, hippocampus dependent associative learning was assessed during re-exposure to the training environment for 600 seconds. Three hours later, mice were exposed to a modified environment (scented with vanilla extract, cleaned with 10% isopropanol instead of 0.5% glacial acetic acid, novel floor texture covering the shock-grid, and rounded walls). They were allowed to habituate for 180 seconds, and then exposed to the cue for a second period of 180 seconds. Associative learning was measured as the percent time spent freezing in response to the contextual environment or the tone. Immediate acquisition of conditioned fear was measured following CS-US pairings. Motion during shock (proprietary index, Med Associates) was measured to account for potential differences in response to the shock during training.
Tissue processing
Mice were administered a ketamine-xylazine-acepromazine cocktail intraperitoneally (0.01ml/g of 25mg/ml ketamine (Sigma, St. Louis, MO), 0.625mg/ml acepromazine (Vetus Animal Health, Rockville Centre, New York), 3.125 mg/ml xylazine (Sigma)), and intracardially perfused with 20 ml of 0.9% Phosphate-Buffered Saline. Brains were removed from the skull, and hemispheres were separated with a razor blade. Hippocampus, amygdala, cortex and cerebellum were manually dissected from the right hemisphere and immediately frozen in liquid nitrogen and stored at −80°C for later analysis. Left hemispheres were immediately drop-fixed in 4% paraformaldehyde and placed in the same fixative at 4°C overnight. The following morning, the left hemipsheres were transferred into a 30% sucrose cryoprotectant solution at 4° C until sectioning. For immunohistochemistry, brains were sectioned coronally at 50 µm into 4 alternate series at −22°C from bregma −0.46mm to −2.80 mm (Franklin and Paxinos 2007) using a HM505E cryostat (Microm). Tissue was placed in cryopreservative at 4° C until immunohistochemistry was performed.
Immunohistochemistry and Quantification
For visualization of 8-hydroxyguanosine, sections were rinsed in phosphate-buffered saline (PBS) and incubated in 4% normal goat serum (NGS) in PBS containing 0.4% triton x-100 (PBS-TX) for 1 hour. After rinsing in PBS, sections were incubated with primary anti-sera (α-8 hydroxyguanosine (1:250, mouse, abcam)) in 4% NGS and PBS-TX overnight at room temperature. Tissue was then rinsed in PBS and incubated for 2.5 hours in donkey-anti mouse antibody conjugated to Texas Red in PBS-TX (1:200; Life Technologies). Subsequently, sections were rinsed in PBS, slide mounted and coverslipped with Vectashield containing Dapi (Vector Labs), and 8-hydroxyguanosine immunoreactivity was analyzed using an Olympus IX81 confocal microscope (Olympus, Center Valley, PA) equipped with SlideBook 5 software (Intelligent Imaging Innovations, Inc., Denver, CO, USA). Three consecutive sections containing the CA1 region of the hippocampus (near bregma −1.94mm) and basolateral amygdala (near bregma −1.46mm) were identified by Dapi nuclear label using a mouse brain atlas (Franklin and Paxinos 2007). Images were captured within these sections using a 20× objective and area occupied by immunoreactivity was quantified within two 75 × 55 µm grids placed within the hippocampal CA1 region and the basolateral amygdala. Area occupied by 8-hydroxyguanosine immunoreactivity was calculated as immunoreactive pixels above a set background threshold level for each brain region.
Right Hemisphere Tissue Preparation
Regional brain tissues from the right hemisphere were homogenized in 200 µl RIPA buffer containing protease inhibitor cocktail (Roche). Immediately after homogenization, samples were divided in aliquots and prepared for their respective assays (pyridine derived thiol scavenger for the glutathione assay, butylated hydroxytoluene (BHT) for lipid peroxidation and protein carbonyl content determination).
Catalase activity
Catalase activity was measured using a discontinuous ferrous oxidation of H2O2 assay. Ammonium ferrous sulfate oxidation in a xylenol orange solution was followed at 560 nm as previously described (Jiang et al., 1990; Ou and Wolff, 1996). Homogenates (50 µl) were combined with 2.2 mM H2O2 (5 µl) and immediately vortexed. At 0 and 10 minutes following addition of 2.2 mM H2O2, 20 µl of sample was added to 400 µl FOX1 reagent to stop the reaction. FOX1 reagent was prepared at a final concentration of 0.1M sorbitol, 250 µM ammonium ferrous sulfate, 100 µM xylenol orange, 25 mM H2SO4. Catalase activity was calculated as Units/ml = [ΔA/min (blank) – ΔA/min (sample)] × d × v/(V × ε), where A = absorbance, d = dilution of original sample, v = volume of reaction, V = Sample volume in catalase reaction, and ε = extinction coefficient of H2O2 in the FOX 1 reagent (2.35 × 105 M–1 cm–1).
GSH/GSSG ratio, Protein Carbonyl Content, and Lipid peroxidation
GSH and GSSG were determined in brain tissue by measuring thiols via reaction with 5,5'-Dithiobis-(2-Nitrobenzoic Acid) (DTNB) acid using a microplate assay (Oxford Biomed, Riviera Beach, Florida) following the manufacturer’s instructions. Protein carbonyl content was determined using a 2,4-dinitrophenylhydrazine assay (49). The extent of lipid peroxidation was estimated as the concentration of two common polyunsaturated fatty acid peroxides, malondialdehyde (MDA) and 4-hydroxyalkenals (HAE), using a microplate assay (Oxford Biomed) using manufacturer’s instructions. Because of limited amygdala tissue, lipid peroxidation was only measured in the hippocampus. Aliquots of hippocampal homogenates (25 µl) were incubated with N-methyl-2-phenylindole (80 µl) and methanesulfonic acid (20 µl) for 1hour at 45 °C, and absorbance of the samples was read at 586 nm using a spectrophotometer.
Mitochondrial isolation
The cortex and hippocampus were dissected on ice from 6 month old MCAT and WT mice and immediately placed in 750 µl of mitochondrial homogenization medium (0.32 M sucrose, 1 mM EDTA, 10mM Tris-HCl, pH 7.8) on ice. The tissue was homogenized with 10 strokes by a Dounce-type glass homogenizer. The homogenate was transferred to a microcentrifuge tube and spun for 10 min at 1,000 g at 4°C. The homogenate was transferred to a new microcentrifuge tube and spun for 10 min at 1,000 g at 4°C to remove the remaining nuclear material. The supernatant was then transferred into a new microcentrifuge tube and spun for 20 min at 13,000 g at 4°C. The resulting pellet was the crude mitochondrial fraction. The supernatant was discarded and the pellet was resuspended in 750 µl of homogenization medium and re-spun for 10 min at 13,000 g at 4°C. The resulting pellet was immediately frozen and stored at −80°C until time of lipid peroxidation analysis, at which point it was re-suspended in 250 µl of PBS. The lipid peroxidation assay was performed as describe above.
Statistics
Data are reported as averages ± SEM. Results were considered significant at p < 0.05. When data were analyzed using one-way ANOVAs, Bonferroni corrections were applied as a post-hoc test to control for multiple comparisons. Non-normal data were analyzed using nonparametric alternatives (Mann-Whitney) if traditional transformations were not sufficient to satisfy requirements of parametric distributions. Novel object preference was first analyzed using two-way ANOVA comparing the novel and familiar object and genotype, followed by t-tests to compare against a hypothetical value (no preference, 50%). Water maze learning curves were analyzed using repeated measures ANOVA. Multivariate statistics were calculated if Mauchly’s test of sphericity was violated but Box’s covariance matrix was preserved. Probe trial analyses utilized one-way ANOVAs with a Dunnet’s post-hoc test to compare non-target quadrants against the target quadrant. Rotarod performance was similarly analyzed as repeated measures ANOVAs. Fear conditioning was assessed using repeated measures ANOVAs to compare between time-points and genotype, with one-way ANOVAs to compare between groups for single measures. Catalase and measures of oxidative stress were analyzed using t-tests with corrections for multiple comparisons.
RESULTS
Open field
There were no genotype differences in activity or measures of anxiety in the open field (Table 1). Over the 4 sessions, there was a linear decrease (F(1,16) = 13.008, p = 0.002) in time spent in the center (effect of session: (F(3,48) = 7.34, p < 0.0001; session 1 > session 3, p = 0.005; session 1 > session 4, p = 0.008); session 2 > session 3, p = 0.002; session 2 > session 4, p = 0.009). Distance moved similarly exhibited a decrease over time (F(3,48) = 131.72, p < 0.0001) which was primarily linear (F(1,16) = 539.40, p < 0.0001) with each subsequent session a lower distance moved than the previous one (Session 1 vs Session 2, Session 2 vs Session 3: p < 0.0001; Session 3 vs Session 4, p = 0.001). The number of crossings between the periphery and center also decreased over time (F(3,48) = 41.36, p < 0.0001) with a trend towards a difference between sessions 1 and 2 (p = 0.054), and a decrease between sessions 2 and 3 (p < 0.0001).
Table 1.
Behavioral performance of MCAT and WT mice in the rotorod, open field, elevated zero maze, fear conditioning, and novel object recognition tests.
| Rotarod |
Open Field |
||||||
|---|---|---|---|---|---|---|---|
|
Fall Latency (s) |
Time in Center (s) |
||||||
| Session 1 |
Session 2 |
Session 3 |
Session 1 |
Session 2 |
Session 3 |
Session 4 |
|
| WT | 33.79 ± 2.83 |
41.74 ± 3.93 |
46.92 ± 4.11 |
145.32 ± 19.75 |
150.36 ± 16.29 |
130.16 ± 25.20 |
109.87 ± 24.08 |
| MCAT | 37.49 ± 4.33 |
42.83 ± 4.39 |
54.52 ± 5.41 |
120.42 ± 14.57 |
124.88 ± 10.79 |
69.11 ± 7.42 |
80.27 ± 11.48 |
|
Zeromaze |
Distance Moved (cm) |
||||||
| Velocity (cm/s) | Crossings* |
% Time in Open* |
Session 1 | Session 2 | Session 3 | Session 4 | |
| WT | 4.46 ± 0.15 |
29.56 ± 3.92 |
7.62 ± 1.08 |
6447.80 ± 429.98 |
4867.29 ± 271.37 |
3865.7415 ± 285.79 |
3763.43 ± 249.96 |
| MCAT | 4.47 ± 0.09 |
39.89 ± 3.39 |
14.40 ± 1.61 |
7186.97 ± 224.72 |
5284.29 ± 241.16 |
4374.05 ± 211.68 |
3199.96 ± 249.94 |
|
Fear Conditioning |
Border Crossings |
||||||
|
Training |
Session 1 |
Session 2 |
Session 3 |
Session 4 |
|||
| Tone 3 |
Tone 4 |
Tone 5 |
|||||
| WT | 6.02 ± 4.37 |
13.19 ± 8.87 |
13.75 ± 4.98 |
70.44 ± 6.24 |
65.89 ± 4.20 |
37.00 ±4.13 |
33.00 ±4.72 |
| MCAT | 10.60 ± 6.02 |
10.09 ± 5.91 |
27.18 ± 0.69 |
75.00 ±8.12 |
61.22 ±4.90 |
38.56 ±4.88 |
33.11 ±4.41 |
|
Novel Object |
|||||||
|
% Baseline Freezing |
% Context Freezing* |
%Cued Freezing |
Exploration Time (Old + Novel) (seconds) |
Time Exploring Novel Object (%) |
|||
| WT | 0.99 ± 0.87 |
4.61 ± 1.44 |
22.02 ± 6.89 |
77.17 ± 11.40 |
61.34 ± 3.04 |
||
| MCAT | 0.10 ± 0.10 |
10.24 ± 1.68 |
31.08 ± 4.47 |
56.86 ± 5.68 |
56.16 ± 3.06 |
||
Novel object recognition
One WT animal was excluded from the analysis because of extremely diminished exploration time (less than 4 seconds). No effect of genotype was observed in total time spent exploring the objects, nor in percentage of time exploring the novel object. (Table 1) Both groups showed robust recognition of the novel object (WT: t(14) = 2.85, p = 0.0128; MCAT: t(16) = 5.28, p < 0.0001).
Elevated zero maze
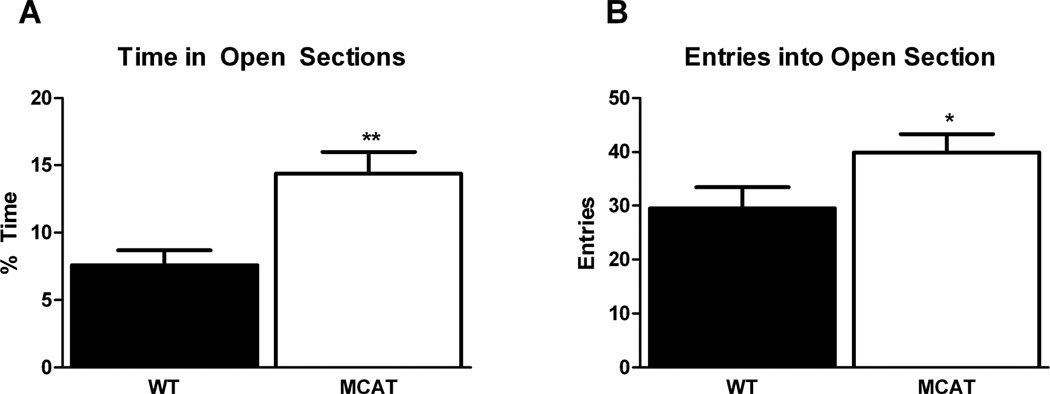
MCAT mice showed reduced measures of anxiety as compared to WT mice. As the data was not normally distributed, a Mann-Whitney U test was used for analysis. MCAT mice spent significantly more time in the open areas of the elevated zero maze than WT (Table 1, Fig. 1A). (MDMCAT = 14.40 ± 1.61, MDWT = 7.62 ± 1.08, U = 7.00, W = −.52000, Z = −2.96, p = 0.003, Fig. 1A), and exhibited significantly greater number of crossings between the closed and open area (F(1,16) = 5.12, p = 0.038)(Table 1, Fig. 1B). There was no genotype difference in velocity in the elevated zero maze (Table 1) suggesting that overall activity between groups was uniform.
Fig. 1.
MCAT mice spent more time in the open areas (A) and crossed into the open areas of the elevated zero maze more frequently (B) than WT mice.**p <0.01;*p < 0.05.
Water maze
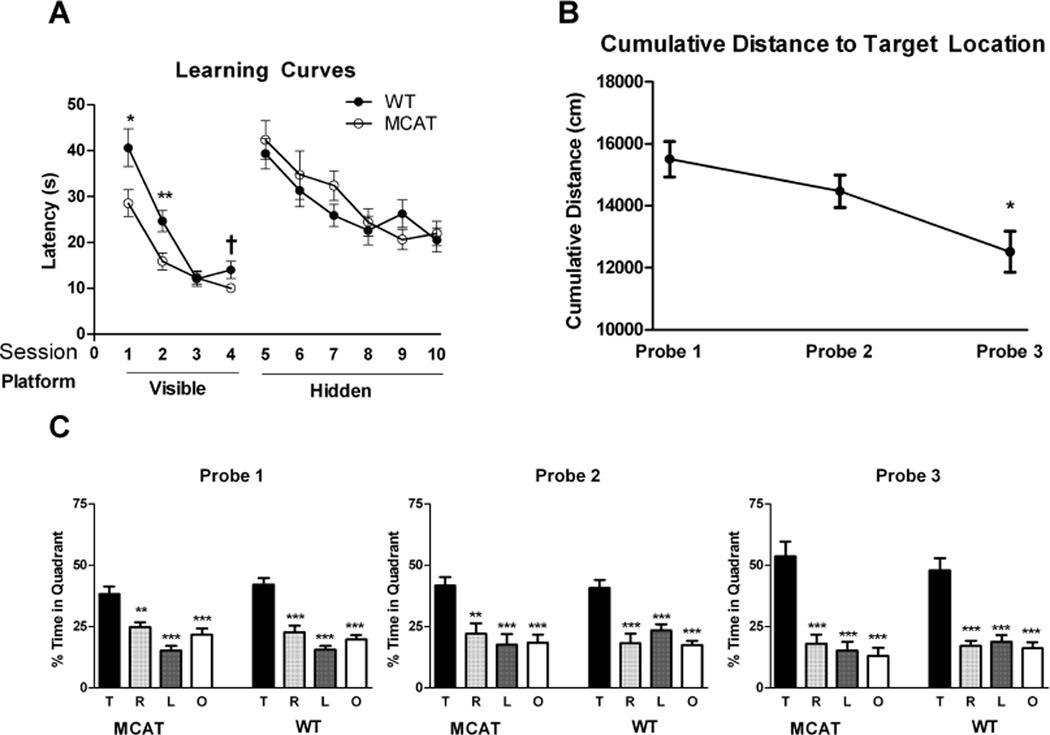
Genotype differences were not found in swim speeds during the visible (WT: 20.92 ± 0.56 cm/s; MCAT: 21.94 ± 0.76 cm/s) or hidden sessions (WT: 18.45 ± 0.62 cm/s; MCAT: 18.54 ± 0.56 cm/s). Both genotypes improved their performance during visible platform training (effect of session: (λ = 0.14, F(3,14) = 28.45, p < 0.0001; session 1 vs session 2, p < 0.001; session 1 vs session 3, p < 0.001, session 1 vs session 4, p < 0.001; session 2 vs session 3, p < 0.01, session 2 vs session 4, p < 0.01)(Fig. 2A). There was also a trend toward a genotype × session interaction (p = 0.089). A between-subjects effect of genotype showed that on average, MCAT mice required less time to locate the escape platform (F(1,16) = 13.95, p = 0.002). A multivariate ANOVA confirmed the group difference (λ = 0.45, F(4,13) = 3.47, p = 0.039), and indicated that the group difference was driven by the sessions 1 (p = 0.030) and 2 (p = 0.009) and possibly session 4 (p = 0.060). These results suggest that while both groups acquire the task, MCAT mice may do so at a faster rate than WT controls.
Fig. 2.
Spatial learning and memory of MCAT and WT mice in the water maze. A. Learning curves during the hidden and visible portions of the water maze. For details, see text. B. Both genotypes exhibited reduced cumulative distance to the target by the third probe trial. C. Both genotypes show a target quadrant bias in the probe trials. ***p < 0.001; **p <0.01;*p < 0.05.
Both genotypes also improved their performance during hidden platform training but there was no genotype difference in performance (Fig. 2A). There was a significant decrease in escape latency (effect of session: λ = 0.33, F(5,12) = 4.85, p = 0.012; session 5 vs session 7, p < 0.01, session 5 vs session 8, p < 0.001; session 5 vs session 9, p < 0.001, session 5 vs session 10, p < 0.001; session 6 vs session 8, p < 0.05, session 6 vs session 9, p < 0.05, and session 6 vs session 10, p < 0.01).
When spatial memory retention was assessed in the probe trials, both genotypes showed robust preference for the target quadrant in each probe trial (MCAT: Probe 1: F(4,9) = 16.14, p < 0.001; Probe 2: F(4,9) = 8.73, p < 0.001; Probe 3: F(4,9) = 20.67, p < 0.0001; WT: Probe 1: F(4,9) = 26.85, p < 0.0001; Probe 2: F(4,9) = 13.47, p < 0.001; Probe 3: F(4,9) = 21.62, p < 0.0001, Fig. 2C), with mice spending significantly more time in the target quadrant than any other quadrant. Performance in the probe trials improved between the first and last session, as measured by a decrease in cumulative distance to the previous location of the platform (F(2,32) = 6.46, p = 0.004, Fig. 2B), with mice swimming significantly closer in the third probe trial than the first (p = 0.022), but there was no genotype difference.
Rotarod
There was no genotype difference in rotarod performance. Both genotypes improved performance with training. Rotarod fall latency increased with each session (F(2,32) = 8.52, p = 0.001) in a linear fashion (F(1,16) = 14.39, p = 0.002) and fall latencies in session 3 were greater than those in session 1 (p = 0.002) and session 2 (p = 0.010) (Table 1).
Fear conditioning
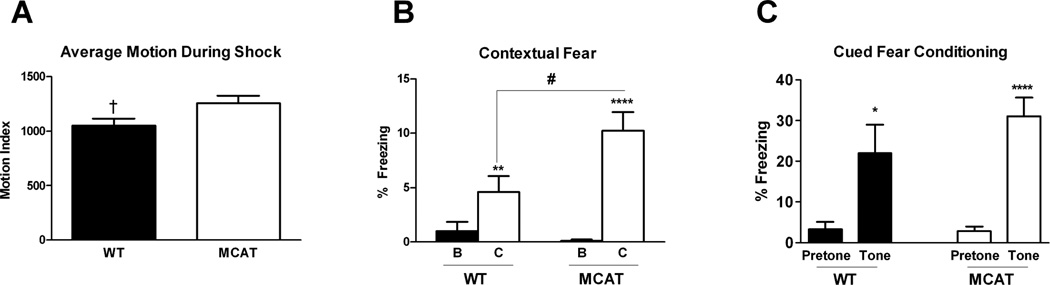
Baseline freezing did not differ between groups (WT: 0.99 ± 0.87%; MCAT: 0.10 ± 0.10%) (Table 1). Freezing data from inter-shock intervals during training were largely nonnormal and could not be analyzed using repeated measures ANOVA. To assess acquisition of contextual fear, the last 110 seconds (after the final shock) of the trial were analyzed instead. There was an increase in freezing from baseline (F(1,16) = 27.32, p < 0.0001), but no interaction with genotype (WT: 19.22 ± 5.73%; MCAT: 18.40 ± 4.42%). Freezing during the first two tone presentations did not differ from baseline in either genotype, so data were analyzed from tones 3 to 5 (Table 1). Freezing during training tones exhibited an effect of tone (F(2,32) = 4.54, p = 0.018) with a linear increase (F(1,16) = 12.45, p = 0.003) such that freezing during tone 5 was significantly elevated compared to that during tone 3 (p = 0.008). There was no interaction with genotype. Motion during shock did not differ between the genotypes and there was no effect of shock order, or interaction thereof with genotype. There was a trend towards an effect of genotype (F(1,16) = 4.48, p = 0.0502) indicated an overall (marginal) elevated motion during shock in MCAT mice (1255.32 ± 73.28) compared to WT (1053.49 ± 60.96) (Fig. 3A)
Fig. 3.
Fear conditioning of MCAT and WT mice. A. Average motion during the shock. B. MCAT mice showed enhanced contextual fear conditioning compared to WT mice. C. Both genotypes showed cued fear conditioning. ***p < 0.001; **p <0.01;*p < 0.05; †p = 0.0502.
Contextual fear conditioning was elevated compared to baseline freezing (F1,16) = 55.54, p < 0.0001) (Table 1, Fig. 3B), and there was an interaction with genotype (F(1,16) = 12.48, p = 0.003). Both groups exhibited a significant increase in freezing (WT: F(1,8) = 22.51, p = 0.001; MCAT: F(1,8) = 36.38, p < 0.0001). A multivariate analysis accompanied by a Bonferroni correction indicated that the genotype interaction stemmed from the freezing values in the contextual test (effect of genotype: λ = 0.223, F(2,15) = 7.49, p = 0.006, between subjects effect for contextual freezing: F(1,16) = 6.75, p = 0.022) with MCAT mice exhibiting significantly greater freezing than WT mice. Contextual freezing did not correlate with motion during shock overall (r2 = 0.01), or within either group (WT: 0.12; MCAT: r2 = 0.01).
Freezing in response to the cued environment did not differ between genotypes (Table 1, Fig. 3C). A repeated measures analysis indicated an effect of the tone (F(1,16) = 74.50, p < 0.0001), but no interaction with genotype. Freezing during the tone presentation did not differ between genotypes.
To determine whether increasing the shock intensity would modulate the genotype difference in contextual fear conditioning, we repeated the fear conditioning experiment and increased the shock intensity from 0.35 to 0.9 mA. Increasing the shock intensity increased the acquisition of conditioned fear (F(1,30) = 17.256, p < 0.0001), but there was no interaction with genotype. When freezing during the tone presentation in training was analyzed, there were no interactions with tone-presentation and either cohort or genotype. The presence of an effect of tone number was preserved (F(2,60) = 7.947, p = 0.001). However, there was a trend towards an interaction with cohort (F(2,60) = 2.761, p = 0.071). Overall, mice shocked with 0.9 mA exhibited greater freezing to the tones (F(1,30) = 5.514, p = 0.026).
Analysis of contextual freezing indicated that increasing the shock amplitude elevated freezing in the WT (F(1,17) = 4.616, p = 0.046) animals, but not in MCAT mice. This elevation may have been due to a small subset of animals (3/10) which exhibited freezing > ~twice the mean. There was no difference between genotypes in contextual freezing under these conditions with the increased shock amplitude. As in the previous experiment, there was also no genotype difference in response to the cue in this experiment.
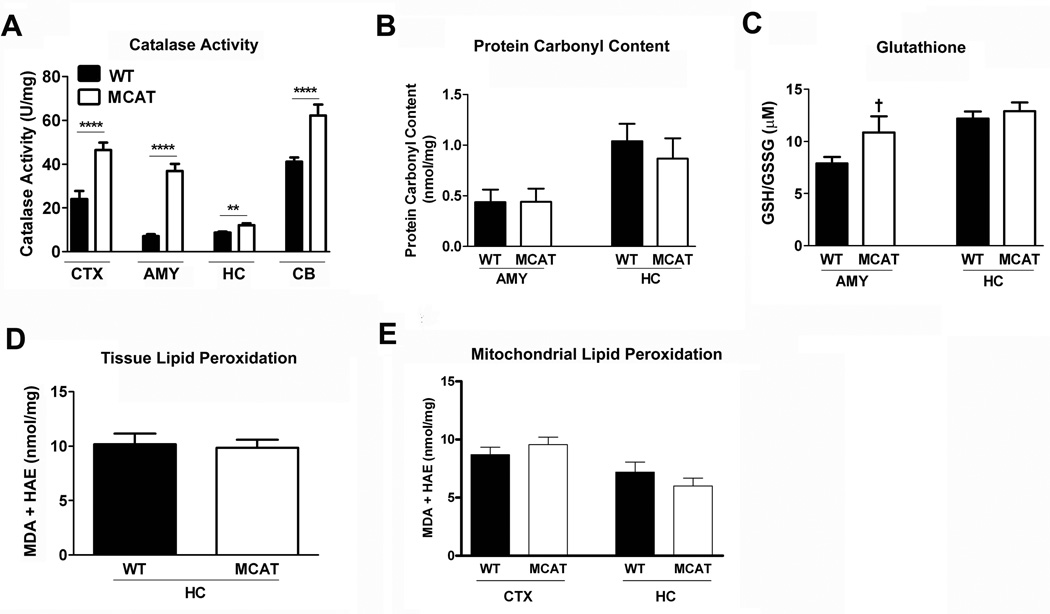
Catalase Activity
Catalase activity was measured in the prefrontal cortex, amygdala, hippocampus and the cerebellum (Fig. 4A). Catalase activity was elevated in MCAT mice compared to WT mice in all brain regions (Prefrontal cortex: t(15) = 4.43, p = 0.0005; Amygdala: t(16) = 8.79, p < 0.0001); Hippocampus: t(16) = 3.63, p = 0.0023; Cerebellum: t(14)= 4.35, p = 0.0007) (Fig. 4A).
Fig. 4.
A. MCAT mice showed enhanced catalase activity in the cortex (CTX), amygdala (AMY), hippocampus (HC), and cerebellum (CB). B. MCAT and WT showed similar protein carbonyl contents in the amygdala and hippocampus. C. MCAT mice showed a trend towards an enhanced GSH/GSSG ratio in the amygdala but not hippocampus. D. MCAT and WT mice show similar lipid peroxidation in the hippocampus. E. There was no genotype difference in lipid peroxidation in mitochondria isolated from the hippocampus or cortex. ***p < 0.001; **p <0.01; +p = 0.093.
Measures of Oxidative Stress
The protein carbonyl content in the amygdala and hippocampus was similar in both genotypes (Fig. 4B). The ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) in the hippocampus did not differ between genotypes (WT: 12.20 ± .68; MCAT: 12.89 ± 0.82, Fig. 4C), but there was a marginal trend towards a difference indicating greater levels of oxidative stress in the amygdala of WT mice (7.76 ± 0.66) compared to the MCAT mice (10.85 ± 1.54)(p = 0.093).
Lipid peroxidation in the hippocampus did not differ between MCAT or WT mice (Fig. 4D). Next, we isolated mitochondria to determine whether there was a genotype difference in lipid peroxidation in the hippocampus or cortex. As shown in Fig. 4E, there was no genotype difference in lipid peroxidation in either the hippocampus or cortex either. Finally, 8-hydroxyguanine immunoreactivity did not differ between genotypes in either brain region (Fig. 5A; see Fig. 5A for a representative image of MCAT CA1).
Fig. 5.
A. Similar 8-Hydroxyguanine immunorectivity in the CA1 region of the hippocampus (CA1) and basolateral nucleus of the amydala (BLA) of MCAT and WT mice. B. Representative image of 8-Hydroxyguanine immunorectivity in the CA1 region of the hippocampus in an MCAT mouse (red) (nuclear counterstain in blue).
DISCUSSION
The results from the present study indicate enhancements in hippocampus-dependent spatial memory in the water maze and contextual fear conditioning, and reduced measures of anxiety in the elevated zero maze in MCAT mice, implicating catalase activity in hippocampal and amydala behavior and cognition. Catalase activity was universally elevated in MCAT mice in all brain regions examined. In contrast to catalase activity, measures of oxidative stress (GSH/GSSG ratio, protein carbonyl content, lipid peroxidation, and 8-hydroxyguanine) did not differ between the genotypes. These results suggest that catalase overexpression is sufficient to produce cognitive enhancements and reduced measures of anxiety even in the absence of alteration in levels of OS. The lack of differences in common markers of OS suggests that the differences observed in this study are likely due to altered redox signaling, a result which has been observed in similar studies (Lee et al. 2012). The presence of such enhancements in these relatively young animals, but a lack of differences in OS experiments utilizing pharmacological methods such as catalase mimetics in similarly aged animals, suggests that effects during development and throughout life or some compensatory mechanism yet to be explored might be required.
Consistent with the genotype difference seen in the elevated zero maze, oxidative stress (Rammal et al, 2008; Bouayed et al. 2009), antioxidant capacity (Filiou et al. 2011), and mitochondrial DNA variation (Gimsa et al. 2009) are related to anxiety-related behavior. However, there was a genotype difference in measures of anxiety in the elevated zero maze but not in the open field. Athough both the open field and elevated zero maze are being used to assess measures of anxiety, the open field is thought to not provide a particularly sensitive measure of anxiety for specific aspects of anxiety, including trait anxiety or anxiety disorders (Prut and Belzung, 2003).
The fear conditioning protocol in this study, employing brief (10 second) tones in rapid succession may make forming contextual associations (rather than just cue-associations) more challenging. This might have resulted in the relatively low levels of freezing in both genotypes. Increasing the shock intensity ameliorated these differences by elevating freezing in the WT animals (as well as freezing immediately following training). Cognitive differences between the genotypes may therefore reflect subtle enhancements apparent under challenging training conditions.
ROS, including H2O2, are important signaling messengers whose effects on cognitive function are not well characterized (Stone and Yang 2006). H2O2 is generated from enzymatic dismutation of superoxide (O2•−) by Super Oxide Dismutase (SOD) (Adam-Vizi 2005). Overexpression of SOD has been shown to modulate cognition (Levin 2005; Lee et al. 2012). Antioxidants, including catalase mimetics, can offset or ameliorate cognitive deficits due to OS in aged animals (Clausen et al., 2010; Clausen et al. 2012), including deficits in fear conditioning (Clausen et al. 2010; Clausen et al. 2012) and performance in the water maze (Lee et al. 2012) and reduced catalase activity is associated with hippocampus-dependent spatial memory impairment (Wang et al. 2009) (Cui et al. 2012). Interestingly, the present study indicates enhanced performance of MCAT mice in these same tests. These studies differ from the present model which utilizes chronic overexpression of catalase targeted specifically to the mitochondria, the primary source of H2O2 involved in neuronal signaling (Bao et al. 2009). The action of catalase on endogenous H2O2 may mediate physiological processes such as synaptic plasticity and LTP and produce the cognitive enhancements observed in MCAT mice.
MT dysfunction is indicated in a growing number of pathologies and behavioral phenotypes. The diverse role that MT play in these outcomes severely limits targeted hypothesis testing at this descriptive level of investigation and as such our analysis remains speculative. Regardless, the absence of measurable OS at gross levels, which have been broadly employed in other studies, promotes the idea that perturbations in physiological redox systems may be sufficient to produce alterations in behavior in the absence of these measures of OS. This possibility suggests that either more sensitive and exact measures of OS should be employed or that increased emphasis should be placed in elucidating effects of altered redox signaling. Nevertheless, the results provide support for an important role for catalase in cognitive function.
In summary, MCAT mice show enhancements in hippocampus-dependent spatial memory in the water maze and contextual fear conditioning, and reduced measures of anxiety in the elevated zero maze as compared to WT mice. These differences were not associated with common measures of OS. Future studies are warranted to determine the molecular and potential signaling mechanisms underlying these effects.
Acknowledgements
This work was supported by NASA NSCOR NNX10AD59G and NIH T32 Grants T32 ES007060 and T32 DA07262. We thank Gregory A. Nelson, Tamaka A. Jones, and Lauren Yeiser for their generous support and assistance with this study.
Footnotes
There are no conflicts of interests to report.
References
- Abou-Sleiman PM, Muqit MMK, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat. Rev. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Adam-Vizi V. Production of Reactive Oxygen Species in Brain Mitochondria: Contribution by Electric Transport Chain and Non-Electric Transport Chain Sources. Antiox. Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Wood SC, Shuman T, Cai DJ, Leduc AD, Zurn KR, Zurn JB, Sage JR, Herrera GM. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Front. Behav. Neurosci. 2010;4:1–11. doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signalling. J. Neurosci. 2009;29:9002–9010. doi: 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Berr C, Balansard B, Arnaud J, Roussel AM, Alpérovitch A. Cognitive decline is associated with systemic oxidative stress: the EVA study. Etude du Vieillissement Artériel. J. Am. Ger. Soc. 2000;48:1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia–ischemia in the developing brain. Free Radical Biol. Med. 2006;40:388–397. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxidat. Med. Cell. Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn. Memory. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA. Oxidative Stress in Neurodegenerative Disorders. Antiox. Redox Signal. 2006;8:1971–1973. doi: 10.1089/ars.2006.8.1971. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MT, Rice ME. H2O2 Is a Novel, Endogenous Modulator of Synaptic Dopamine Release. J. Neurophysiol. 2001;85:2468–2476. doi: 10.1152/jn.2001.85.6.2468. [DOI] [PubMed] [Google Scholar]
- Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol. Aging. 2010;31:425–433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen A, Xu X, Bi X, Baudry M. Effects of the Superoxide Dismutase/Catalase Mimetic EUK-207 in a Mouse Model of Alzheimer's Disease: Protection Against and Interruption of Amyloid and Tau Pathology and Cognitive Decline. J. Alz. Dis. 2012;30:183–208. doi: 10.3233/JAD-2012-111298. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chloriazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Cui Y, Shu Y, Zhu Y, Shi Y, Le G. High-Fat Diets Impair Spatial Learning of Mice in the Y-Maze Paradigm: Ameliorative Potential of α-Lipoic Acid. J. Med. Food. 2012;15:713–717. doi: 10.1089/jmf.2011.1970. [DOI] [PubMed] [Google Scholar]
- D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Rev.: Mol. Cell. Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Davis M. The Role of the Amygdala in Fear and Anxiety. Ann. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior systems responsible for fear. Psychon. Bull. Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Filiou MD, Zhang Y, Teplytska L, Reckow S, Gormanns P, Maccarrone G, Frank E, Kessler MS, Hambsch B, Nussbaumer M, Bunck M, Ludwig T, Yassouridis A, Holsboer F, Landgraf R, Turck CW. Proteomics and Metabolomics Analysis of a Trait Anxiety Mouse Model Reveals Divergent Mitochondrial Pathways. Biol. Psychiatr. 2011;70:1074–1082. doi: 10.1016/j.biopsych.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Holland, Amsterdam: Elsevier; 2007. [Google Scholar]
- Gemma C, Vila J, Bachstetter A, Bickford PC. Oxidative Stress and the Aging Brain: From Theory to Prevention. In: Riddle DR, editor. Brain Aging: Models, Methods and Mechanisms. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- Gimsa U, Kanitz E, Otten W, Ibrahim SM. Behavior and Stress Reactivity in Mouse Strains with Mitochondrial DNA Variations. Ann. New York Acad. Sci. 2009;1153:131–138. doi: 10.1111/j.1749-6632.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- Good M. Spatial Memory and Hippocampal Function: Where are we now? Psicológica. 2002;23:109–138. [Google Scholar]
- Harman D. The biological clock: the mitochondria? J. Am.Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Honey RC, Watt A, Good M. Hippocampal lesions disrupt an associative mismatch process. J. Neurosci. 1998;18:2226–2230. doi: 10.1523/JNEUROSCI.18-06-02226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2009;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Paradoxical Actions of Hydrogen Peroxide on Long-Term Potentiation in Transgenic Superoxide Dismutase-1 Mice. J. Neurosci. 2003;23:10359–10367. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp LT, Klanne E. Role of Reactive Oxygen Species in Hippocampal Long-Term Potentiation: Contributory or Inhibitory. J. Neurosci. Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Lee W-H, Kumar A, Rani A, Herrera J, Xu J, Someya S, Foster TC. Influence of Viral Vector-Mediated Delivery of Superoxide Dismutase and Catalase to the Hippocampus on Spatial Learning and Memory During Aging. Antiox. Redox Signal. 2012;16:339–350. doi: 10.1089/ars.2011.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED. Extracellular Superoxide Dismutase (EC-SOD) Quenches Free Radicals and Attenuates Age-Related Cognitive Decline: Opportunities for Novel Drug Development in Aging. Curr. Alz. Res. 2005;2:191–196. doi: 10.2174/1567205053585710. [DOI] [PubMed] [Google Scholar]
- Levin ED, Christopher C, Crapo JD. Memory Decline of Aging Reduced by Extracellular Superoxide Dismutase Overexpression. Behav. Genet. 2005;35:447–453. doi: 10.1007/s10519-004-1510-y. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu I, Bi X, Thompson RF, Doctro SR, Malfroy B. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Nat. Acad. Sci. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian Fear Conditioning. Ann. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B. 2005;1:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Miguel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp. Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur. J. Biochem. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- Oliveira JM. Nature and cause of mitochondrial dysfunction in Huntington's disease: focusing on huntingtin and the striatum. J. Neurochem. 2010;114:1–12. doi: 10.1111/j.1471-4159.2010.06741.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Praticò D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Archiv. Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Prutt L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav. Immun. 2008;22:1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Molecular Psychiatry. 2011;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre J, Pallardó V, Viña J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. Life. 2000;49:427–435. doi: 10.1080/152165400410281. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity. Ageing Res. Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology 1994. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen Peroxide: A Signaling Messenger. Antiox. Redox Signal. 2006;8:244–282. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Ide T, Kinugawa S. Mitochondrial Oxidative Stress, DNA Damage, and Heart Failure. Antiox. Redox Signal. 2006;8:1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- Wang W, Li S, Dong HP, Lv S, Tang YY. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci. 2009;85:127–135. doi: 10.1016/j.lfs.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Yan LJAnalysis of oxidative modification of proteins. Analysis of Oxidative Modification of Proteins. Curr. Protoc. Protein Sci. 2009;56:14.4.1–14.4.28. doi: 10.1002/0471140864.ps1404s56. [DOI] [PubMed] [Google Scholar]