Abstract
Food advertisements often promote choices that are driven by inferences about the hedonic pleasures of eating a particular food. Given the individual and public health consequences of obesity, it is critical to address unanswered questions about the specific neural systems underlying these hedonic inferences. For example, although regions such as the orbitofrontal cortex (OFC) are frequently observed to respond more to pleasant food images than less hedonically pleasing stimuli, one important hedonic brain region in particular has largely remained conspicuously absent among human studies of hedonic response to food images. Based on rodent research demonstrating that activity in the ventral pallidum underlies the hedonic pleasures experienced upon eating food rewards, one might expect that activity in this important ‘hedonic hotspot’ might also track inferred food pleasantness. To date, however, no human studies have assessed this question. We thus asked human subjects to undergo fMRI and make item-by-item ratings of how pleasant it would be to eat particular visually perceived foods. Activity in the ventral pallidum was strongly modulated with pleasantness inferences. Additionally, activity within a region of the orbitofrontal cortex that tracks the pleasantness of tastes was also modulated with inferred pleasantness. Importantly, the reliability of these findings is demonstrated by their replication when we repeated the experiment at a new site with new subjects. These two experiments demonstrate that the ventral pallidum, in addition to the OFC, plays a central role in the moment-to-moment hedonic inferences that influence food-related decision-making.
Keywords: Food, pleasure, ventral pallidum, orbitofrontal cortex
INTRODUCTION
The rise in obesity and its concomitant health concerns can ultimately be traced to an imbalance between individuals’ energy intake and expenditure. Although factors related to energy expenditure, such as sedentary lifestyles, may contribute to overweight and obesity, evidence suggests that over-consumption of energy-dense food bears relatively more responsibility for the growing rates of obesity (Swinburn et al. 2011). Evolutionary pressures promoted the development of reward systems in the brain to respond to the ingestion of energy-dense foods with hedonic pleasure, and to associate that pleasure with the preceding environmental cues, such as the visual perception of those energy-dense foods (Berridge et al. 2010). Once these associations are in place, simply seeing a familiar food stimulus will result in the automatic and obligatory retrieval of information about the hedonic reward associated with eating that food, as well as its other salient properties such as its taste (Simmons et al. 2005). Because the retrieval of this reward information precedes feeding, it constitutes a hedonic inference about the pleasures afforded by the food’s ingestion.
Translational neuroscience research has revealed much about the neural bases of the hedonic pleasure that attends eating (Berridge et al. 2010), including those brain regions where activity is modulated with the pleasure experienced by humans upon ingesting rewarding tastes (Kringelbach et al. 2003). It is similarly important to know which brain regions underlie the pleasure expected by humans upon visual perception of a rewarding food. Said another way, it is important to understand which brain regions underlie hedonic inferences about the foods we see. This is made all the more urgent by the fact that the neural systems supporting hedonic inferences evolved in a very different food environment from the current one, in which consumers are constantly enticed with images of palatable foods, which are then supplied nearly ubiquitously. Given the individual and public health consequences of obesity (Finkelstein et al. 2005; Reilly et al. 2003; Wang et al. 2011), it is critical to understand the neural systems underlying hedonic inferences to visually perceived foods.
In previous research, two brain regions in particular have been frequently highlighted for the roles they play in representing the hedonic potential of visual food cues: namely the ventral pallidum and the orbitofrontal cortex (OFC). Rodent studies have demonstrated that the ventral pallidum in particular plays an important role underlying the hedonic pleasure associated with food rewards (Aldridge and Berridge 2010). For example, damage to the ventral pallidum results in a diminution of the hedonic experience associated with food rewards (Cromwell and Berridge 1993). Although it is part of the mesolimbic dopamine pathway, hedonic-related activity in the ventral pallidum is not strictly dependent on dopamine, but rather is mediated by opioid-sensitive neurotransmission. This is demonstrated by studies that use either mu-opioid agonists or antagonists to modulate ventral pallidum activity and associated hedonically-related behavioral responses to food rewards (Smith and Berridge 2005).
In contrast to the ventral pallidum, where activity appears to underlie hedonic experience per se, activity in the OFC instead appears to code for the reward value generally of food cues (Noonan et al. 2010; Rushworth et al. 2011). For example, although numerous human fMRI studies have demonstrated that the OFC responds reliably to images of pleasurable foods (Killgore et al. 2003; LaBar et al. 2001; Simmons et al. 2005; van der Laan et al. 2011) and its activity tracks pleasantness inferred from verbal food descriptions (Arana et al. 2003; Hinton et al. 2004; Piech et al. 2009), lesions to the OFC do not result in a loss of the hedonic experience associated with pleasurable foods. Importantly, unlike the OFC findings cited above, to date no human studies have assessed whether ventral pallidum activity tracks the inferred pleasantness of visually perceived foods. This gap in the literature is important for two reasons. First, it remains unclear whether ventral pallidum activity is merely related to the pleasure experienced upon eating pleasurable foods, or whether its activity might also underlie inferences about the pleasure one can expect from those foods as well. Second, as described above, the ventral pallidum has somewhat different neurotransmitter sensitivities than the OFC and other regions in the mesolimbic dopamine pathway (Haber and Knutson 2010; Smith and Berridge 2005) and thus may offer different pharmacological targets for influencing hedonic responses to food cues.
To address this gap in the literature, we asked subjects to undergo fMRI while viewing a diverse set of food images and make item-by-item ratings of how pleasant it would be to eat that particular food at that moment. Here we show that ventral pallidum activity in humans is indeed modulated by inferences about food pleasantness. We additionally demonstrate that activity within a region of the OFC previously shown to track the pleasantness of actual tastes is also modulated with inferences of food pleasantness, a finding that agree with much of the previous literature on OFC responses to food images. Importantly, the reliability of these findings is demonstrated by their replication when we repeated the experiment at a new site with new subjects performing exactly the same task in a new scanner. Together, the two experiments reported here demonstrate that the ventral pallidum, in addition to the OFC, plays a central role in moment-to-moment inferences of how pleasant it will be to eat a particular food.
METHODS
Subjects
In Experiment 1, twenty-two right-handed, native Englishspeaking healthy volunteers (12 male, average age 27.7 years, age range 21— 39 years) from the greater Washington D.C. area were recruited for monetary compensation to participate in research at the National Institutes of Health Clinical Center in Bethesda, Maryland. Subjects had no previous or current neurological or psychiatric disorders and had stable body mass indices (BMIs; Mean BMI 22.54, range 17.85—27.41) as defined by no more than a 5kg increase/decrease over the previous 6 months. Appropriate procedures and potential risks were explained in detail to all subjects, and each provided informed consent in accordance with the approved Institutional Review Board standards. All subjects were monitored on an inpatient basis at the National Institutes of Health Clinical Center for at least 48 hours prior to scanning. Subjects were fed highly controlled diets balanced for macronutrients and were in a eucaloric state at the time of scanning. All subjects ate a standardized meal 4.5 hours prior to the fMRI scan session.
In the replication study, 18 right-handed, native English-speaking healthy volunteers (3 male, average age 26.8 years, age range 18—42 year; Mean BMI 22.8, range 18.5—27.8) from the Tulsa Oklahoma metropolitan area were recruited for monetary compensation to participate in research at the Laureate Institute for Brain Research. All subjects provided written informed consent prior to participation in accordance with the approved Institutional Review Board standards. All subjects completed detailed physical and mental health evaluations using both structured and unstructured diagnostic interviews. Exclusion criteria included a prior history of major medical or psychiatric disorders, head injury or neurological disorders, current pregnancy, lifetime history of substance dependence, substance abuse within one year, exposure within 3 weeks to psychotropic or other medications expected to influence cerebral blood flow or function, or general MRI exclusions. All subjects were asked to eat a meal that was typical of their diet at their usual mealtime prior to their scan session.
Experimental Methods
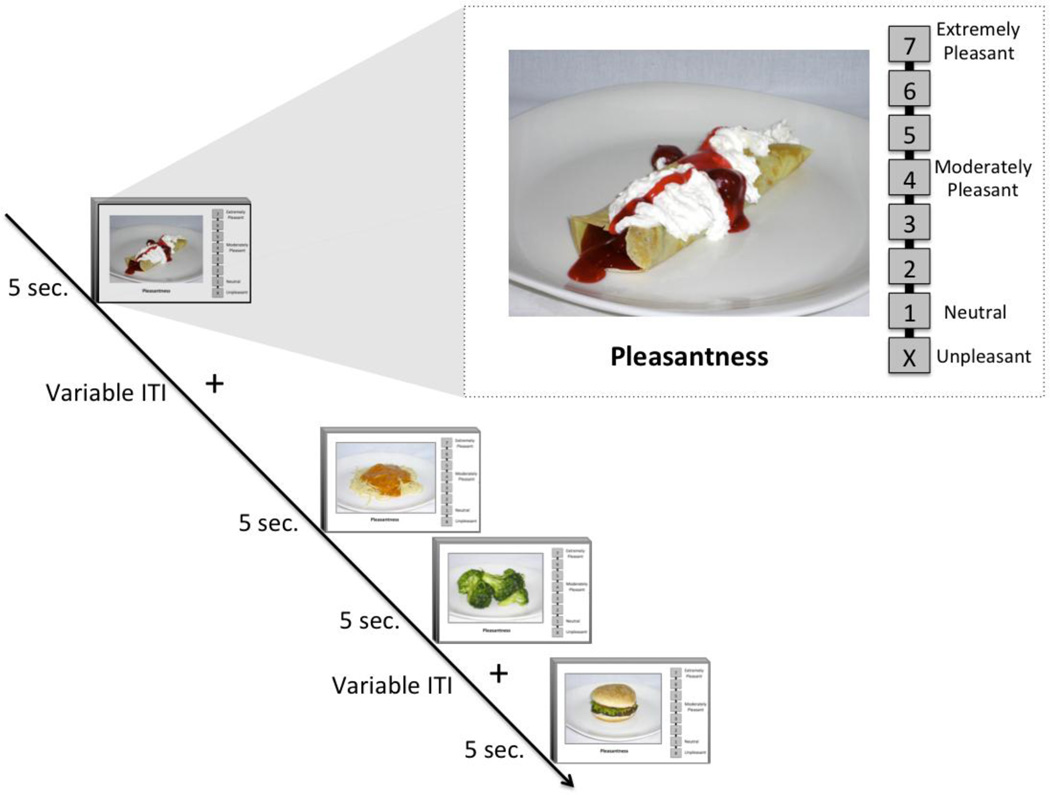
While undergoing functional magnetic resonance imaging (fMRI), subjects rated food images, each presented for 5-seconds. For each food image, subjects were asked to answer the following question: “If given the opportunity right now, how pleasant would it be to eat this food?” Subjects provided their responses by manipulating an MR-compatible scroll wheel to select values along a number line positioned next to the food image (Figure 1). The pleasantness rating scale ranged from 1 to 7, with 1 depicted as “neutral” and 7 as “extremely pleasant”. The pleasantness scale also included an “unpleasant” option represented by the letter “X” located below the number line. Subjects were instructed to select the “X” if they believed the depicted food would be at all unpleasant to eat. A fixation cross was presented for varying durations (Mean ISI = 3.7 seconds; duration 2.5–7.5 seconds) during the intervening periods between food images.
Fig 1. Food Pleasantness Rating Task.
Subjects viewed food pictures for 5 seconds, during which time they provided ratings of “how pleasant it would be to eat this particular food right now.” Using a handheld scroll wheel, subjects indicted their pleasantness ratings by clicking on a number located directly to the right of the food picture.
The food photographs presented in the scanner were taken with a high-resolution digital camera. The depicted food items were prepared by laboratory personnel, which allowed for high control of stimulus presentation, lighting, portion size, and a standard background (white plate against a grey backdrop photographed within a photobox). Over the course of the experiment, subjects viewed 3 different exemplars of forty-eight distinct classes of food items. Because we were interested in tracking brain activity across a wide range of inferred pleasantness, these 144 food images depicted many different varieties of foods, including highly processed, high calorie foods (e.g., cheeseburgers, French fries, pizza, cake, cinnamon rolls, ice cream, etc.) to uncooked fruits and vegetables (grapes, strawberries, cauliflower, broccoli, carrots, etc.). Prior to the experiment, we collected normative scaling data with a different group of subjects who rated the food pictures on nameability, typicality, perceived fat content, and sweetness.
In addition to providing food pleasantness ratings, subjects were also asked to view a different collection of food photographs from those shown in the pleasantness task and provide ratings of how much self-control it would take to not eat the depicted foods (Simmons et al., in preparation). Subjects performed the self-control task during the same scan session, but not at the same time, as the food pleasantness task. Subjects experienced 4 runs of the tasks, which were counterbalanced for order of presentation.
MRI Data Acquisition
Food images were displayed to subjects through the use of Eprime software (www.pstnet.com). Images were projected onto a screen in the scanner bore behind the subject’s head and viewed through a mirror mounted to the head coil. In Experiment 1, all subjects were scanned at 18:00 in the evening. In the replication study, all subjects were scanned between the hours of 12:00 and 16:00.
In Experiment 1, two hundred and six echoplanar magnetic resonance (MR) volumes were acquired with a 3T General Electric scanner and a GE 8-channel receive-only head coil. Each echoplanar image (EPI) consisted of 44 2.8-mm slices (echo time [TE] = 27 ms, repetition time [TR] = 2500 ms, flip angle = 90 degrees, voxel size = 3.4375×3.4375×2.8 mm). High-resolution anatomical images were collected prior to functional scanning runs (TE = 2.7 ms, TR: 7.24 ms, flip angle: 12 degrees, voxel size: 0.937×0.937×1.2 mm). All structural and functional images were collected with a Sensitivity Encoding (SENSE) factor of 2 used to reduce image collection time (for structural images) or minimize image distortions (in functional images) while reducing gradient coil heating over the course of the scan session.
In the replication study, two-hundred and six echoplanar magnetic resonance (MR) volumes were acquired with a 3T General Electric MR750 scanner and a NOVA 32-channel receive-only head coil. Each echoplanar image (EPI) consisted of 38 2.9-mm slices (echo time [TE] = 22 ms, repetition time [TR] = 2500 ms, flip angle = 70 degrees, voxel size = 1.72×1.72×2.9). High-resolution anatomical images were collected prior to functional scanning runs (TE = 1.9 ms, TR = 5 ms, flip angle: 10 degrees, voxel size: .93×.93×1.2 mm). All structural and functional images were collected with a Sensitivity Encoding (SENSE) factor of 2.
fMRI Pre-Processing
All imaging data were preprocessed and analyzed using the AFNI software package. In both experiments, all subjects’ anatomical scans were spatially transformed to the stereotaxic array of Talairach and Tournoux using AFNI’s automated algorithm. The resulting transformation parameters were then applied to the functional data during preprocessing. All functional volumes were aligned to a common base EPI represented by the third volume of the first functional run. The first 3 volumes of each EPI run were trimmed to allow the fMRI signal to reach steady state. A slice-time correction was applied to all functional volumes, which were also smoothed with a 6-mm full width half max Gaussian kernel. Additionally, the signal value for each EPI volume was normalized to the percent signal change from the voxel’s mean signal across the time-course.
Statistical Analysis
All individual subject data was checked for quality assurance, and outlying time points resulting from head motion were censored out of the analyses. At the single-subject level, multiple regression was used to analyze the data, with regressors of non-interest included in the model to account for each run’s signal mean, linear, quadratic, and cubic signal trends, as well as 6 motion parameters (3 translations and 3 rotations) saved from the image registration step during pre-processing. The food pleasantness task regressor was constructed by convolving a box-car function with a width of 5 seconds beginning at the onset of the food image with a gamma-variate function to adjust the predictor variable for the delay and shape of the BOLD response. Next, using amplitude modulation (AM) regression in AFNI, the subject’s pleasantness ratings for each food item were mean-normalized and entered into the regression equation as auxiliary behavioral covariates associated with each food image, thereby scaling the predicted BOLD response for each food item by the individual subject’s pleasantness rating for that item. The resulting regression coefficients thus indicate the extent to which brain activity varies proportionally with the ratings. Additionally, a gamma-variate-convolved regressor was included in the model to account separately for those items that the subject indicated were unpleasant.
To implement group-level random effects analyses, the individual subjects’ pleasantness amplitude-modulated beta maps were included in a one-sample t-test against a mean of 0 to identify voxels where activity across subjects was reliably modulated by the pleasantness ratings.
All statistical maps were corrected for multiple comparisons at the p < .05 level using cluster size corrections implemented via Monte Carlo simulations in AFNI’s 3dClustSim. There is good reason to predict a priori that regions implicated in reward processing may also track the inferred pleasantness of visually perceived foods. As such, small volume corrections were implemented within the orbitofrontal cortex and striatal-pallidal neurocircuit, with a voxel-wise p < .005 and cluster size threshold to achieve correction for multiple comparisons at p < .05. Outside of the a prior-defined regions of interest (ROI) a voxel-wise threshold of p < .0005 was used with cluster size thresholding to achieve correction for multiple comparisons at p < .05.
The OFC ROI was anatomically defined posteriorly by a line drawn at the anterior portion of the subgenual frontal cortex (Y = 28 in on the AFNI Talairach N27 atlas brain). Anteriorly, the OFC ROI was defined by the frontal pole. The ventral extent of the OFC ROI was defined by the ventral surface of the cortex, and the dorsal extent was defined by the fundus of the transverse orbital sulcus (Chiavaras et al. 2001). The medial extent of the ROI was defined by the lateral edge of the olfactory sulcus, which defines the medial edge of the medial orbital gyrus (Chiavaras et al. 2001). The lateral extent was defined by either the lateral intermediate orbital sulcus or the lateral orbital sulcus, whichever was located more medially.
The ventral striatum ROI was defined using the procedure outlined by Mawlawi and colleagues (Mawlawi et al. 2001). Briefly, the dorsal-lateral boundary of ventral striatum ROI, separating it from the dorsal caudate and putamen, was defined in each coronal slice by “a line joining the intersection between the outer edge of the putamen with a vertical line going through the most superior and lateral point of the internal capsule and the center of the portion of the AC transaxial plane overlying the striatum. This line was extended to the internal edge of the caudate” (Mawlawi et al. 2001). The remaining boundaries in each coronal slice were easily identifiable by the marked image intensity differences compared to the surrounding structures. The anterior extend of the ventral striatum ROI was defined by the appearance of the caudate. It’s posterior extent was defined by the appearance of the anterior commissure in the coronal plane.
The anterior extent of the ventral pallidum ROI was defined by the anterior commissure1, with its posterior boundary defined at 7mm posterior to the commissure. The dorsal extent of the ventral pallidum was defined by the anterior commissure, and ventrally included all of the subcommissural space (Haber and Knutson 2010). Laterally, the ventral pallidum was defined by a vertical line drawn at 15 mm from the midline, which is the lateral-most extent of the ventral pallidum in the Mai atlas (Mai, Paxinos, and Voss, 2007). Medially, the ventral pallidum was defined by a vertical line drawn at 5 mm from the midline, which appears to be the lateral-most extent of the hypothalamus in the Mai atlas. This rather conservative medial boundary of the ventral pallidum was chosen to ensure that any areas of activation were distinguishable from activity that might be observed in the hypothalamus.
RESULTS
Behavioral Ratings
In both Experiments 1 and the replication study, subjects underwent fMRI while providing ratings on a seven-point scale of how pleasant it would be to eat visually presented foods (see Figure 1). Participants in the two Experiments provided very similar ratings (Experiment 1 mean pleasantness = 4.6, SD = 2.0; replication study mean pleasantness = 4.3, SD = 2.3). Each subject’s ratings were used as auxiliary behavioral information to modulate the predicted amplitude of a fMRI blood oxygenation level dependent (BOLD) response model, onto which the subject’s BOLD fMRI data were then regressed. This so-called amplitude modulation regression approach allowed us to identify voxels where activity was modulated with subject’s ratings on an item-by-item basis.
Experiment 1
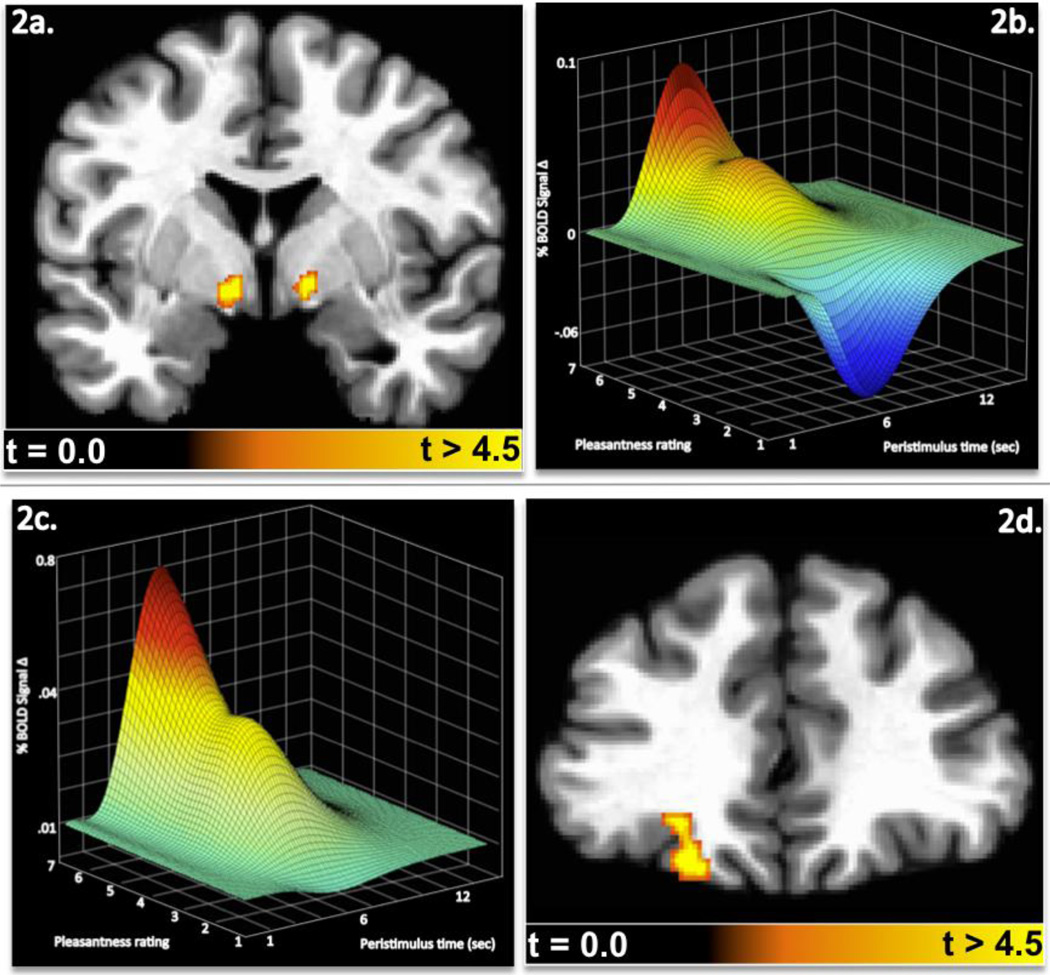
In Experiment 1, activity in both the left and right ventral pallidum was positively correlated with subjects’ rating of inferred food pleasantness (Figures 2a and 2b). The peak response in the left ventral pallidum was located at Talairach coordinates -9,-5,-4 (peak T = 4.45) and in the right hemisphere at +11,−3,−4 (peak T = 4.28). As subjects rated a particular food more pleasant, both regions exhibited a corresponding increase in activity. Foods receiving low pleasantness ratings produced decreases in ventral pallidum activity relative to the signal baseline, while foods that were rated as highly pleasant produced marked increases in activity.
Fig 2. Ventral Pallidum and Orbitofrontal Cortex Activity modulate inferred food pleasantness in Experiment 1.
The coronal slice (Y = −4) in panel 2a shows bilateral regions of the ventral pallidum where activity was reliably associated with subjects ratings of inferred food pleasantness (p<.05 corrected) in Experiment 1. The surface mesh graph in Panel 2b demonstrates how ventral pallidum activity over peristimulus time was related to the subjects’ food pleasantness ratings. Panel 2c and 2d show the corresponding OFC (Y = 33) activity and peristimulus response mesh as a function of pleasantness ratings in Experiment 1.
In addition to the ventral pallidum, activity in the left OFC was also positively correlated with subjects’ ratings of inferred food pleasantness (Figures 2c and 2d), with activity increasing along with ratings. The peak response in the left OFC was located at Talairach coordinates −19,+35,−14 (peak T = 4.39). Foods receiving high pleasantness scores produced a marked increase in activity, while those that received low scores did not produce the marked negative deflection from baseline observed in the ventral pallidum.
Replication Study
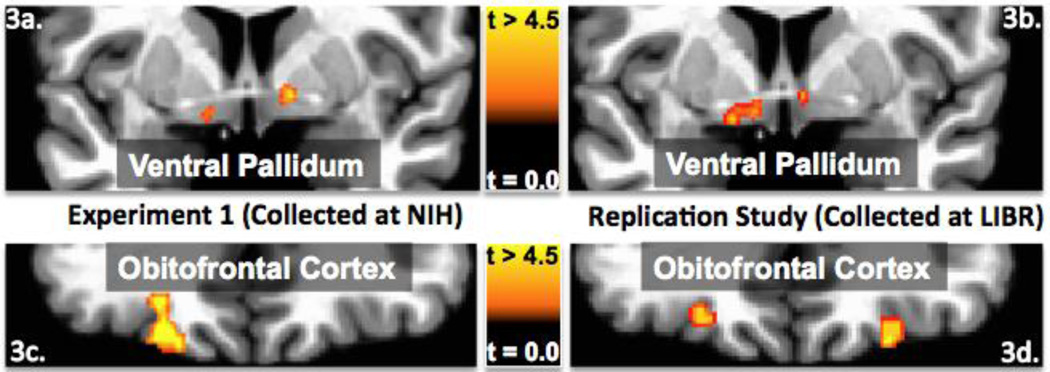
In the replication study, using the same stimuli and analyses as in Experiment 1, but with a different group of participants, in a different location, using different scanning hardware, we again observed that activity in both the left and right ventral pallidum predicted subjects’ ratings of inferred food pleasantness (see Figures 3a and 3b). The peak response in the left ventral pallidum was located at Talairach coordinates -6,0,-3 (peak T = 3.98) and in the right hemisphere at +6,0,−1 (peak T = 3.98). In addition, we again observed that OFC activity predicted inferred food pleasantness. Although nearly identical regions of the left OFC were observed in both studies, in the replication study we also observed reliable activity in the corresponding region of the right OFC (see Figures 3c and 3d). The peak response in the left OFC was located at Talairach coordinates −22,+36,−8 (peak T = 3.55), and in the right OFC at +25,+34,−15 (peak T = 4.29).
Fig 3. Ventral Pallidum and Orbitofrontal Cortex Findings in Experiment 1 and the Replication Study.
The coronal images on the left (3a and 3c) exhibit regions of the ventral pallidum and OFC where activity in Experiment 1 reliably modulated subjects’ food pleasantness ratings (p < .05 corrected). The coronal images on the right (3b and 3d) show that in the replication study the activity within the same regions of the ventral pallidum and OFC were also reliably modulated by subjects’ food pleasantness ratings (p < .05 corrected). The two sets of images (3a/3b and 3c/3d) are depicted on the same coronal slices (ventral palldium Y = −1; OFC Y = 33) and have the same statistical thresholds applied. The remarkably similar results were obtained even though the two datasets were collected using the same task and stimuli at different scan centers (NIH Intramural Scan Center, Laureate Institute for Brain Research), with different scanning hardware, and different subjects.
Given that ventral pallidum and OFC both exhibited activity correlated with subjects’ inferred pleasantness ratings, their activity timecourses should also be highly correlated. To assess this possibility, and to further test the replicability of the findings across studies, we used the ventral pallidum and OFC clusters identified in Experiment 1 as regions of interest on the individual subjects’ data in the Replication study to determine the timecourse correlations between the two regions. We observed that the ventral pallidum and OFC ROIs (defined in Experiment 1) exhibited a strong positive correlation in their activity timecourses in the independent replication study (Mean Pearson r = 0.64, p < .005).
DISCUSSION
Although numerous cognitive, emotional, and metabolic processes influence decisions about what to eat, in economically developed societies where diverse food supplies are readily available, food hedonics play an out-sized role in daily food decisions (Eertmans et al. 2001). Food advertisements capitalize on this by presenting compelling images of palatable energy-dense foods, thereby powerfully influencing unhealthy eating behaviors (Borzekowski and Robinson 2001; Dixon et al. 2007; Harris et al. 2009). Given the individual and public health consequences of overweight and obesity, it is critical that we develop detailed neurobiological models of the interaction between food perception and food hedonics. This interaction cannot be understood without knowing precisely which brain regions underlie inferences about the hedonic pleasures offered by foods encountered through visual media.
In recent years, research has revealed that food perception and conceptual representation depend on a distributed collection of brain regions, including visual object recognition pathways in the ventral temporal lobe (Martin and Simmons 2008), the insula to represent taste property information and feeding-relevant interoceptive states (Simmons et al. 2005; Simmons et al. In Press; Small 2010), emotional arousal or salience in the amygdala (Zald 2003), and OFC and anterior cingulate cortex for food reward and motivation (Rolls 2008). Additionally, studies have specifically examined food-related decision-making in response to food images, including such topics as the regions underlying willingness to pay for food, food value, and food preference (Hare et al. 2009; Litt et al. 2011; Plassmann et al. 2007; Plassmann et al. 2010).
Although these studies have shed light on which brain regions underlie food perception, food motivation, and food decision-making, they do not specifically tell us which neural systems support inferences about the gradations of pleasure one might experience should one eat a particular visually-perceived food. These hedonic inferences come into play every time we scan the options depicted on a fast food restaurant menu and deliberate about whether to order the salad or the cheeseburger and milk shake combo. To this end we sought to identify which brain regions track on a stimulus-by-stimulus basis the inferred pleasantness of visually perceived foods. We observed two brain regions where activity in both experiments was modulated with inferred food pleasantness. One of these regions, the medial OFC, has been frequently reported in human imaging studies relating activity to the pleasantness of food stimuli (Arana et al. 2003; de Araujo et al. 2003; Grabenhorst et al. 2010b; Grabenhorst et al. 2010a; Hinton et al. 2004; Killgore et al. 2003; LaBar et al. 2001; O'Doherty et al. 2000; Piech et al. 2009). The second region where we observed activity modulated by inferred food pleasantness was the ventral pallidum. It is this second finding that represents the main contribution of the present studies.
Much of what we know about the ventral pallidum’s functions in reward processing comes from rodent studies of food and drug ‘liking’ (i.e., the hedonic pleasure associated with a reward) and ‘wanting’ (i.e., the motivational salience of a rewarding stimulus) (Berridge 1996). Foundational research by Berridge, Aldridge, and colleagues has demonstrated that the ventral pallidum is an important “hedonic hotspot” in the brain, containing neurons who’s activity signals the degree to which a particular stimulus is hedonically pleasant (Smith et al. 2009), thereby imbuing that stimulus with what these authors have described as a “pleasure gloss” (Aldridge and Berridge 2010). This ‘liking’-related activity in the ventral pallidum appears be mediated by opioid transmission as injection of a mu-opioid agonist in the region brings about multi-fold increases in behavioral liking responses to sucrose (Smith and Berridge 2005).
In addition to rodent studies, ventral pallidum activity has been observed in human fMRI studies of monetary reward (Pessiglione et al. 2007), and the region responds more to olfactory stimuli associated with immediately available sucrose solutions (Small et al. 2008). Ventral pallidum activity to food stimuli is correlated with individual differences in personality traits related to disgust and reward sensitivity (Beaver et al. 2006; Calder et al. 2007). What has heretofore remained unclear is whether ventral pallidum activity is related to inferences about the pleasure one can expect from a particular visually perceived food. The two studies presented here demonstrate that it is. Ventral pallidum neurons fire faster for hedonically pleasing tastes, and slower when a taste is disliked (Tindell et al. 2006). Likewise, in the current studies ventral pallidum activity increased with subjects’ ratings of how pleasant it would be to eat a particular food, and exhibited decreased activity as foods received lower pleasantness ratings (see Figure 2b). It thus appears that in humans the ventral pallidum’s role in representing food pleasantness is not restricted to hedonic experience during food consumption that has been demonstrated in rodents, but also underlies graded expectations of food pleasantness.
In addition to the ventral pallidum, we also observed that an OFC region generally within the medial orbital sulcus of BA 11l also exhibited activity correlated with subjects’ ratings of food pleasantness. Extensive human and non-human primate literatures demonstrate that this region of the OFC codes for the reward value of encountered stimuli (Barbas 2007; Noonan et al. 2010; Rushworth et al. 2011), but probably does not underlie the hedonic experience of pleasure (Berridge and Kringelbach 2008).
OFC reward value codes are highly dynamic, constantly changing along with an organism’s homeostatic state and the rewards available to it (Scott et al. 1995; Small et al. 2001). One example of dynamic reward valuation in the OFC can be found in stimulus-specific satiety. Although neurons in the OFC will fire vigorously when a monkey is provided a rewarding liquid stimulus, their activity will quickly attenuate with repeated presentations of that stimulus (Rolls et al. 1989). In addition, lesions of the OFC both in monkeys and humans can result in a loss of satiety effects (Baxter et al. 2000; Woolley et al. 2007). Importantly for the present study, Kringelbach and colleagues (2003) demonstrated that the dynamic reward valuations in OFC underlying changes in satiety are also related to subjective food pleasantness (Kringelbach et al. 2003). In their study, the researchers showed that activity in the OFC correlated with subjects’ pleasantness ratings for received tastes and that this response was modulated by satiety. Interestingly, the peak voxel exhibiting a correlation with taste pleasantness in their study was only approximately 4mm from the peak OFC voxels observed in the present study. The study by Kringelbach and colleagues, with its satiety manipulation, is important as it shows that this region of the OFC dynamically codes for the reward value of tastes, which in turn is related to subjects’ experience of hedonic pleasure. The present study builds on this important finding, showing that activity in this same region of the OFC likewise codes for subjects’ inferences of hedonic pleasure, a finding that is consistent with many earlier reports from studies comparing OFC responses to food and non-food images, or foods of varying hedonic value due to satiety manipulations (van der Laan et al. 2011). Although we cannot rule-out that the activity in this region underlies hedonic experience per se, we believe the correlation between ratings and actual tastes, like the one observed by Kringelbach and colleagues (2003) and subsequently by Plassmann and colleagues (Plassmann et al. 2008), likely reflect valuations of expected reward. This interpretation of the data finds support in the extensive literature about the OFC’s role in reward valuation, including studies like those described above where lesions to the OFC result in the loss of dynamic reward valuation, but not hedonic experience. Our interpretation of the OFC activity in the present study thus stands in contrast to how we conceptualize the activity within the ventral pallidum. Like the OFC, activity in the ventral pallidum is correlated with ratings of expected pleasantness. Unlike the OFC, however, damage to the region of the ventral pallidum appears to greatly diminish the capacity for hedonic experience (Cromwell and Berridge 1993). This suggests to us that the ventral pallidum activity is related to the hedonic experience associated with the food.
Conclusion and Future Directions
Food decision-making is implemented by a complex set of processes, many of which appear to be anchored in regions of the prefrontal cortex, such as the OFC, anterior cingulate cortex, and dorsolateral prefrontal cortex (Camus et al. 2009; Hare et al. 2009; Izquierdo et al. 2004). The food reward representations and food motivations upon which these decision processes operate are themselves influenced by factors spanning from the level of metabolism all the way up to culture and socialization. Research in this area has grown tremendously, owing largely to the significance of individuals’ food choices on both personal and public health.
In the present study we observed that activity in the ventral pallidum, in addition to the OFC, was modulated with subjects’ pleasantness ratings. The ventral pallidum and OFC findings were observed in maps corrected for multiple comparisons in two separate experiments, at two different research facilities, on two different scanners, with two different groups of subjects. Additionally, the robustness of the findings is further supported by the fact that both regions were observed in studies with participants who were on different diet regimens.
In light of the findings reported here, future research on inferred pleasantness should attempt to address additional key questions. First, studies should assess whether pharmacological interventions aimed at the ventral pallidum might attenuate pleasantness inferences, thereby rendering food images less enticing and improving diet maintenance and weight loss, or reducing pathological feeding such as binge eating in bulimia nervosa. An important related point is that research in rodents has identified regions in the anterior ventral pallidum where microinjections suppress both hedonic responses and food intake. These regions appear to be separate from those described earlier where opioid microinjections enhance the hedonic impact of stimuli (Aldridge and Berridge 2010). Unfortunately, the image resolution of most 3 Tesla fMRI studies is not conducive to rigorously mapping sub-regions within a structure the size of the ventral pallidum. Nonetheless, future studies at higher magnetic field strengths could potentially observe regional specialization for hedonic hotspots and coldspots. This approach might be a useful adjunct to the pharmacological interventions described above aimed at either attenuating or potentiating hedonic responses to food stimuli.
Second, future research should be done to disambiguate the activity of regions that simply code for reward value, from those that directly underlie the phenomenological experience of pleasure (Berridge and Kringelbach 2008). Relatedly, the experience of pleasantness may be relatively context-dependent and influenced by multiple sources of information, including food taste, food nutrition versus homeostatic dietary needs, immediacy of food rewards, and even socio-cultural factors such body image. It may therefore be important to undertake studies to determine if ventral pallidum activity in humans is related equally to manifestations of pleasure experienced within these varied contexts. Likewise, variable manifestations and determinants of food pleasure could also explain important group differences. For example, although a growing body of research demonstrates important differences in obese and lean subjects during food perception (Carnell et al. 2012), future research should also address whether group differences exist in the coupling between subjects’ ratings of inferred food pleasantness and the activity of the ventral pallidum and OFC, as well as whether specific dimensions of pleasantness are differentially weighted in obesity and influence food-related decision making. In so doing, these findings might thus provide a neurobiological account for why obese children are more susceptible to food advertisements than their lean counterparts (Halford et al. 2008b; Halford et al. 2008a), and provide targets for interventions aimed at ameliorating their relative vulnerability.
Acknowledgements
This research supported by the Intramural Research Programs of the National Institute of Mental Health (NIMH), and the National Institute of Diabetes and Digestive and Kidney Diseases, as well as NIMH grant 1K01MH096175-01 and an Oklahoma Tobacco Research Center grant to Kyle Simmons. We would like to thank both Dr. Steve Gotts and Dr. Wayne Drevets for helpful comments on this manuscript and research.
Footnotes
Based on immunohistochemistry studies and retrograde tracer findings, the ventral pallidum is now considered to encompass the medial rostral internal segment of the globus pallidus (see Haber and Knutson, 2010). It is for this reason that we allowed the ventral pallidum ROI to extend to Y = −7, even though the clusters of activity observed in the present study are actually anterior to this and were located completely within the first 5mm behind the commissure.
Conflict of Interest
The authors declare no competing financial interests
References
- Aldridge JW, Berridge K, editors. Pleasures of the Brain. New York, NY: Oxford University Press; 2010. Neural coding of pleasure: "rose-tinted glasses" of the ventral pallidum. [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(29):9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Annals of the New York Academy of Sciences. 2007;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(11):4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(19):5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and biobehavioral reviews. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho C-Y, Richard JM, Difeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzekowski DL, Robinson TN. The 30-second effect: an experiment revealing the impact of television commercials on food preferences of preschoolers. J Am Diet Assoc. 2001;101(1):42–46. doi: 10.1016/S0002-8223(01)00012-8. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25(11):3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Camus M, Halelamien N, Plassmann H, Shimojo S, O'Doherty J, Camerer C, Rangel A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. Eur J Neurosci. 2009;30(10):1980–1988. doi: 10.1111/j.1460-9568.2009.06991.x. [DOI] [PubMed] [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavaras MM, LeGoualher G, Evans A, Petrides M. Three-dimensional probabilistic atlas of the human orbitofrontal sulci in standardized stereotaxic space. Neuroimage. 2001;13(3):479–496. doi: 10.1006/nimg.2000.0641. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain research. 1993;624(1–2):1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Tasteolfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18(7):2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Dixon HG, Scully ML, Wakefield MA, White VM, Crawford DA. The effects of television advertisements for junk food versus nutritious food on children's food attitudes and preferences. Soc Sci Med. 2007;65(7):1311–1323. doi: 10.1016/j.socscimed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Eertmans A, Baeyens F, Van den Bergh O. Food likes and their relative importance in human eating behavior: review and preliminary suggestions for health promotion. Health Educ Res. 2001;16(4):443–456. doi: 10.1093/her/16.4.443. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Ruhm CJ, Kosa KM. Economic causes and consequences of obesity. Annu Rev Public Health. 2005;26:239–257. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cerebral cortex. 2010a;20(5):1082–1091. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 2010b;20(5):1082–1091. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Boyland EJ, Cooper GD, Dovey TM, Smith CJ, Williams N, Lawton CL, Blundell JE. Children's food preferences: effects of weight status, food type, branding and television food advertisements (commercials) Int J Pediatr Obes. 2008a;3(1):31–38. doi: 10.1080/17477160701645152. [DOI] [PubMed] [Google Scholar]
- Halford JC, Boyland EJ, Hughes GM, Stacey L, McKean S, Dovey TM. Beyond-brand effect of television food advertisements on food choice in children: the effects of weight status. Public Health Nutr. 2008b;11(9):897–904. doi: 10.1017/S1368980007001231. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28(4):404–413. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20(5):1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(34):7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cerebral cortex. 2011;21(1):95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Martin A, Simmons WK. The structural basis of semantic memory. Learning and Memory: A Comprehensive Reference. 2008:113–130. [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11(4):893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316(5826):904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech RM, Lewis J, Parkinson CH, Owen AM, Roberts AC, Downing PE, Parkinson JA. Neural correlates of appetite and hunger-related evaluative judgments. PLoS ONE. 2009;4(8):e6581. doi: 10.1371/journal.pone.0006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(37):9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiologica Hungarica. 2008;95(2):131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger Modulates the Responses to Gustatory Stimuli of Single Neurons in the Caudolateral Orbitofrontal Cortex of the Macaque Monkey. Eur J Neurosci. 1989;1(1):53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Scott TR, Yan J, Rolls ET. Brain mechanisms of satiety and taste in macaques. Neurobiology (Bp) 1995;3(3–4):281–292. [PubMed] [Google Scholar]
- Simmons W, Martin A, Barsalou L. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery J, Barcalow J, Bodurka J, Drevets W, Bellgowan PS. Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. doi: 10.1002/hbm.22113. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM. Taste representation in the human insula. Brain structure & function. 2010;214(5–6):551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57(5):786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain : a journal of neurology. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose "liking" and food intake. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(38):8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196(2):155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. Journal of Neurophysiology. 2006;96(5):2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55(1):296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL. Binge eating is associated with right orbitofrontal-insularstriatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]