Abstract
Objectives
To determine whether early treatment with cysteamine affects cognitive functioning in patients with nephropathic cystinosis.
Study design
Individuals (n = 46, ages 3–18 years) with cystinosis underwent cognitive testing to determine intelligence, visual spatial abilities, and visual motor skills. An age-matched control group (n = 85, age range 2–22 years) received the same tests. Age at diagnosis and age at treatment onset with cysteamine were recorded at the time of testing.
Results
Patients with cystinosis treated at or after 2 years of age (late-treatment patients) scored significantly lower on verbal, performance, and full-scale IQ measures, as well as on a test of visual spatial skills when compared to early treatment patients (treatment onset <2years old) and to controls. Regardless of the age of treatment, both groups of subjects with cystinosis showed impairment in visual motor skills compared with controls; early-treatment patients showed no advantage in this area.
Conclusion
Early treatment with cysteamine appears to improve intellectual function in nephropathic cystinosis. However, the fact that visual motor function was not improved with early cysteamine treatment suggests that the mechanisms underlying visual motor performance may be different from other areas of cognition in this disorder.
Keywords: cysteamine, cognitive function
Nephropathic cystinosis is an autosomal recessive disorder that occurs in approximately 1 per 100,000 live births1. The defect has been mapped to a mutation in chromosome 17p13, which encodes the lysosomal membrane transport protein cystinosin (CTNS). Multiple mutations have been identified, most leading to the inactivation of the cystinosin protein2–3. With the inactivation of CTNS, the amino acid cystine is incapable of crossing the lysosomal membrane, causing accumulation of cystine crystals in virtually all organs, including the brain1.
The kidney is the first organ affected by the disease, usually within the first 6 to 12 months of life. Other complications arise over time. These may include thyroid dysfunction, growth retardation, progressive visual impairment, pancreatic dysfunction1, myopathy4, and in rare cases, dementia5–6.
Treatment with cysteamine (beta-mercaptoethylamine, MEA) generally begins as soon as the diagnosis is made. This medication effectively decreases the rate of cystine buildup within cells1. Life expectancy has increased markedly and quality of life is significantly improved with cysteamine treatment7.
Collective data from MRI scans, CT scans, and autopsies has revealed that cystinosis is associated with altered brain structure and increased levels of cystine in many areas of the brain8–12. Despite the structural changes in the brains of patients with cystinosis, neurological and cognitive changes have generally been relatively mild. Patients with the disease fall within the range of normal intelligence and have normal language, verbal learning, and reading skills13–15. The primary cognitive deficits in patients with cystinosis are in visuospatial abilities, visual motor coordination, and short-term visual memory. Neurological difficulties consist primarily of impaired gross and fine motor skills and seizures14–20. Patients with cystinosis may experience academic challenges, especially in spelling and math15.
The underlying cause of the cognitive impairments is not clear. One possible explanation is that progressive cystine accumulation in the brain over time causes functional as well as structural damage. If this is the case, then early treatment with cysteamine should reduce cognitive deficits. The present study was conducted to examine whether early treatment with cysteamine was associated with more favorable cognitive outcomes.
Methods
Forty-six children and adolescents with nephropathic cystinosis ages 3 through 18 years (mean age, 7.3 ± 4.5 years) participated in the study. This testing was part of a larger, longitudinal study of brain structure and function in cystinosis. Each participant was diagnosed by his or her nephrologist with nephropathic cystinosis, based on clinical presentation and by assays documenting elevated leukocyte cystine concentrations. Patients with other medical issues (ie, untreated thyroid dysfunction, uncorrected vision problems, and patients in renal failure) were excluded, as these factors may adversely affect cognitive performance. Individuals were also excluded if they were on dialysis, were acutely ill, or had any other condition that might adversely affect cognitive function. Only one subject was excluded for these reasons.
Subjects were recruited at family conferences organized by one of the cystinosis foundations (Cystinosis Research Network, Cystinosis Research Foundation and Cystinosis Foundation). Individuals were recruited from around the country, and either traveled to San Diego for testing, or were tested while attending the family conferences. Background and demographic information was obtained on each participant, including the age at start of treatment with cysteamine. On the basis of this information, subjects were divided into early treatment (prior to or by the age of 2 years) or later treatment (after the age of 2 years) for later analyses. The rationale for choosing 2 years as a cutoff for “early” vs. “late” treatment is that many children are not diagnosed until at least 12–18 months of age and so treatment is often not instituted prior to about 2 years of age21.
We were unable to acquire specific information about renal function on most of the patients for various reasons. However, some of these children participated in another study at the same time as ours, and all had estimated glomerular filtration rates (eGFRs) between 79 and 122 mL/min per 1.73 m2 or stage 1 or 2 kidney disease22.
A control group consisting of 85 individuals ages 2 through 22 (mean age, 8.3 ± 4.8 years) was used for comparison. Controls were group-matched to subjects with cystinosis on the basis of age and socio-economic status. All control participants were free of neurological, psychiatric and developmental problems, and did not have any other underlying condition that might affect cognitive function. Control subjects were recruited through IRB-approved advertisements in local parent magazines, through flyers placed in pediatricians’ offices, and through the UCSD subject pool. For all subjects, informed consent was obtained from the parent and/or subject prior to testing, as well as an assent from children over the age of 7 years, in accordance with UCSD Human Subjects Protection Program policies.
Intelligence was measured using an age-appropriate Wechsler Intelligence Scale. These tests are well normed and have a mean of 100 and standard deviation of 1523–25.
The Spatial Relations Test from the Woodcock-Johnson Psychoeducational Battery26 was used to measure visual spatial ability. This test does not require motor involvement. Subjects were shown pictures containing a series of shapes from which they were required to select component parts in order to make a whole. Raw scores were converted to standard scores with a mean of 100 and a standard deviation of 15. This test is normed for 2 years of age and older.
Visual motor skills were measured with the Beery-Buktenica Developmental Test of Visual Motor Intelgration (VMI), 5th Edition27. The VMI required each participant to copy a series of increasingly complex geometric figures. Raw scores were converted to standard scores with a mean of 100 and a standard deviation of 15. This test is also normed for 2 years of age and older.
Data Analyses
Multivariate Analysis of Variance techniques were used to compare early- and late-treated subjects with cystinosis with controls on VIQ, PIQ and FS IQ. Post-hoc independent-sample t-tests were performed using SPSS statistical analysis software. For Spatial Relations and VMI tasks, analysis of variance was used to compare early- vs. late-treatment cystinosis groups.
Results
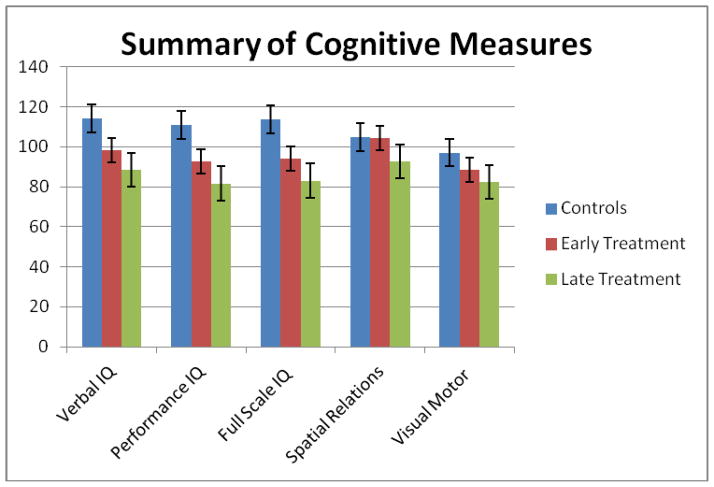
Individuals with cystinosis in the early-treatment group scored significantly higher on performance IQ testing than did the subjects in the late-treatment group. Both groups were significantly lower than controls on all IQ measures. Importantly, however, the scores of the early-treatment group, despite being lower than the controls, still fell within the normal range, whereas the late-treatment mean scores were below the normal range for PIQ and in the low normal range for FSIQ (Table). Scores on the Spatial Relations test were within the normal range for both treatment groups, although the late-treated group scored significantly lower than the early treated group (Figure).
Table 1.
Mean, standard deviation and p values of cognitive tests in individuals with cystinosis treated before or after age 2 years.
| Cognitive Measure | Controls | Early-Treatment | Late-Treatment | P value* |
|---|---|---|---|---|
| Verbal IQ | 114.0 ± 14.8 n = 85 |
98.2 ± 11.1 n = 32 |
88.6 ± 15.2 n = 14 |
.022 |
| Performance IQ | 110.8 ± 13.2 n = 85 |
92.7 ± 15.3 n = 32 |
81.6 ±13.2 n = 14 |
.023 |
| Full Scale IQ | 113.6 ± 13.2 n = 85 |
94.0 ± 12.5 n = 32 |
83.0 ± 15.1 n = 14 |
.013 |
| Spatial Relations | 104.7 ± 16.1 n = 81 |
104.5 ± 12.0 n = 29 |
92.7 ± 22.6 n = 11 |
.038 |
| Visual Motor (VMI) | 97.1 ± 13.2 N = 81 |
88.5 ± 17.5 n = 30 |
82.3 ± 13.1 n = 11 |
NS |
| Picture Completion | 11.43 ± 2.92 n = 75 |
9.04 ± 3.49 n = 27 |
7.18 ± 3.43 n = 11 |
.144 |
| Block Design | 12.45 ± 3.26 n = 82 |
8.88 ± 2.91 n = 33 |
6.71 ± 3.56 n = 14 |
.035 |
| Symbol Search | 12.33 ± 2.88 n = 75 |
7.85 ± 2.64 n = 27 |
7.09 ± 2.39 n = 11 |
.414 |
Statistical comparisons of Early- vs. Late-Treatment groups.
p values for cystinosis groups compared with controls were all <.001.
Figure.
Comparison of scores on primary cognitive tests for controls, early-and late-treated patients with cystinosis (mean ± standard error)
An analysis of selected subtest scores for Performance IQ measures further demonstrated a pattern of overall poorer performance by the children with cystinosis compared with controls (Table). Scores on the Block Design subtest, a spatially mediated task, were significantly lower in the late-treatment than the early-treatment group, whereas scores on the Picture Completion subtest, which assesses visual perception, were not significantly different between the two cystinosis groups.
There were no significant differences between early and late treatment groups on the test of visual motor ability, with both groups scoring well below the expected mean. The mean for the late-treatment group fell more than one standard deviation below the normal range, however. There were also no significant differences between the cystinosis groups on another task of speeded motor function, the Symbol Search subtest, with both groups scoring well almost one standard deviation below the expected mean of 10.
Discussion
The introduction of cysteamine for the treatment of nephropathic cystinosis has made a remarkable impact on the disease. Multiple studies have demonstrated significant benefits of cysteamine with regard to preserved renal function, growth, and other systemic manifestations1,7. Cysteamine treatment has also been shown to halt the progression of dementia that has been shown to develop in adults with cystinosis6. The current study documents a beneficial effect of cysteamine on cognitive function in children with cystinosis. Children with nephropathic cystinosis who were treated prior to 2 years of age had significantly higher scores on intelligence tests and on a test of spatial relations than did children treated after 2 years of age. Interestingly, however, visual motor skills did not benefit from early treatment, suggesting that the underlying mechanism by which visual motor skills are adversely affected in cystinosis may be different from those involved in other aspects of neuro-cognitive function.
The fact that early treatment results in preserved intellectual function in cystinosis suggests that the cognitive impairment may at least in part be due to progressive accumulation of cystine in the brain over the first years of life, during a crucial time in brain development. Cystine accumulation may have a destructive impact on myelination and other microstructural elements of white matter in the brain, which may lead to alterations in the development of fiber networks important for cognitive, motor, and sensory abilities. In fact, a recent study from our laboratory demonstrated abnormalities in white matter integrity in both parietal lobes of children with cystinosis28. That finding, in conjunction with those of the current study, lends support to the hypothesis that early cystine accumulation may underlie some of the neuropathology of this condition. However, it is also possible that there is damage to other developing neural elements as a result of early cystine accumulation. Currently, the mechanism by which cystine damages cells is unknown, but it is reasonable to speculate, based on the current study, that introducing a cystine-depleting agent into the body as early as possible may afford the brain some protection against such injury.
An alternative explanation for the better cognitive performance of the early-treatment group relates to the impact of treatment on renal dysfunction. The natural history of untreated cystinosis is that of progressive renal failure ultimately requiring dialysis and eventually kidney transplant before the age of ten. If renal complications remain untreated over the first several years of life, a child’s cognitive functioning may be adversely impacted due to progressive renal dysfunction29. Reducing the severity of renal complications earlier may allow a child’s brain to develop more normally. A limitation of the current study was the lack of information on the degree of renal damage prior to onset of treatment with cysteamine in our subjects. We also did not have information about renal function at the time of our testing, and although parents reported that their children’s renal function was “acceptable” based on their personal physicians’ reports, it is possible that different degrees of renal impairment were present between the two cystinosis groups. This would not, however, explain the differential performance on visual spatial vs. visual perceptual tasks, because renal dysfunction is more likely to be associated with global cognitive deficits than with specific types of impairments29.
Although the beneficial effect of early treatment on cognitive function is clearly demonstrated in the present study, some problems still remain. Specifically, visual motor skills are impaired in both the early- and late-treated cystinosis groups. It is possible that these are more complex tasks tapping several cognitive skills, and are able to reveal subtle persistent deficits that less complex tasks do not. It is also possible that the underlying cause of the visual motor deficit is different from that of general intellectual function. Given that visual motor dysfunction is evident even in young children after treatment20, it is possible that either pre-natal cystine accumulation is responsible for such neurological changes, or that the gene itself exerts an as yet unknown effect on brain development. Further studies are needed to identify the precise mechanisms by which cystinosis produces aberrations in brain development. Whatever the ultimate mechanism, however, this study demonstrates the importance of early diagnosis and treatment of cystinosis in order to better preserve cognitive functioning in these individuals.
Acknowledgments
Supported by National Institute of Neurological Disorders and Stroke (NS043135 to D.T.). L.V. received support from the National Institutes of Health Minority Access to Research Careers U-STAR Award (T34 GM087193).
We would like to thank the Cystinosis Research Foundation, the Cystinosis Research Network, and the National Cystinosis Foundation for their support of our work, and the parents and children who participated in the study.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nesterova G, Gahl WA. Cystinosis: The evolution of a treatable disease. Pediatr Neprhol. 2013;28:51–59. doi: 10.1007/s00467-012-2242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDowell GA, Gahl WA, Stephenson LA, Schneider JA, Weissenbach J, Polymeropoulos MH, et al. Linkage of the gene for cystinosis to markers on the short arm of chromosome 17. Nat Genet. 1995;10:246–248. doi: 10.1038/ng0695-246. [DOI] [PubMed] [Google Scholar]
- 3.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–24. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 4.Charnas LR, Luciano CA, Dalakas M, Gilliatt RW, Bernardini I, Ishak K, et al. Distal vacuolar myopathy in nephropathic cystinosis. Ann Neurol. 1994;35:181–8. doi: 10.1002/ana.410350209. [DOI] [PubMed] [Google Scholar]
- 5.Vogel DG, Malekzedah MH, Cornford ME, Schneider JA, Shields WD, Vinters HV. Central nervous system involvement in nephropathic cystinosis. J Neuropathol Exp Neurol. 1990;49:591–599. doi: 10.1097/00005072-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Broyer M, Tete MJ. Central nervous system complications in cystinosis. In: Broyer M, editor. Cystinosis. Elsevier; Paris: 1999. pp. 75–80. [Google Scholar]
- 7.Brodin-Sartorius A, Tête MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–89. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 8.Enrich JHH, Stoeppler L, Offner G, Brodehl J. Evidence for cerebral involvement in nephropathic cystinosis. Neuropaediat. 1979;10:128–137. doi: 10.1055/s-0028-1085319. [DOI] [PubMed] [Google Scholar]
- 9.Cochat P, Drachman R, Gagnadoux MF, Pariente D, Broyer M. Cerebral atrophy and nephropathic cystinosis. Arch Dis Child. 1986;61:401–403. doi: 10.1136/adc.61.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine S, Paparo G. Brain lesions in a case of cystinosis. Acta Neuropathologica. 1982;57:217–220. doi: 10.1007/BF00685392. [DOI] [PubMed] [Google Scholar]
- 11.Jonas AJ, Conley SB, Marshall R, Johnson RA, Marks M, Rosenberg H. Nephropathic cystinosis with central nervous system involvement. Amer J Med. 1987;83:966–970. doi: 10.1016/0002-9343(87)90661-9. [DOI] [PubMed] [Google Scholar]
- 12.Nichols S, Press GA, Schneider JA, Trauner DA. Cortical atrophy and cognitive performance in infantile nephropathic cystinosis. Pediat Neurol. 1990;6:379–381. doi: 10.1016/0887-8994(90)90004-k. [DOI] [PubMed] [Google Scholar]
- 13.Williams BLH, Schneider J, Trauner DA. Global intellectual deficits in cystinosis. Amer J Med Genet. 1994;49:83–87. doi: 10.1002/ajmg.1320490115. [DOI] [PubMed] [Google Scholar]
- 14.Trauner DA, Chase C, Scheller J, Katz B, Schneider J. Neurologic and cognitive deficits in children with cystinosis. J Pediatr. 1988;112:912–914. doi: 10.1016/s0022-3476(88)80214-2. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne A, Scarvie K, Trauner D. Academic achievement in individuals with infantile nephropathic cystinosis. Amer J Med Genet. 1997;74:157–161. doi: 10.1002/(sici)1096-8628(19970418)74:2<157::aid-ajmg8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Scarvie KM, Ballantyne AO, Trauner DA. Visual-motor performance in children with infantile nephropathic cystinosis. Perceptual Motor Skills. 1996;82:67–75. doi: 10.2466/pms.1996.82.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Colah S, Trauner DA. Deficits in tactile recognition in nephropathic cystinosis. Devel Med Child Neurol. 1997;39:409–413. doi: 10.1111/j.1469-8749.1997.tb07455.x. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne AO, Trauner DA. Neurobehavioral consequences of a genetic metabolic disorder: Visual processing deficits in infantile nephropathic cystinosis. Neuropsychiat, Neuropsychol Behav Neurol. 2000;13:254–263. [PubMed] [Google Scholar]
- 19.Trauner D, Spilkin A, Williams J, Babchuck L. Specific cognitive deficits in young children with cystinosis: evidence for an early effect of the cystinosin gene on neural function. J Ped. 2007;151:192–196. doi: 10.1016/j.jpeds.2007.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trauner DA, Williams J, Ballantyne AO, Spilkin AM, Crowhurst J, Hesselink J. Neurological impairments in nephropathic cystinosis: Motor coordination deficits. Ped Nephrol. 2010;25:2061–6. doi: 10.1007/s00467-010-1589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med. 1993;328:1157–62. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- 22.Dohil R, Ganfioti JA, Cabrera BL, Fidler M, Schneider JA, Barshop BA. Long-term treatment of cystinosis in children with twice-daily cysteamine. J Pediatr. 2010;156:823–827. doi: 10.1016/j.jpeds.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler Intelligence Scale for Children-third edition (WISC-III) San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 24.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio, TX: Psychological Corporation; 2002. (WPPSI-III) [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. (WAIS-III) [Google Scholar]
- 26.McGrew KS, Woodcock RW. Woodcock-Johnson psychoeducational battery and scales of independent behavior. Allen, TX: DLM Teaching Resources; 1985. [Google Scholar]
- 27.Beery KE, Beery NA. The Beery-Buktenica developmental test of visual-motor integration (VMI) 5. Minneapolis, MN: NCS Pearson; 2004. [Google Scholar]
- 28.Bava S, Theilmann RJ, Sach M, May SJ, Frank L, Hesselink J, et al. Developmental changes in cerebral white matter microstructure in a disorder of lysosomal storage. Cortex. 2010;46:206–16. doi: 10.1016/j.cortex.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1824–1830. doi: 10.2215/CJN.09751110. [DOI] [PMC free article] [PubMed] [Google Scholar]