Abstract
As the newest plant hormone, strigolactone research is undergoing an exciting expansion. In less than five years, roles for strigolactones have been defined in shoot branching, secondary growth, root growth and nodulation, to add to the growing understanding of their role in arbuscular mycorrhizae and parasitic weed interactions.1 Strigolactones are particularly fascinating as signaling molecules as they can act both inside the plant as an endogenous hormone and in the soil as a rhizosphere signal.2-4 Our recent research has highlighted such a dual role for strigolactones, potentially acting as both an endogenous and exogenous signal for arbuscular mycorrhizal development.5 There is also significant interest in examining strigolactones as putative regulators of responses to environmental stimuli, especially the response to nutrient availability, given the strong regulation of strigolactone production by nitrate and phosphate observed in many species.5,6 In particular, the potential for strigolactones to mediate the ecologically important response of mycorrhizal colonization to phosphate has been widely discussed. However, using a mutant approach we found that strigolactones are not essential for phosphate regulation of mycorrhizal colonization or nodulation.5 This is consistent with the relatively mild impairment of phosphate control of seedling root growth observed in Arabidopsis strigolactone mutants.7 This contrasts with the major role for strigolactones in phosphate control of shoot branching of rice and Arabidopsis8,9 and indicates that the integration of strigolactones into our understanding of nutrient response will be complex. New data presented here, along with the recent discovery of phosphate specific CLE peptides,10 indicates a potential role for PsNARK, a component of the autoregulation of nodulation pathway, in phosphate control of nodulation.
Keywords: strigolactones, nodulation, autoregulation, mycorrhizae, nitrate, phosphate, pea
Strigolactones as Potential Endogenous Signals during Symbioses
Until recently, strigolactones were characterized as a rhizosphere signal in the establishment of mycorrhizal symbiosis. Plant host roots secrete strigolactones which activate the mycorrhizal fungal partner, stimulating both germination and hyphal branching through enhanced fungal mitochondrial activity.2,11,12 Consistent with this, mutants with defects in strigolactone biosynthesis secrete little strigolactone and have reduced mycorrhizal colonization, which can be increased with exogenous strigolactones.3-5,13 Our recent results using a pea mutant with defects in strigolactone signaling indicate that in addition to the rhizosphere role, strigolactones may also play a role as an endogenous hormone inside the root during mycorrhizal establishment. The rms4 mutant of pea is not deficient in strigolactones but is strigolactone insensitive and contains a mutation in an F-box protein that may form a component of a SCF complex required for strigolactone response.5,14 Total mycorrhizal colonization was significantly reduced in rms4 mutant plants, with reductions in both hyphal colonization and arbuscule formation, although the arbuscules that did form appeared to be normal.5 An independent paper exploring the mycorrhizal phenotypes of a strigolactone insensitive mutants of rice was consistent with this result. As we found in pea, rice mutants with defects in the OsD3 F-box protein, orthologous to PsRMS4, also exhibited a strong reduction in mycorrhizal colonization, including reduced hyphal growth and arbuscule number, but normal arbuscule shape.15 Clearly, this component of the strigolactone perception pathway enhances mycorrhizal colonization. The simplest interpretation of this is that strigolactones not only need to be produced by the root and exuded into the rhizosphere to enhance the fungal partner, but that strigolactones must also be perceived by the plant itself via the RMS4/D3 F-box pathway to enhance mycorrhizal colonization (Fig. 1). Consistent with a role for strigolactones inside the root during mycorrhizal colonization, expression of a recently identified ABC strigolactone transporter is elevated in root tissue flanking mature arbuscules.13
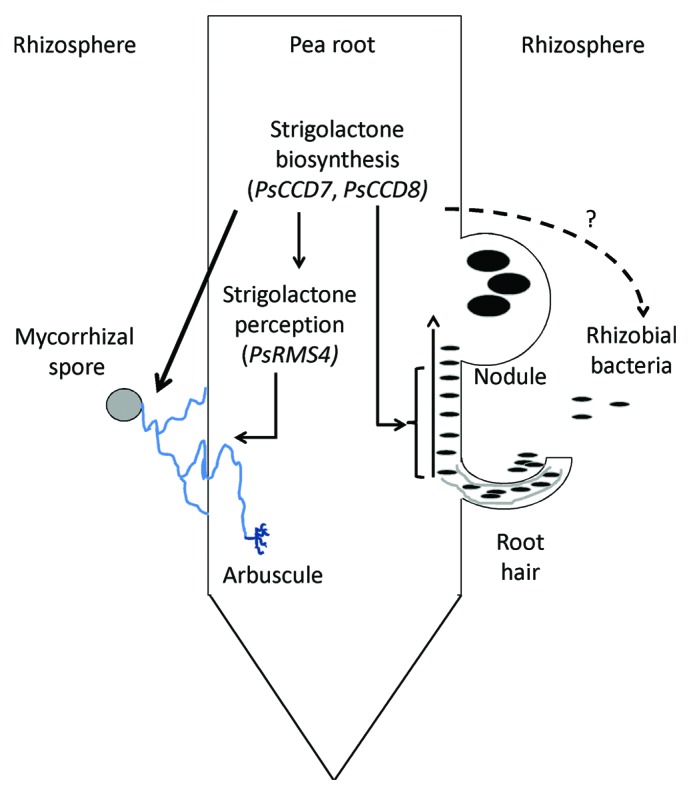
Figure 1. Proposed roles of strigolactone biosynthesis genes (PsCCD7, PsCCD8) and signaling gene (PsRMS4) in nodulation and mycorrhizal development of pea roots. All arrows drawn have been tested to some degree; solid arrows indicate relationships supported by experimental evidence; dashed arrows indicate relationships not supported by experimental evidence.
Strigolactones are thought to act as an endogenous plant hormone during the related legume symbiosis with rhizobial bacteria. Application of synthetic strigolactone directly to pea- and Medicago sativa-specific rhizobial cell cultures failed to enhance growth, stimulate nod factor production or induce calcium spiking, all processes that are strongly induced by flavonoids, which are nodulation-specific rhizosphere signals.16,17 Our recent work with mutants indicate that endogenous strigolactones are required to enhance nodulation, as strigolactone-deficient mutants form significantly fewer nodules and this can be restored by synthetic strigolactones.5,18 Strigolactones appear to influence nodule development after root hair curling but before visible nodule emergence, as strigolactone-deficient mutants exhibited wild type numbers and length of roots hairs18 and similar levels of root hair curling in response to rhizobia (unpubl. data Hugill, Foo, Reid) but no abnormal or small nodules (Fig. 1).18 We observed an interesting difference between the role of the RMS4 F-box protein in nodulation and mycorrhizal symbioses. In contrast to the prediction that rms4 mutants may have reduced nodulation as observed in the strigolactone biosynthesis mutants, we found rms4 mutants did not exhibit reduced nodule number. This is the reverse of what was seen in mycorrhizal studies5 (Fig. 1). One interpretation of this finding is that the influence of strigolactones on nodulation is not inside the root but external to it. However, as indicated above, all the data available suggests strigolactones do not influence the growth or behavior of the bacteria. It must be noted that changes in root size or root architecture do not appear to be the cause of the altered fungal and bacterial symbioses observed in the strigolactone pea mutants, as such small changes are accounted for in the sampling methods used.5,18
The Role of Strigolactones in the Response to Phosphorus and Nitrogen
The exudation of the hormone strigolactone is highly sensitive to nutrient levels in the soil, with all the species tested so far exhibiting a strong increase in strigolactone production under low phosphate conditions, with some species also responding to low nitrogen.1,5,6 Indeed, it has been suggested in the literature that this strong regulation of strigolactones may be the mechanism through which plants mediate changes in shoot and root growth and architecture in response to phosphate availability.7-9 Further, it has been discussed that this response may form part of the strict regulation of (metabolically expensive) symbioses with the mycorrhizal fungi.6,19,20 Studies with strigolactone-deficient or -insensitive mutants offer the most elegant method for testing such hypotheses and have recently yielded some contrasting results that require careful consideration.
We found that strigolactones are not essential for the regulation of mycorrhizal symbioses in response to phosphate, as strigolactone-deficient and -insensitive mutants can still enhance mycorrhizal colonization in response to low phosphate.5 Similarly, regulation of nodulation in response to nitrate and phosphate is also not dependant on strigolactones.5 Whether they play any part in the responses is not clear but the response of nodulation to low phosphate is in the wrong direction for such a role. This is consistent with the fact that exogenous strigolactone cannot enhance mycorrhizal colonization under high phosphate19,20 and is significant as it indicates additional genetic programs must be in place for these responses. One such system is the autoregulation of nodulation (AON) pathway that has been defined in several legume species and regulates nodule number and mycorrhizal colonization.21,22 The suppression of nodulation in response to nitrate acts in part through the NARK protein, an LRR receptor kinase that is a component of the autoregulation system, and this includes the action of a number of CLE peptides.23,24
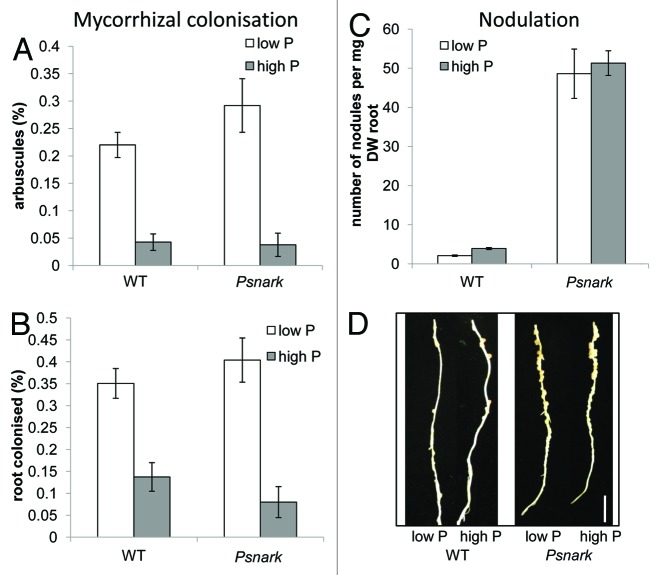
We have found preliminary evidence that in pea NARK may play some role in the phosphate response of nodulation but not mycorrhizal colonization. Mutants defective in PsNARK,25,26 exhibited a similar suppression of mycorrhizal colonization in response to phosphate as observed in wild type pea plants (Fig. 2a,b), as has been reported for the orthologous soybean Gmnark mutant.27 As observed previously,28 Psnark mutants grown under low phosphate may exhibit a small increase in mycorrhizal colonization compared with wild type plants, although in this case the effect was not significant (Fig. 2a). In contrast to the suppression of mycorrhizal colonization by high phosphate observed in both genotypes, the suppression of nodulation by low phosphate observed in wild type plants (Fig. 2c,d)29 was impaired in Psnark mutants. A highly significant approximately 2-fold reduction in nodule number was observed in wild type plants grown under low phosphate compared with high phosphate (Fig. 2c; p < 0.001). In contrast, Psnark mutants exhibited a clear supernodulating phenotype under both phosphate treatments, with no significant reduction in nodule number in response to phosphate limitation. With the recent description of phosphate-induced CLE peptides,10 the potential for integration of phosphate response in the AON pathway is an intriguing avenue for future research.
Figure 2. Effect of phosphate fertilisation on the development of mycorrhizal symbiosis (A and B) and nodulation (C and D) in Psnark (autoregulation mutant, sym29) and wild type pea (cv Frisson). Plants were grown as described previously5 with thrice weekly fertilisation with 0.05 (low P) or 5 mM (high P) NaH2PO4. For (A−C) values are mean ± s.e., n = 6 - 10. (A and B) Mycorrhizal colonization after 7 weeks growth with Glomus intraradices expressed as percentage of the root containing (A) arbuscules or (B) any fungal structure. (C and D) Nodulation of 3 week old plants inoculated at 1 week with Rhizobium leguminosarum bv viciae. (C) Nodule number per mg root dry weight and (D) photo of nodules on secondary root of wild type and Psnark mutant plants (tertiary roots have been removed), scale bar = 1 cm.
Since the publication of our investigation into the role of strigolactones in nutrient regulation of symbioses, a paper examining the role of strigolactones in the phosphate control of Arabidopsis root growth has been published. A mutant approach was also used to examine if strigolactone-deficient or -insensitive mutants have impaired ability to modify aspects of seedling root development or gene expression in response to phosphate.7 The enhancement of root hair number in response to low phosphate was found to be impaired in strigolactone deficient and insensitive mutants, but only at 48 h post-germination and not at later stages of seedling development. Other aspects of root growth that respond strongly to phosphate in wild type plants, including main root length and lateral root formation, were also found to be significantly affected by phosphate in max2 mutants, which are strigolactone-insensitive due to a lesion in an F-box protein orthologous to PsRMS4 and OsD3. However, the authors point to the small but significant impairment in main root length and lateral root density in response to phosphate limitation observed in max2 mutants. Some impairment in the induction of phosphate-responsive genes was also observed in the strigolactone mutants. Data are also presented that suggests auxin may play a role in this relatively small contribution of strigolactones to the phosphate root response. Taken together, we would interpret these results to indicate that strigolactones are not essential for the root growth response to phosphate, as mutants unable to produce or perceive strigolactones still respond strongly to phosphate. We would agree with the authors of this paper that the small and sometimes transient impairments in phosphate response observed in the mutants would suggest a small (modulating) role for strigolactones in early seedling responses to phosphate and that this indicates additional pathways are the main contributor to the phosphate response of root growth at later stages of seedling development.
In contrast to the two examples given above, where strigolactones may only play a minor role in nutrient response, there is evidence for a significant role for strigolactones in regulation of shoot branching in response to phosphate. In both rice and Arabidopsis, there is a correlation between low phosphate, high strigolactone levels and reduced bud outgrowth, with strigolactone-deficient mutants in these species failing to suppress bud outgrowth under low phosphate.8,9 This substantial reduction in response in strigolactone-deficient mutants is surely the strongest indication that strigolactones are required for this response.
Conclusions
The recent discovery of a potential endogenous role for strigolactones in nodulation and mycorrhizal development5,15,18 opens up a myriad of exciting avenues for future research. Two such recent developments are the discovery of a potential interaction between strigolactones and auxin in mycorrhizal development30 and a novel role for gibberellin in mycorrhizal symbiosis.31 Integration of strigolactones into the symbiosis pathways will be achieved through the use of more detailed anatomical studies, molecular markers and symbiosis mutants. These studies will help us to understand how legumes in particular strike the delicate balance required in their interaction with bacterial and fungal symbiotic partners.
Acknowledgments
We acknowledge the support of Australian Research Council and the University of Tasmania for financial support and Kaori Yoneyama was supported by a JSPS Research Fellowship for Young Scientists. We would like to thank A/Prof John Ross for helpful comments on the manuscript and Caine Barlow, Tracey Winterbottom and Michelle Lang for assistance with plant husbandry.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23168
References
- 1.Foo E, Reid JB. Strigolactones: new physiological roles for an ancient signal. J Plant Growth Regul. 2012 doi: 10.1007/s00344-012-9304-6. [DOI] [Google Scholar]
- 2.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–7. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–94. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 4.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 5.Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant. 2012 doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- 6.Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, et al. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta. 2012;235:1197–207. doi: 10.1007/s00425-011-1568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayzlish-Gati E, De-Cuyper C, Goormachtig S, Beeckman T, Vuylsteke M, Brewer PB, et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 2012;160:1329–41. doi: 10.1104/pp.112.202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010;51:1118–26. doi: 10.1093/pcp/pcq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, et al. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155:974–87. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funayama-Noguchi S, Noguchi K, Yoshida C. kawaguchi M. Two CLE genes are induced by phosphate in roots of Lotus japonicus. J Plant Res. 2012;124:155–63. doi: 10.1007/s10265-010-0342-5. [DOI] [PubMed] [Google Scholar]
- 11.Besserer A, Bécard G, Jauneau A, Roux C, Séjalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008;148:402–13. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, et al. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006;4:e226. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, et al. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–4. doi: 10.1038/nature10873. [DOI] [PubMed] [Google Scholar]
- 14.Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA, et al. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 2006;142:1014–26. doi: 10.1104/pp.106.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida S, Kameoka H, Tempo M, Akiyama K, Umehara M, Yamaguchi S, et al. The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol. 2012;196:1208–16. doi: 10.1111/j.1469-8137.2012.04339.x. [DOI] [PubMed] [Google Scholar]
- 16.Soto MJ, Fernandez-Aparicio M, Castellanos-Morales V, Garcia-Garrido JA, Delgado MJ, Vierheilig H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa) Soil Biol Biochem. 2012;42:383–5. doi: 10.1016/j.soilbio.2009.11.007. [DOI] [Google Scholar]
- 17.Moscatiello R, Squartini A, Mariani P, Navazio L. Flavonoid-induced calcium signalling in Rhizobium leguminosarum bv. viciae. New Phytol. 2010;188:814–23. doi: 10.1111/j.1469-8137.2010.03411.x. [DOI] [PubMed] [Google Scholar]
- 18.Foo E, Davies NW. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–81. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- 19.Balzergue C, Puech-Pagès V, Bécard G, Rochange SF. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot. 2011;62:1049–60. doi: 10.1093/jxb/erq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, et al. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 2010;64:1002–17. doi: 10.1111/j.1365-313X.2010.04385.x. [DOI] [PubMed] [Google Scholar]
- 21.Reid DE, Ferguson BJ, Hayashi S, Lin Y-H, Gresshoff PM. Molecular mechanisms controlling legume autoregulation of nodulation. Ann Bot. 2011;108:789–95. doi: 10.1093/aob/mcr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staehelin C, Xie Z-P, Illana A, Vierheilig H. Long-distance transport of signals during symbiosis: are nodule formation and mycorrhization autoregulated in a similar way? Plant Signal Behav. 2011;6:372–7. doi: 10.4161/psb.6.3.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid DE, Ferguson BJ, Gresshoff PM. Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol Plant Microbe Interact. 2011;24:606–18. doi: 10.1094/MPMI-09-10-0207. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, et al. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009;50:67–77. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- 25.Sagan M, Duc G. Sym28 and Sym29, two new genes involved in regulation of nodulation in pea (Pisum sativum L.) Symbiosis. 1996;20:229–45. [Google Scholar]
- 26.Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–6. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- 27.Wyss P, Mellor RB, Wiekmen A. Mutants of soybean (Glycine max) unable to suppress nodulation in the presence of nitrate retain the ability to suppress mycorrhization in the presence of phosphate. J Plant Physiol. 1990;136:507–9. doi: 10.1016/S0176-1617(11)80045-3. [DOI] [Google Scholar]
- 28.Morandi D, Sagan M, Prado-Vivant E, Duc G. Influence of genes determining supernodulation on root colonization by the mycorrhizal fungus Glomus mosseae in Pisum sativum and Medicago truncutula mutants. Mycorrhiza. 2000;10:37–42. doi: 10.1007/s005720050285. [DOI] [Google Scholar]
- 29.Tsvetkova GE, Georgiev GI. Changes in phosphate fractions extracted from difference organs of phosphorus starved nitrogen fixing pea plants. J Plant Nutr. 2007;30:2129–40. doi: 10.1080/01904160701700616. [DOI] [Google Scholar]
- 30.Foo E. Auxin influences strigolactones in pea mycorrhizal symbiosis. J Plant Physiol 2012; dio.10.1016/j.jplph.2012.11.002 [DOI] [PubMed]
- 31.Foo E, Ross JJ, Jones WT, Reid JB. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann Bot 2013; In Press [DOI] [PMC free article] [PubMed]