Abstract
In higher plants, cellulose is synthesized by cellulose synthase complexes, which contain multiple isoforms of cellulose synthases (CESAs). Among the total 10 CESA genes in Arabidopsis, recessive mutations at three of them cause the collapse of mature xylem cells in inflorescence stems of Arabidopsis (irx1cesa8, irx3cesa7 and irx5cesa4). These CESA genes are considered secondary cell wall CESAs. The others (the function CESA10 is still unknown) are thought to be specialized for cellulose synthesis in the primary cell wall. A split-ubiquitin membrane yeast two-hybrid system was used to assess interactions among four primary CESAs (CESA1, CESA2, CESA3, CESA6) and three secondary CESAs (CESA4, CESA7, CESA8). Our results showed that primary CESAs could physically interact with secondary CESAs in a limited fashion. Analysis of transgenic lines showed that CESA1 could partially rescue irx1cesa8 null mutants, resulting in complementation of the plant growth defect, collapsed xylem and cellulose content deficiency. These results suggest that mixed primary and secondary CESA complexes are functional using experimental set-ups.
Keywords: cellulose, cellulose synthase complex (CSC), primary cell wall, secondary cell wall, promoter swap
Primary cell walls differ from secondary cell walls in the composition of polysaccharides, the mechanical and structural properties of microfibrils, and their specific roles during plant growth.1 Primary cell walls are synthesized during cell expansion. Therefore primary cell walls are highly extensible and incorporative. Secondary cell walls are synthesized after the cell ceases its expansion and they provide tensile strength and rigidity, but not extensibility. Although cellulose is a major component of both primary and secondary cell walls, the degree of polymerization, the amount of cellulose relative to other wall polymers and the dimensions of microfibrils differs in primary and secondary walls.2 These differences may arise from the composition and/or activity of cellulose synthase complexes (CSCs) and their interactions with cytosolic components.3 Consistent with this idea, CSCs for primary wall cellulose synthesis have different sets of CESA proteins from those for secondary wall cellulose synthesis. For primary wall cellulose synthesis, CSCs are composed of CESA1, 3, 6 or 6-like primary CESA proteins, whereas CESA4, 7 and 8 are required to form functional CSCs during secondary wall cellulose synthesis.4-6
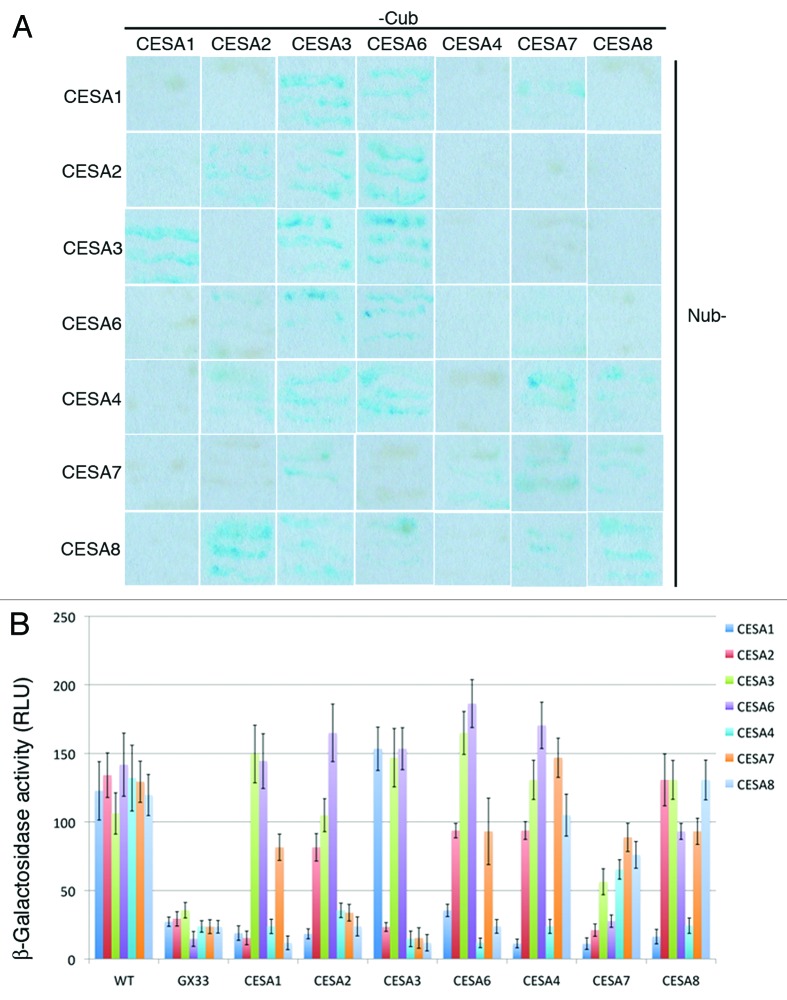
In this short communication, pair-wise interactions were tested between four primary CESAs (CESA1, 2, 3 and 6) and three secondary CESAs (CESA4, 7 and 8) using the split-ubiquitin yeast two-hybrid (Y2H) system.7 Each of the primary CESAs investigated were able to form homodimers except for CESA1 (Fig. 1). For primary CESAs, the following interactions were observed: CESA1 with CESA3 and CESA6, CESA3 with CESA2 and CESA6, and CESA2 with CESA6. Two of the three secondary CESAs, CESA7 and CESA8, were able to form homodimers. Although CESA4 was not able to form homodimers, it was able to interact with CESA7 and CESA8. CESA7 and CESA8 were also able to interact with one another. Interactions were also observed between primary and secondary CESAs. CESA7 interacted with both CESA1 and CESA3. CESA4 and CESA8 were each able to interact with the each of the primary CESAs under investigation except for CESA1. These results indicate that primary CESAs can physically interact with secondary CESAs in a limited fashion.
Figure 1. Interactions between CESA isoforms. (A) The growth and β-galactosidase expression of yeast transformed with plasmids containing both N-terminal fusions of Nub to a CESA (indicated by the labels of the top row) and C-terminal fusions of Cub to a CESA (indicated by the labels in the right column). (B) β-galactosidase activity was quantified colorimetrically in colonies expressing pairs of Nub and Cub CESA fusions. Cub CESA fusions were also expressed with WT Nub or mutated Nub (GX33) as a positive and negative control, respectively. The error bars indicate standard deviation.
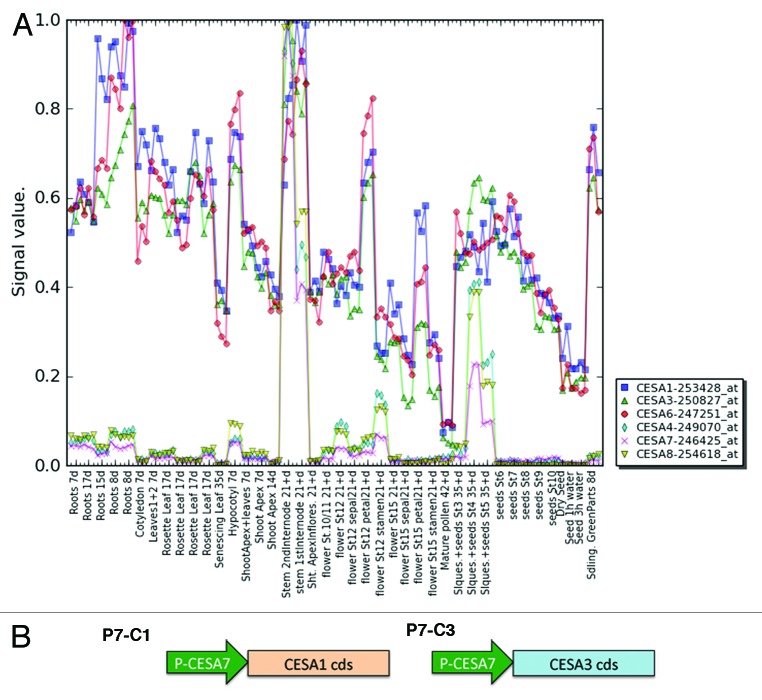
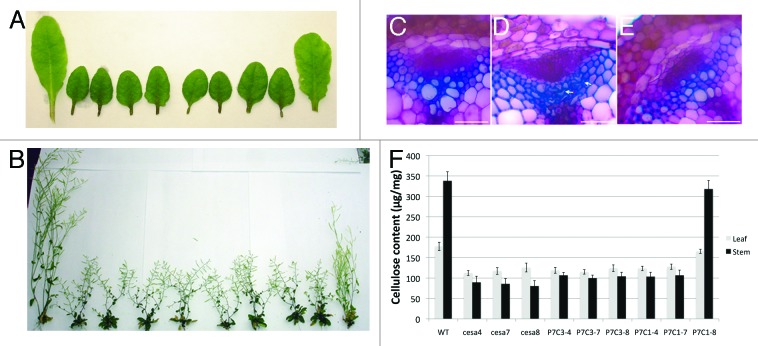
To test whether mixed primary and secondary CESAs are functional in planta, we generated a series of promoter-swap constructs. Analysis of the expression profile of primary and secondary CESA indicates that secondary CESAs are more tightly controlled.8 Since the three secondary CESA genes have similar expression profiles (Fig. 2A), the CESA7 promoter was chosen to drive the expression of primary CESAs (CESA1 and CESA3) in cells synthesizing secondary cell walls. CESA1 and CESA3 were chosen for the promoter-swap experiment because these two CESA isoforms are believed to be essential for primary CSCs. Two constructs, P7C1 and P7C3 (Fig. 2B, P7 refers to the CESA7 promoter and C1 and C3 refer to CESA1 and CESA3 cDNA, respectively), were transformed into three secondary CESA mutants, irx5cesa4, irx3cesa7 and irx1cesa8.9-11 Since these are null mutants, it is possible to determine whether P7C1 or P7C3 can complement the function of any of the individual secondary CESA mutants without any interference from the endogenous secondary CESA of interest. The growth morphology was first examined in all transgenic lines. P7C1 partially complemented several irx1cesa8 growth defects such as dwarf stature, reduced fertility, reduced leaf size and short siliques (Fig. 3A-B). irx1cesa8 is also known to have defects in xylem cells.9,10 Analysis of the cross section of P7C1 irx1cesa8 stems showed that P7C1 completely rescued the collapsed xylem defect of irx1cesa8, indicating P7C1 can incorporate in the secondary CSCs (Fig. 3C-E). The growth defects in the adult stem and leaves of irx1cesa8 are consistent with reduced cellulose content in these tissues (Fig. 3F). P7C1 recovered the cellulose content deficiency phenotype of irx1cesa8 whereas the rest of transgenic lines did not affect the cellulose content in their respective mutant background. Interestingly, the only interaction partner of CESA1 in the split-ubiquitin yeast two-hybrid assay was CESA7, suggesting that CESA1 may substitute for CESA8 in a way that differs from the native functional secondary CSCs. This observation may explain the partial complementation of irx1cesa8 growth defects by P7C1.
Figure 2. Design for promoter swap constructs. (A) The expression of the primary and secondary CESAs during different developmental stages in diverse organs (Schmid et al., 2005). (B) Schematic diagram of primary CESAs driven by the secondary CESA7 promoter. Arrows indicate promoter regions. Rectangular boxes indicate the coding sequence of primary CESAs.
Figure 3. P7C1 partially complements morphological and molecular defect in irx1cesa8. (A) Leaf morphology of secondary cesa mutants and various promoter-swap transmormants in secondary cesa mutants. From the left to right, wild type (WT), irx5cesa4, irx3cesa7, irx1cesa8, P7C3 in irx5cesa4 (P7C3–4), P7C3 in irx3cesa7 (P7C3–7), P7C3 in irx1cesa8 (P7C3–8), P7C1 in irx5cesa4 (P7C1–4), P7C1 in irx3cesa7 (P7C1–7), P7C1 in irx1cesa8 (P7C1–8). (B) Whole-plant morphology of secondary cesa mutants and various promoter-swap transgenic lines. Plants were arranged in the same order as shown in (A). (C−E) Transverse sections stained with toluidine blue were taken from the base of stem. Wild type (C), irx1cesa8 (D) and P7C1 irx1cesa8 (E). White arrows indicate the collapsed metaxylem. Bar = 50 μm. (F) Cellulose content in leaf or stem from wild type and various transformants in secondary cesa mutants. Error bars represent SE, n = 5. The figure is modified from Carroll et al., 2012 (www.plantphysiol.org, Copyright American Society of Plant Biologists).
Carroll et al. reported that all primary CESAs (CESA1, CESA3 and CESA6) interacted with secondary CESAs (CESA4, CESA7 and CESA8) in a split-ubiquitin Y2H assay.12 We cannot confirm this observation with our split-ubiquitin Y2H assay. This discrepancy may arise from the ability of our split-ubiquitin system to optimize the expression of Cub-CESAs under the control of MET25 promoter.7 We tested interactions on different methionine concentrations to keep the background to the minimum. Moreover, different from Carroll et al., our system generates a C-terminal fusion of Cub for the bait CESA protein and an N-terminal fusion of Nub for the prey CESA protein. The positions of the fusions could potentially alter the interaction abilities. The split-ubiquitin Y2H is prone to false positives and false negatives, therefore it is crucial to validate all possible fusion combinations in planta. Nevertheless, our results indicate that there are limited cross-interactions between primary and secondary CESAs, suggesting that CESAs have preferential interaction partners in the CSC complex that may impact the assembly or activity of CSCs.
Although many studies have shown unique components in primary (CESA1, 3, 6 and 6-like) and secondary CESA complexes (CESA4, 7 and 8), our results suggest mixed primary and secondary CESA complexes are functional in Arabidopsis. The primary and secondary CESAs are thought to be expressed at different times in plants according to microarray analysis (Fig. 2). However, there may be a limited period of time when both primary and secondary CESA genes are coexpressed. There are several reports supporting a role of primary CESAs during secondary cell wall formation. For example, the primary CESA clades (CESA1, 3, 5 and 6) are required for secondary wall synthesis in Arabidopsis trichomes.13 Another example came from the most recent finding that primary CESAs have function in the formation of secondary cell walls during seed coat development.14,15 Specifically, CESA5 is responsible for mucilage attachment and CESA2, 5 and 9 contribute to secondary cell wall biosynthesis in the columella cells.16 These findings suggest that the distinction between primary and secondary CESAs might not be as strict as initially defined. Moreover, defects in secondary cell wall cellulose biosynthesis showed complex alterations in the primary wall structure in cesa7mur10 and cesa4brittle cum11, suggesting that the formation of primary cell wall and secondary cell wall might not be entirely separated or that an integrity sensing mechanism exists from the secondary wall to the primary wall synthesis and remodeling machinery.15,17-19 The molecular details of the primary to secondary cell wall transition and mechanisms that underlie the cross talk between primary and secondary cell wall biosynthesis are largely unknown.
Our results suggest that mixed primary and secondary CSCs are functional using experimental setups. The question remains if mixed primary and secondary complexes exist in planta. Nevertheless, our findings may provide insight on the organization or position of CESA isoforms within CSCs.
Acknowledgments
This work is supported by the Center for LignoCellulose Structure and Formation, an Energy Frontier Research Center funded by the US. Department of Energy, Office of Science under Award Number DE-SC0001090.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23179
Reference
- 1.Cosgrove DJ, Jarvis MC. Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci. 2012;3:204. doi: 10.3389/fpls.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somerville C. Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol. 2006;22:53–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- 3.Lei L, Li S, Gu Y. Cellulose synthase complexes: composition and regulation. Front Plant Sci. 2012;3:75. doi: 10.3389/fpls.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc Natl Acad Sci USA. 2001;98:10079–84. doi: 10.1073/pnas.191361598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doblin MS, Kurek I, Jacob-Wilk D, Delmer DP. Cellulose biosynthesis in plants: from genes to rosettes. Plant Cell Physiol. 2002;43:1407–20. doi: 10.1093/pcp/pcf164. [DOI] [PubMed] [Google Scholar]
- 6.Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci USA. 2003;100:1450–5. doi: 10.1073/pnas.0337628100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, et al. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA. 2004;101:12242–7. doi: 10.1073/pnas.0404467101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, et al. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37:501–6. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Zeef LA, Ellis J, Goodacre R, Turner SR. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell. 2005;17:2281–95. doi: 10.1105/tpc.105.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell. 1997;9:689–701. doi: 10.1105/tpc.9.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor NG, Scheible WR, Cutler S, Somerville CR, Turner SR. The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. Plant Cell. 1999;11:769–80. doi: 10.1105/tpc.11.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll A, Mansoori N, Li S, Lei L, Vernhettes S, Visser RG, et al. Complexes with mixed primary and secondary cellulose synthases are functional in Arabidopsis plants. Plant Physiol. 2012;160:726–37. doi: 10.1104/pp.112.199208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betancur L, Singh B, Rapp RA, Wendel JF, Marks MD, Roberts AW, et al. Phylogenetically distinct cellulose synthase genes support secondary wall thickening in arabidopsis shoot trichomes and cotton fiber. J Integr Plant Biol. 2010;52:205–20. doi: 10.1111/j.1744-7909.2010.00934.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendu V, Griffiths JS, Persson S, Stork J, Downie AB, Voiniciuc C, et al. Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiol. 2011;157:441–53. doi: 10.1104/pp.111.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stork J, Harris D, Griffiths J, Williams B, Beisson F, Li-Beisson Y, et al. CELLULOSE SYNTHASE9 serves a nonredundant role in secondary cell wall synthesis in Arabidopsis epidermal testa cells. Plant Physiol. 2010;153:580–9. doi: 10.1104/pp.110.154062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan S, Ralet MC, Berger A, Diatloff E, Bischoff V, Gonneau M, et al. CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol. 2011;156:1725–39. doi: 10.1104/pp.111.179077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song D, Shen J, Li L. Characterization of cellulose synthase complexes in Populus xylem differentiation. New Phytol. 2010;187:777–90. doi: 10.1111/j.1469-8137.2010.03315.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Deng L, Qian Q, Xiong G, Zeng D, Li R, et al. A missense mutation in the transmembrane domain of CESA4 affects protein abundance in the plasma membrane and results in abnormal cell wall biosynthesis in rice. Plant Mol Biol. 2009;71:509–24. doi: 10.1007/s11103-009-9536-4. [DOI] [PubMed] [Google Scholar]
- 19.Bosca S, Barton CJ, Taylor NG, Ryden P, Neumetzler L, Pauly M, et al. Interactions between MUR10/CesA7-dependent secondary cellulose biosynthesis and primary cell wall structure. Plant Physiol. 2006;142:1353–63. doi: 10.1104/pp.106.087700. [DOI] [PMC free article] [PubMed] [Google Scholar]