Abstract
Melatonin was found in the fresh water characeae Chara australis. The concentrations (~4 μg/g of tissue) were similar in photosynthesizing cells, independent of their position on the plant and rhizoids (roots) without chloroplasts. Exogenous melatonin, added at 10 μM to the artificial pond water, increased quantum yield of photochemistry of photosystem II by 34%. The increased efficiency appears to be due to the amount of open reaction centers of photosystem II, rather than increased efficiency of each reaction center. More open reaction centers reflect better functionality of all photosynthetic transport chain constituents. We suggest that melatonin protection against reactive oxygen species covers not only chlorophyll, but also photosynthetic proteins in general.
Keywords: antioxidants, chlorophyll fluorescence, Characeae, melatonin, photosynthesis, reactive oxygen species
Introduction
Melatonin was first discovered in plants in two surveys of common fruits and vegetables from the market.1,2 In 1997, melatonin was reported in growing plants3 and a putative pathway for melatonin synthesis in plants was described in 2000.4 It was hypothesized that melatonin levels in plants may be timing mechanisms for circadian rhythm or seasonality with the highest level in dark periods and the lowest level during daylight.5 A diurnal rhythm in melatonin with a maximum in the dark phase and low levels during the day has been reported in the short-day plant Chenopodium rubrum, but changing the duration of the dark phase did not change the melatonin fluctuation.6 There was a 15–30-fold higher level of melatonin in etiolated (dark grown) seedlings as compared with light adapted tissues of Hypericum perforatum and Arabidopsis thaliana.7 Melatonin levels were higher in Glycyrrhiza uralensis grown under red light than those plants grown under green or blue light.8 In water hyacinths a peak in melatonin was found late in the light phase, near sunset,9 while in field grown Vitis vinifera L. cv Malbec the peak occurred at dawn.10 If the grapes were shielded from sunlight throughout the day, the concentration of melatonin remained high.10 More recently, Byeon et al. found that increased melatonin and activity of the genes associated with melatonin biosynthesis were detected in detached rice leaves under constant high light with lower concentrations observed in constant darkness.11 These researchers hypothesized that melatonin biosynthesis is dependent on a light signal.11 However, none of the systems studied to date have provided definitive evidence of the role of melatonin in the circadian rhythms, plant light/dark responses or the timing mechanisms of plants.12-15
The current research was designed to investigate the potential interactions between melatonin and photosynthetic reactions in plant cells. For these studies, a model system was required that: (1) contains melatonin; (2) is responsive to melatonin signaling; (3) has active, independent and complete photosynthetic apparatus; and (4) allowed for separation of individual cells to reduce cell-to-cell signaling. We developed a model system using fresh water characeae Chara australis.
The plants consist of large cells up to 1 mm in diameter and several cm in length. Single cells are easily excised from the plants and contents of cell compartments can be extracted and analyzed. Thus the effects of melatonin can be studied on single cell level. The endogenous melatonin content of cells and cell compartments can be measured and accurate doses of exogenous melatonin can be easily administered. These studies have wide applicability to other plant studies since the Characeae are the sister group to the ancestors of all land plants16 and their use as experimental system has already established the fundamentals of plant cell electrophysiology.17
Based on the recently reported findings, we hypothesized that melatonin antioxidant properties facilitate greater photosynthetic efficiency. Our objectives were: (1) to establish that Chara sp contain melatonin and respond to melatonin exposure; and (2) to determine the rate and mechanism of melatonin effects on photosynthesis in Chara. The results of our study show that melatonin is highly conserved across ancient and modern plants with quantifiable concentrations of melatonin in Chara.
Results
Endogenous melatonin in Chara cells
Melatonin was detected in cells of Chara (see Table 1). There were no significant differences in the amount of melatonin quantified in different parts of the plant, with similar amounts in green axial and leaf internodal cells and non-photosynthetic rhizoids (roots).
Table 1. The amounts of melatonin detected in samples from Chara australis plants.
| Tissue | Melatonin μg/g |
|---|---|
| Top leaf Chara cells with fruit |
4.2 |
| Top leaf Chara cells without fruit |
4.2 |
| Fruiting bodies (male) |
4.1 |
| Internodes top of plant |
2.9 |
| Cytoplasmic fragments top of plant |
4.2 |
| Internodes middle plant |
3.6 |
| Cyto fragments middle plant |
4.5 |
| Internodes bottom of plant |
4.4 |
| Rhizoids (roots) | 4.5 |
Photosynthesis
An effect of addition of exogenous melatonin on photosynthetic function of Chara australis cells was investigated by measurements of chlorophyll a fluorescence induction curves with control (no treatment) and melatonin-treated cells from which several parameters were evaluated. Typical courses of ΦL(PSII), ΦL(F,D) and ΦL(NPQ) parameters during 3 min illumination of the samples are shown in Figure 1. At the beginning of the experiment ΦL(F,D) showed no difference whether melatonin was present or not. The steady-state levels after 3 min illumination were close (0.42 for the control and 0.38 after melatonin exposure). On the other hand, ΦL(PSII), which measures the actual quantum yield of photochemistry of PSII, was by 34% higher for melatonin-treated cells (0.51) at the end of the experiment than that for the control cells (0.38). ΦL(PSII) is a product of two terms: the coefficient of photochemical quenching, qP, which is a measure of amount of open reaction centers of PSII during the illumination, and quantum yield of photochemistry of PSII in these open PSII reaction centers. As the maximal difference of the later term between the control and melatonin-treated cells was very small (up to 4%, data not shown), it is the amount of open reaction centers of PSII, which is responsible for difference in ΦL(PSII). Therefore, higher ΦL(PSII) in melatonin-treated cells means higher amount of the open PSII reaction centers in these cells, i.e., a better photosynthetic function in Chara cells supplied by exogenous melatonin. The higher amount of open reaction centers of PSII is not only a property of PSII itself, but it also reflects a better functionality of all photosynthetic electron transport chain constituents (see below) in melatonin-treated cells. The quantum yield of regulated non-photochemical energy quenching is lower by about 53% at the end of measurements for the melatonin-treated cells (0.11) than for the control cells (0.21). This is a consequence of the fact that sum of all the quantum yields [ΦL(PSII), ΦL(F,D), ΦL(NPQ)] is unity. The measurements were also performed for different intensities of excitation actinic light and the results always followed the same trends (control vs. melatonin-treated cells).
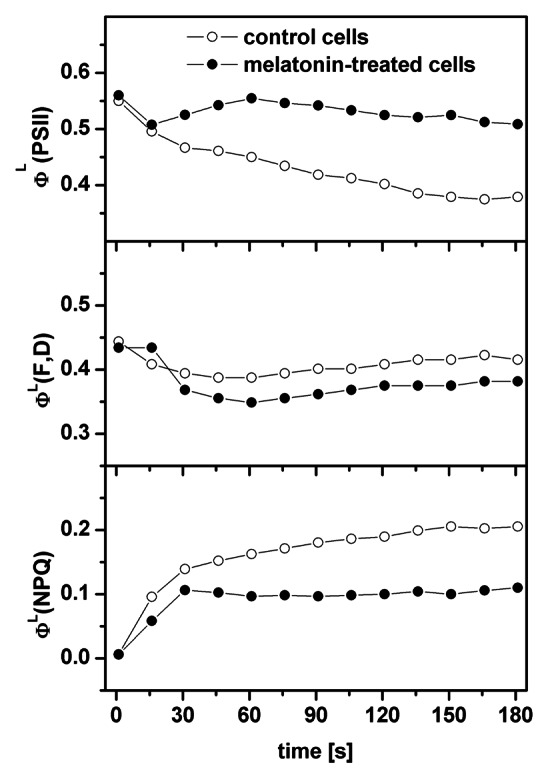
Figure 1. Time courses of the ΦL(PSII), ΦL(F,D) and ΦL(NPQ) parameters evaluated with the help of the saturation pulse method during illumination of control or melatonin-treated cells. The readers are reminded that the sum of all the quantum yields [ΦL(PSII), ΦL(F,D), ΦL(NPQ)] is unity.
Before the measurement of quenching parameters, the fast O-J-I-P fluorescence transients were also measured for the dark-adapted cells. These measurements revealed that the maximal quantum yield of photochemistry of PSII for dark-adapted cells, ΦD(PSII), was almost unaffected by melatonin treatment (0.75 for the control and 0.77 for melatonin-treated cells), but the total performance index, PItotal, was by about 75% higher in the melatonin-treated cells (5.49) than in the control cells (3.13). The higher PItotal in melatonin-treated cells is in agreement with the higher amount of open PSII centers in these cells as mentioned above. Interestingly (but see discussion), we obtained a lower value of ABS/RC parameter for melatonin-treated cells (1.01) than for the control cells (1.20) indicating that apparent antenna size of PSII was lower in melatonin-treated cells than for the control cells.
Discussion
Endogenous melatonin in Characeae
Melatonin was found in many land plant species and in algae divisions of Chlorophyta, Rhodophyta and Dinoflagellata.27 We have now measured it in the important division of Charophyta in the characeae family, which is thought to have given rise to land plants.16 Concentrations of melatonin were similar to previously reported for leaf and shoot portions of higher plants such as St. John’s wort,4 Datura25 and Scutellaria28 but lower than has been observed in fruit such as grapes26 or cranberries.29 In contrast, concentrations in Chara were murch greater than has been reported for lupin with the highest concentrations of melatonin found in root tissues.30 Tan et at. suggests that melatonin production dates back to purple nonsulfur bacteria and cyanobacteria, the precursors of mitochondria and chloroplasts, respectively.31 Thus finding melatonin in characeae is not a great surprise.
The role of exogenous melatonin in photosynthesis
To our knowledge, there are only two papers in which an effect of melatonin was explored by means of measurements of chlorophyll fluorescence.32,33 Wang et al. subjected detached apple leaves to dark-induced senescence. They found that ΦD(PSII) decreased more gradually in leaves supplied with 10 mM melatonin compared with controls, potentially indicating a role for melatonin in protection of the photosynthetic apparatus.32 Zhang et al. applied melatonin (at concentration of 50, 100, 300 or 500 μM) to cucumber seeds, which were also water-stressed by application of polyethylene glycol (PEG), i.e., melatonin was not applied to unstressed samples.33 Nevertheless the authors obtained very similar results as in our case: very small changes of ΦD(PSII) and of quantum yield of photochemistry of open reactions centers of PSII for light-adapted state, but an increase of qP and hence also of ΦL(PSII) for the PEG-melatonin-treated samples as compared with the PEG-treated samples.33 The authors also observed a decrease of non-photochemical quenching of excitation energy, expressed by the NPQ parameter, for the PEG-melatonin treated samples.33
In agreement with other studies (reviewed in ref. 34), Zhang et al. also observed an increase of chlorophyll content (per fresh weight) when samples were treated by melatonin33 and Wang et al. and Arnao et al. reported that exogenous application of melatonin slowed the degradation of chlorophyll during senescence of apple32 and barley35 leaves, respectively. These findings are explained in literature as a protective role of melatonin against reactive oxygen species (ROS), which damages (oxidizes) the chlorophylls. However, we have measured a decrease of apparent antenna size of PSII (expressed by the ABS/RC parameter) in melatonin-treated cells, which might indicate a lower amount of chlorophylls in these cells. This seemingly contradictory result can be explained by the fact that ROS does not damage (oxidize) only chlorophylls, but also other components (proteins in general, including PSII and other photosynthetic proteins) of photosynthetic electron transport chain. Thus the application of exogenous melatonin lowers ROS damage of many photosynthetic components. If melatonin offers more protection to PSII proteins than to chlorophyll molecules, the antenna size might appear lower after melatonin exposure. The decreased amount of damaged photosynthetic proteins in melatonin-treated cells is also in agreement with an increased value of the qP parameter in these cells. The qP parameter is a measure of how many reaction centers of PSII are open during the illumination. The amount of open reaction centers reflects the functionality of PSII, as well as functionality of all other photosynthetic proteins. Hence if fewer proteins are damaged (oxidized) by ROS due to protective role of melatonin, more PSII are functional (open) in the light. This explanation is also in agreement with increased value of PItotal parameter in the melatonin-treated cells. To sum up, melatonin offers protection against ROS for chlorophylls as well as for the photosynthetic proteins in general.
Materials and Methods
Measurement of photosynthetic parameters
The excited chlorophyll molecules dispose of the absorbed light energy by: (1) transfer to a reaction center for photosynthetic primary charge separation, (2) fluorescence with longer wavelength; and (3) heat dissipation. The first process and the following photochemical electron transport reactions store energy into chemical bonds. Process (3) can be further divided into ever-present non-regulated heat dissipation (3-a) and regulated additional heat dissipation (3-b) as protection against excess light energy.
Chlorophyll a fluorescence signal was measured by modular version of Dual-PAM-100 (Walz) and by AquaPen (Photon Systems Instruments) fluorometers. Dual-PAM-100 was used for measurements of the quenching analysis by means of the saturation pulse method (reviewed in ref. 18) and the AquaPen was used for measurements of the O-J-I-P fluorescence rise curves (reviewed in ref. 19). To characterize the process: (1) we measured maximal quantum yield of photochemistry of photosystem II (PSII) for dark-adapted state ΦD(PSII)20 and the actual quantum yield of photochemistry of PSII for light-adapted state ΦL(PSII).21 The sum of quantum yield of non-regulated (constitutive) non-photochemical energy quenching by thermal dissipation and of energy quenching by fluorescence emission for light-adapted state ΦL(F,D)22 measures processes (2) and (3-a). The quantum yield of regulated (also called protective) non-photochemical energy quenching by thermal dissipation for light-adapted state ΦL(NPQ) isolated process (3-b).22 The ABS/RC and PItotal parameters of the so-called JIP test reflect apparent antenna size (amount of functional chlorophyll) of PSII and potential for energy conservation of photons absorbed by PSII to reduction of photosystem I end electron acceptors, respectively.23 AquaPen was used for measurement of ΦD(PSII), ABS/RC and PItotal and Dual-PAM-100 for ΦL(PSII), ΦL(F,D) and ΦL(NPQ).
Melatonin was first diluted in less than 1% methanol and then in artificial pond water (APW in mM: 1.0 NaCl, 0.1 KCl, 0.1 CaCl2) to obtain the final melatonin concentration of 10 μM. Chara cells were treated with melatonin solutions for 20 h in darkness in a glass Petri dish. The cells were exposed to daylight for 1 h before start of the measurements. Both control and melatonin-treated cells were dark-adapted for 5 min. The measurement by AquaPen was performed, followed by the dark-adaptation again and by measurement by Dual-PAM-100. Each measurement was performed on 5–7 internodal Chara australis cells to increase the strength of the signal. Thus each point represents an average value. To evaluate the quenching analysis parameters their time course was followed, with the final steady-state value being the most important. In our measurements we assumed that the parameters reached their steady-state values after 3 min illumination. The results were obtained for intensities of excitation actinic and saturation light, respectively, at 70 μmol photons (of red light, λ = 635 nm) m-2 s-1 (Dual-PAM-100 measurements) and 1,500 μmol photons (of blue light, λ = 450 nm) m-2 s-1 (AquaPen measurements).
Measurement of endogenous melatonin
Chara cells were collected from a fresh water pond at Little Bay golf course in April 2011. The plants were more than meter high and the internodal cells about 1 mm in diameter. Sample cells were taken from top, middle and bottom part of the plants and placed into separate labeled containers. Some plants were pulled out carefully from the mud to isolate rhizoids (roots). Samples were brought to the laboratory. The cytoplasm enriched fragments were made by spinning long internodal cells at ~1 g for 30 min.24 The cytoplasm was moved to one end of the cell and the cells were quickly wilted and the cytoplasm-rich end was tied off with thread. The plants had a lot of male fruiting bodies attached to top branches. Samples included one plant top with fruiting bodies, one without and some fruiting bodies separately. The cells and fragments were taken from their medium and plunged into liquid nitrogen, then transferred into eppendorf tubes and stored in -80°C freezer until they could be sent in dry ice to Murch laboratory. The sample harvesting and preparation was done over 8 h of daylight without recording the time of day.
The melatonin concentration was measured using previously published methods.25,26 In brief, melatonin and serotonin were extracted from frozen samples in complete darkness using only a single red light for safety. Individual samples were accurately weighed in 1.5 ml microfuge tubes, homogenized for 3 min in 100 ul methanol:water:formic acid (80:20:1 v/v) using a cordless motor Pellet Pestle grinder (Kontes) and disposable pestles (Kontes) and centrifuged for 3 min at 16,000 g (VWR, Galaxy 16DH Microcentrifuge) to settle particulate matter. The resulting supernatant was filtered (0.2 µm, Ultrafree-MC filtered centrifuge tubes; Millipore). Compounds were separated using a reverse phase C18 column (Waters BEH C18 column (2.1, 150 mm, 1.7 µm) and elution with 1% aqueous formic acid:acetonitrile (0.0–4.0 min, 95:5–5:95 v/v, 4.0–4.5 min, 95:5–95:5 v/v, 4.5–5.0 min, 95:5 v/v) at 0.25 ml/min using an Acquity ultraperformance liquid chromatography (UPLC) at 30°C over a 5 min gradient with a 2 min cleaning and re-equilibration period. Eluted indoleamines were detected by time-of-flight mass spectrometry (ToF-MS; LCT Premier, Micromass, Waters Ltd.) using previously published optimized parameters26 and quantified by comparison to authenticated standards. The limits of detection (LOD) were 51 pg/µl and 430 pg/µl for melatonin and serotonin, respectively. The lower limits of quantification (LOQ) for melatonin was 543 pg/µl and serotonin had an LOQ of 978 pg/µl. Recovery of spiked melatonin in the sample matrix through the preparation and separation was 98% and recovery of serotonin was 86% as determined at 80% of the highest point in the linear quantification range.
Acknowledgment
D.L. was supported by grant No. ED0007/01/01—Centre of the Region Haná for Biotechnological and Agricultural Research and by grant No. EE2.3.20.0057—Progress and internationalization of biophysical research at Faculty of Science, Palacky University in Olomouc.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23279
References
- 1.Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35:627–34. [PubMed] [Google Scholar]
- 2.Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res. 1995;18:28–31. doi: 10.1111/j.1600-079X.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 3.Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. Lancet. 1997;350:1598–9. doi: 10.1016/S0140-6736(05)64014-7. [DOI] [PubMed] [Google Scholar]
- 4.Murch SJ. KrishnaRaj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19:698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 5.Pöggeler B, Balzer I, Hardeland R, Lerchl A. Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulax polyedra. Naturwissenschaften. 1991;78:268–9. doi: 10.1007/BF01134354. [DOI] [Google Scholar]
- 6.Wolf K, Kolář J, Witters E, van Dongen W, van Onckelen H, Macháčková I. Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J Plant Physiol. 2001;158:1491–3. doi: 10.1078/0176-1617-00561. [DOI] [Google Scholar]
- 7.Murch SJ. Neurotransmitters, neuroregulators and neurotoxins in plants. In: Baluska F, Mancuso S, Volkmann D., eds. Communication in Plants: Neuronal Aspects of Plant Life. Berlin, D:Springer-Verlag, 2006:137-48. [Google Scholar]
- 8.Afreen F, Zobayed SMA, Kozai T. Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV-B radiation. J Pineal Res. 2006;41:108–15. doi: 10.1111/j.1600-079X.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 9.Tan D-X, Manchester LC, Di Mascio P, Martinez GR, Prado FM, Reiter RJ. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J. 2007;21:1724–9. doi: 10.1096/fj.06-7745com. [DOI] [PubMed] [Google Scholar]
- 10.Boccalandro HE, González CV, Wunderlin DA, Silva MF. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: evidence of its antioxidant role in fruits. J Pineal Res. 2011;51:226–32. doi: 10.1111/j.1600-079X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 11.Byeon Y, Park S, Kim Y-S, Park D-H, Lee S, Back K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J Pineal Res. 2012;53:107–11. doi: 10.1111/j.1600-079X.2012.00976.x. [DOI] [PubMed] [Google Scholar]
- 12.Kolár J, Machácková I. Melatonin in higher plants: occurrence and possible functions. J Pineal Res. 2005;39:333–41. doi: 10.1111/j.1600-079X.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 13.Cao J, Murch SJ, O’Brien R, Saxena PK. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2006;1134:333–7. doi: 10.1016/j.chroma.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 14.Cao J, Cole IB, Murch SJ. Neurotransmitters, neuroregulators and neurotoxins in the life of plants. Can J Plant Sci. 2006;86:1183–8. doi: 10.4141/P06-034. [DOI] [Google Scholar]
- 15.Park WJ. Melatonin as an endogenous plant regulatory signal: Debates and perspectives. J Plant Biol. 2011;54:143–9. doi: 10.1007/s12374-011-9159-6. [DOI] [Google Scholar]
- 16.McCourt RM, Delwiche CF, Karol KG. Charophyte algae and land plant origins. Trends Ecol Evol. 2004;19:661–6. doi: 10.1016/j.tree.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Hope AB, Walker NA. The physiology of giant algal cells. Cambridge, UK:Cambridge University Press, 1975. [Google Scholar]
- 18.Schreiber U. Pulse-Amplitude-Modulation (PAM) fluorometry and saturation pulse method: an overview. In: Pepageorgiou C, Govindjee, eds. Chlorophyll a fluorescence: a signature of photosynthesis. Dordrecht, NL:Springer, 2004:279-319. [Google Scholar]
- 19.Lazár D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biol. 2006;33:9–30. doi: 10.1071/FP05095. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima M, Butler WL. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975;376:105–15. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- 21.Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta, Gen Subj. 1989;990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 22.Hendrickson L, Furbank RT, Chow WS. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res. 2004;82:73–81. doi: 10.1023/B:PRES.0000040446.87305.f4. [DOI] [PubMed] [Google Scholar]
- 23.Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta. 2010;1797:1313–26. doi: 10.1016/j.bbabio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Beilby MJ, Shepherd VA. Cytoplasm-enriched fragments of Chara: structure and electrophysiology. Protoplasma. 1989;148:150–63. doi: 10.1007/BF02079334. [DOI] [Google Scholar]
- 25.Murch SJ, Alan AR, Cao J, Saxena PK. Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res. 2009;47:277–83. doi: 10.1111/j.1600-079X.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 26.Murch SJ, Hall BA, Le CH, Saxena PK. Changes in the levels of indoleamine phytochemicals during véraison and ripening of wine grapes. J Pineal Res. 2010;49:95–100. doi: 10.1111/j.1600-079X.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- 27.Paredes SD, Korkmaz A, Manchester LC, Tan D-X, Reiter RJ. Phytomelatonin: a review. J Exp Bot. 2009;60:57–69. doi: 10.1093/jxb/ern284. [DOI] [PubMed] [Google Scholar]
- 28.Murch SJ, Rupasinghe HPV, Goodenowe D, Saxena PK. A metabolomic analysis of medicinal diversity in Huang-qin (Scutellaria baicalensis Georgi) genotypes: discovery of novel compounds. Plant Cell Rep. 2004;23:419–25. doi: 10.1007/s00299-004-0862-3. [DOI] [PubMed] [Google Scholar]
- 29.Brown PN, Turi CE, Shipley PR, Murch SJ. Phytochemical discovery in large cranberry (Vaccinium macrocarpon Ait.) and small cranberry (Vaccinium oxycoccus L. and Vaccinium vitis-idaea L.) in British Columbia. Planta Med. 2012;78:1–11. doi: 10.1055/s-0031-1298239. [DOI] [PubMed] [Google Scholar]
- 30.Hernández-Ruiz J, Arnao MB. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J Agric Food Chem. 2008;56:10567–73. doi: 10.1021/jf8022063. [DOI] [PubMed] [Google Scholar]
- 31.Tan D-X, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res. 2012 doi: 10.1111/jpi.12026. In press. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Yin L, Liang D, Li C, Ma F, Yue Z. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res. 2012;53:11–20. doi: 10.1111/j.1600-079X.2011.00966.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang N, Zhao B, Zhang H-J, Weeda S, Yang C, Yang Z-C, et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.) J Pineal Res. 2012;54:15–23. doi: 10.1111/j.1600-079X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 34.Tan D-X, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 2012;63:577–97. doi: 10.1093/jxb/err256. [DOI] [PubMed] [Google Scholar]
- 35.Arnao MB, Hernández-Ruiz J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res. 2009;46:58–63. doi: 10.1111/j.1600-079X.2008.00625.x. [DOI] [PubMed] [Google Scholar]


