Abstract
The role of sucrose as a signaling molecule in plants was originally proposed several decades ago. However, recognition of sucrose as a true signal has been largely debated and only recently this role has been fully accepted. The best-studied cases of sucrose signaling involve metabolic processes, such as the induction of fructan or anthocyanin synthesis, but a large volume of scattered information suggests that sucrose signals may control a vast array of developmental processes along the whole life cycle of the plant. Also, wide gaps exist in our current understanding of the intracellular steps that mediate sucrose action. Sucrose concentration in plant tissues tends to be directly related to light intensity, and inversely related to temperature, and accordingly, exogenous sucrose supply often mimics the effect of high light and cold. However, many exceptions to this rule seem to occur due to interactions with other signaling pathways. In conclusion, the sucrose role as a signal molecule in plants is starting to be unveiled and much research is still needed to have a complete map of its significance in plant function.
Keywords: sucrose signaling, low temperature, light
The role of sucrose (Suc) as a signaling molecule in plants was put forward several decades ago by Pontis,1 and more recently by Koch2 and Wind et al.3 Yet, a long debate has taken place regarding whether Suc truly deserves such status. Unlike glucose (Glc), which has been recognized as a signaling molecule in plants for long, especially in relation to the widespread hexokinase (HK) signaling pathway, Suc role as such has been rather neglected. It has been argued that the reason for not giving Suc a signaling role is that the molecule is rapidly metabolized and thus it is uncertain whether plant responses are attributable to this molecule by itself or to the product of its degradation (i.e., Glc).4 However, Suc is relatively a stable molecule (it is transported between different plant organs and even stored for long periods) when compared with monosaccharides, which are promptly metabolized and are seldom transported between cells or accumulated. A more likely reason for neglecting Suc a signaling role is the fact that in several physiological events regulated by Suc, the molecule is also the substrate for polysaccharide synthesis, so that it has proven to be extremely difficult to distinguish the signaling role from its contribution as mere building blocks for reserve and structural polysaccharide synthesis. The situation has nevertheless changed lately, since evidence accumulated indicating that many mRNAs and enzymes are synthesized de novo when the level of this disaccharide exceeds a certain threshold.5 The dual role of Suc has been most clearly evidenced in experiments with the direct addition of Suc to plants in which the responses could not be mimicked by the addition of hexoses. While both Suc and Glc are included in what is generally termed ‘sugar sensing’, both sugars play very different roles in plant function. Glc is associated with early organ growth, playing an important role in osmotic contribution to expansion of recently divided cells. Glc signaling is therefore prevalent during those stages.2 Glc is also produced from the degradation of carbon reserves (such as starch), and it plays a signaling role in the induction of senescence processes,6 which commonly involve remobilization of reserves. Suc, on the other hand, is more associated with the maturity and full functionality of plant organs,2 and its signaling roles are generally to be found among those processes as well.
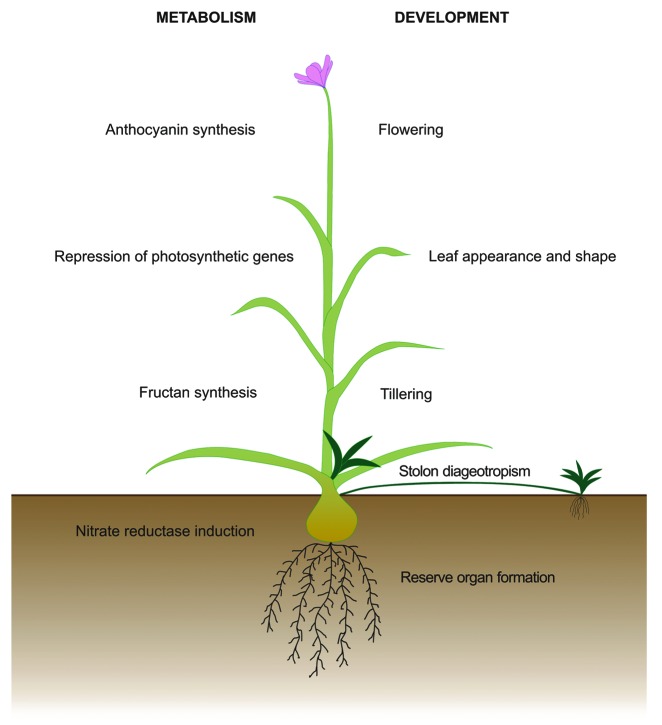
While most well studied Suc driven processes affect general metabolism of plant and take place in different tissues and organs simultaneously, some others, which have been mostly neglected, appear to occur in meristems, giving raise to changes in developmental patterns. Developmental processes in which Suc has been widely recognized as a signaling molecule are still few, including phloem development7 and embryonic cell division in carrot and spruce.3 However, a compelling amount of scattered information clearly indicates that a wide array of plant developmental processes are controlled by Suc, and in this work we attempt to give a short but comprehensive review of these processes. Examples of both metabolic and developmental responses are illustrated in Figure 1 and will be discussed separately.
Figure 1. Examples of processes regulated by the endogenous Suc concentration, as schematized in an hypothetic plant. Metabolic and developmental processes are shown at the left and right of the scheme, respectively.
Sucrose Signaling in Plant Cell Metabolism
The effects of Suc on various aspects of plant metabolism have received more attention and are in general better known than those on plant development. Suc signaling has been involved in carbon and nitrogen assimilation and transport. Regarding carbon metabolism, one case that has been long studied is the induction of fructan (polymers of Fru) synthesis in grasses. Although in Nature fructan metabolism is mainly induced during periods of low temperature, the effect of cold is not direct but through its role in increasing cell Suc concentration due to lower carbon utilization.8 It has been shown that, at warm temperature, light induces fructan accumulation in detached leaves of different grass species, and that Suc mimics the light effect.9 Although Glc supply (and other sugars as well) can also induce fructan synthesis, the efficiency of these sugars is much lower than that of Suc. This fact, together with results from the application of various sugar analogs, led to the conclusion that in Nature, Suc is most likely the molecule which initiates the signaling cascade leading to the induction of fructan synthesizing enzymes.9,10
Sucrose also appears to act as a signaling molecule that initiates/activates starch synthesis. Many reports have shown the upregulation by Suc of diverse genes related to the starch biosynthetic pathway, such as those that encode specific subunits of ADP-Glc pyrophosphorylase (AGPase) in different species.11-17 While in those reports it was not clear whether this induction was specific of Suc signaling or not, evidence that Suc is the only sugar capable of inducing the AGPase large subunits (iAGPLI-1 and ApL3) gene expression in sweet potato and Arabidopsis thaliana was provided by Harn et al.11 and Nagata et al.12 It has also been reported that both starch synthase (GBSSI) and β-amylase genes are induced by Suc in sweet potato,13,14 in the first case the induction was Suc-specific since Glc and Fru could not elicit the same response. Moreover, Suc activates, independently of its metabolism, the AGPase enzyme by post-translational redox modification in growing potato tubers.15 Although Glc could also produce the same effect, the signaling pathway elicited by Suc is different from that of the hexose, involving Snf1-related protein kinases (SnRK) and HK respectively.16 This redox regulation occurs also in leaves of pea, potato and A. thaliana.17 Increased levels of Suc have also been found to enhance expression of a Glc-6-phosphate/phosphate translocator18 which is related to carbohydrate uptake and starch synthesis in heterotrophic tissues.19
Regarding photosynthesis, the downregulation of CO2 fixation by Suc is a widely known phenomenon. However, it is not always clear whether Suc plays a signaling function or if it exerts a feedback effect as an end product. In 1990, Sheen20 reported that several photosynthetic genes, including PEP carboxylase, malic enzyme, CAB and Rubisco of maize protoplasts were repressed by Suc. Also, Van Oosten and Besford21 found that Suc decreased Rubisco content in tomato leaves. However, in both works, Glc exerted similar or even stronger effects, which cast doubt whether Suc is the true signaling molecule in vivo regarding these effects. The action of Suc on photosynthetic genes seems to be very complex since environmental conditions which lead to Suc accumulation may be associated with either downregulation (i. e. CO2 enrichment) or upregulation (high light and cold) of Rubisco content.22-24 Besides, Nielsen et al.,25 working with tobacco and Chenopodium cell cultures demonstrated that Rubisco repression by Suc occurs solely under nitrogen and phosphate limiting conditions. Suc repression of CAB and LHCB1 transcript accumulation in Brassica napus and A. thaliana, respectively were reported by Harter et al.26 and Cottage et al.27 However, a rather intriguing increase in CAB transcripts with Suc supply has recently been reported for A. thaliana plants grown in vitro.28
Sucrose may control its own synthesis, at least indirectly. An A. thaliana ugp gene that encodes the UDP-Glc pyrophosphorylase (UGPase) which is substrate for the action of Suc-phosphate synthase (SPS), was found to be upregulated by Suc in excised leaves.29 The Suc effect on gene expression and activity of UGPase was apparently specific and was mimicked by cold and by exposure of dark-adapted leaves to light.29 Besides, ugp regulation was shown to be independent of Glc signaling by HK.29
Not only carbohydrate synthesis but also its partitioning is regulated by Suc. It was reported that the mRNA levels and activity of Suc symporters from sugar beet source leaves drastically decreased by Suc treatment.30 Hexoses did not elicit the same response, while mannoheptulose, a HK inhibitor, did not block the Suc effect.30 The authors proposed that this was a Suc-specific response pathway, and thus that Suc can control assimilate partitioning at the level of phloem translocation.30,31 On the other hand, the nonfunctional Suc symporter from potato source leaves StSUT2, which is specifically enhanced by Suc, has been suggested to act as a Suc sensor.32
Sucrose appears to control chlorophyll and non-photosynthetic pigment synthesis. It was reported long ago that supplying Suc to the in vitro media prevented chlorophyll accumulation in carrot callus culture.33 Suc was later proposed to affect synthesis of the chlorophyll precursor 5-aminolevulinic acid.34 It must be taken into account that decreased chlorophyll synthesis not necessarily leads to less green plants, since Kumar et al.35 reported an increased chloroplast number in explants of carrot roots treated with Suc. On the other hand, a well-known case of Suc regulation is the induction of the anthocyanin biosynthesis. While this effect was described many decades ago,36 the strong specificity of the Suc signal was demonstrated by Solfanelli et al.37 in experiments with A. thaliana seedlings supplied with different sugar analogs and hexoses. These authors showed that most of the genes coding for enzymes involved in anthocyanin and flavonoid biosynthesis are induced by this sugar. They also suggested that the effect of Suc is performed through the specific induction of the transcription factor PAP1. Besides, there is evidence suggesting that Suc influences carotenoid levels, as shown by Legha et al.38 on callus cultures of Calendula officinalis. In general, it appears that Suc plays a role inducing responses associated with free radical scavenging. Among these responses, which in general appear to be Suc-specific since Glc does not elicit similar effects, are included increased ascorbate levels, mitigation of anoxia and photo-oxidative stress related to the herbicide atrazine.39-43
The role of Suc on nitrogen metabolism signaling is also very important, since this sugar appears to control not only nitrogen assimilation and transport but also carbon:nitrogen balance. It has long been reported that Suc elicits an increase of nitrate reductase (NR) mRNA accumulation in dark-adapted green A. thaliana plants44 and other dycotiledons.45,46 Suc increases not only NR gene expression, but also activity46 and post-translational activation of the enzyme.45,46 The effect of Suc on NR expression is so determinant that it may override the well-known upregulation of nitrate on NR expression.47 Furthermore, Suc stimulates the amino acid biosynthetic pathways.46,48 Suc stimulates the flow of carbon from glycolysis into organic acids, since a decrease of 3-phosphoglycerate and PEP and a large increase of α-oxoglutarate were found in tobacco plants fed with Suc.46 Accordingly, the in vivo net rate of ammonium assimilation doubled after feeding detached tobacco leaves with Suc.46 The authors suggested that it is Suc rather than Glc the signal that regulates nitrogen and respiratory metabolism after feeding tobacco with different sugars. Suc promotion of asparagine synthetase1 and proline dehydrogenase gene expression was shown to occur through regulation of the transcription factor bZIP11.48 It was suggested that bZIP11 is a direct regulatory link between Suc-mediated signaling and amino acid metabolism.48 In contrast, an inverse correlation appears to exist between amino acid biosynthesis and Suc content in potato tubers,49 which suggests differences between Suc signaling pathways between source and sink tissues. While free amino acid content is reduced by Suc in potato tubers,49 the reserve proteins sporamin and patatin are induced by the sugar.14,50 Nitrogen transport also appears to be regulated by Suc. A case in which the ammonium transporter gene, CitAMT1, is specifically induced by Suc has been reported for citrus plants.51 Besides, it has been shown that the A. thaliana nitrate and ammonium transporter genes (NRT and AMT, respectively) are induced after the addition of Suc; however, it is uncertain whether Suc is acting as a signal molecule in this response since hexoses are effective as well.52
A particularly important protein in carbon and nitrogen metabolisms is PII, which coordinates the regulation of nitrogen assimilation in response to nitrogen, carbon and energy availability.53 The expression of the gene (GLB1) that encodes PII protein is induced by light and Suc in dark-adapted A. thaliana plants.54 This effect is not trigger by mannitol or non-metabolizable carbon source.
Regarding other mineral nutrients, the expression of genes encoding for ion transporters for phosphate, sulfate and potasium may be upregulated by Suc.55,56 Suc appear to modify the expression of a number of genes related to P starvation which lead to an altered root physiology.57 Suc also plays an important role in control of copper homeostasis through sugar-responsive miRNAs in A. thaliana.58
Sucrose Signaling in Plant Development
The fact that the developmental pattern of a plant may be affected by Suc concentration in tissues was demonstrated several decades ago in the pioneering works by Lawrence and Barker59 and Montaldi.60 Although not always recognized, enough evidence has been gathered to support the role of Suc as a signal molecule acting on a wide array of plant developmental processes that take place throughout the whole life cycle of the plant. Suc appears to affect both plant growth and differentiation, giving raise to profound changes in plant shape.
A notorious effect of Suc on plant growth is the increase in plant size after exogenous supply of this sugar, which has been found for a large variety of species.61 Growth promotion by Suc must be at least in part the consequence of increased cell number, and this sugar has been reported to promote cell division in apical meristems as demonstrated long ago in studies with Pisum root meristems.62 In cell cultures of A. thaliana, removal of Suc from the growth medium leads to the cessation of cell cycle, while Suc readdition has been used to generate partially synchronous cultures.63 Further studies with A. thaliana have showed that Suc enhances the expression of cyclins B and D,64 and promotes ribosome synthesis.65
Besides influencing plant size, Suc may also modify whole plant morphology by controlling the activation of different types of meristems, in both aerial and subterranean parts. The involvement of Suc as a signal molecule acting in the coordination of cell division within the shoot apical meristem (SAM) has been suggested by Francis and Halford.66 The SAM increases in size through cell division, and then forms a bulge on its side which becomes the next leaf primordium,66 and several evidences suggest that Suc may induce shortening the time interval between the appearance of two successive leaves, this is, the phyllochron. Evidences include shortening of the phyllochron in plants subjected to conditions that favor Suc synthesis or accumulation such as high light and low temperature67 (in the latter case, in thermal time units). In accordance with this, wheat plants that accumulate photoassimilates after treatment with an inhibitor of gibberellin synthesis exhibit shorter phyllochrons.68 Leaf shape also appears to be controlled by Suc. In monocots, leaf extension was found to be reduced by Suc while the opposite was observed under sugar restriction60,69 and the latter effect was similar to what was found by shading.70 In dicots, Hanson et al.71 reported an inhibition of lateral expansion of A. thaliana leaf epidermal cells in sugar-treated seedlings, which is mediated by the transcription factor ATHB13.
Regarding stems, the possibility that Suc promotes branching through the release of dormant axillary buds in grasses (i.e., tillering) has been proposed for long time and is known as the nutrition hypothesis of apical dominance, which states that the development of those buds is directly related to assimilate availability.72 Although experiments with direct injection of Suc have not given convincing results73 evidence of a correlation between tiller bud outgrowth and photoassimilate availability has been provided.74 Again, Suc response seems to mimic the effects of high light and low temperature on tillering. Other known example of the effects of Suc on stem meristems is the control of the gravitropic response.60 Willemoës et al.75 found that diagravitropic (this is, horizontal) growth of stolons in Cynodon and other grass species was stimulated by Suc, while Glc and fructose (Fru) did not give similar results. This effect of Suc on plastic growth favors plant propagation, since it allows plants to explore adjacent territories, and is analogous to what is observed under high light intensity.70 Furthermore, Digby and Firn76 reported a photosynthetic effect on the Tradescantia gravitropism, which the authors related to either a direct effect of Suc or an indirect one, through the Suc regulation of PhyA gene expression. In any case, these effects of Suc are coherent with an environmental situation in which photoassimilates are abundant and plant invests them in colonization of new spaces.
Sucrose has been long related to the promotion of root growth.77-79 However, the addition of Suc to whole plants in several species has caused no increase in the root to shoot ratio.61 Thus, Suc might not cause a differential root vs shoot growth promotion. Lateral root formation has also been shown to be promoted by Suc in A. thaliana80 but this might not be a true Suc signaling case, but rather an effect linked to Suc metabolism. A further putative role of Suc in root meristems concerns cambium activity. The involvement of Suc in phloem differentiation in in vitro grown plants was showed many decades ago81 and accordingly, it has been found that conditions that favor Suc accumulation also induce the development of phloem parenchyma (which is the most important sink for assimilates) in carrot roots.82 In agreement with the role of promoting reserve structures formation, Suc has been found to induce the development of storage organs in different species. The most well-known case is that of potato tuber induction. In potato, Lawrence and Barker59 showed that the level of sugars in the medium, notably Suc, affected tuberization in vitro. It has been found that Suc regulates tuber formation by influencing the levels of gibberellic acid (GA), which is a potent inhibitor of tuber formation.83 This agrees with the findings of Park,84 who reported that Suc induced the expression of tuber-specific genes and that the sensitivity toward Suc was modulated by GA. Contrary to the effect of Suc on tuberization, Mares et al.85 detected an increased level of reducing sugars with the application of GA. More recently, it has been suggested86 that GA inhibits tuberization downstream of the inductive effects of Suc and other positive factors in spontaneous tuberizing potato mutants. Besides tuberization, the formation of other underground storage organs may also be promoted by Suc. This is the case of bulb formation in onion and leek87 and rhyzome formation in Bambusa bambos.88
Many other developmental processes, including flowering, regulation of the circadian clock and senescence also appear to depend on Suc signaling. After the early finding by Friend et al.89 that flowering of Brassica campestris grown in vitro occurred earlier when Suc was added to the medium, the participation of Suc in flower evocation was shown in many other dicotyledons, such as Sinapis alba,90 A. thaliana91,92 and Vitis vinifera.93 These effects of Suc are in agreement with the well-known promoting effect of irradiance on flowering. Nevertheless, the participation of Suc in the differentiation at the apical meristem is very complex, and the steps that are regulated by this sugar are just recently being unveiled. For example, Roldán et al.94 have reported that Suc addition to the medium promotes flower development in the dark in late-flowering A. thaliana ecotypes, but Suc is not always effective promoting flowering.95 The use of mutants for different genes involved in flower development has led to the suggestion that Suc-mediated signals are incorporated into the photoperiod flowering pathway, probably downstream of CONSTANS but upstream of FLOWERING LOCUS T genes.96 It is also likely that similar roles of Suc in flower differentiation may take place in monocots such as maize.97 Recent reports show that Suc regulates the circadian clock in A. thaliana,98 particularly in the dark. The authors demonstrated that the circadian oscillator GIGANTEA is required for the Suc response, being part of the Suc signaling pathway. Regarding senescence, a clear separation between the effects of hexoses and Suc can be observed, since leaf senescence is induced by Glc and Fru but not by Suc.99 In the rose cultivar Super Star, Suc retarded while abscisic acid (ABA) promoted senescence in cut flowers.100 The authors proposed that ABA accelerates senescence of cut roses by promoting petal growth and respiration, thus decreasing the carbohydrate level in the petals and triggering the chain of metabolic processes leading to aging. A delayed leaf senescence in transgenic poplar with elevated SPS activity, and therefore enhanced Suc content toward the end of the vegetative cycle, was shown by Park et al.101
Finally, several aspects of seed development are also controlled by Suc. In developing seeds of Vicia faba high Suc levels have been associated with end of embryo cell division and increasing cell differentiation, expansion and reserve accumulation.102 Also, radicle growth of carrot seeds was found to be inhibited by this sugar in a similar fashion than it occurs under natural dormancy.103 These authors also demonstrated that hexoses did not mimic the effect of Suc; and furthermore, HK signaling was ruled out.
Intracellular Sucrose Signaling
The nature of the Suc receptor that may initiate the signaling pathway is largely unknown. It has been suggested that symporter SUT2 may act as Suc sensor in tomato and in A. thaliana.32 This possibility was mainly based on the close structural similarity with the yeast Glc sensors SNF3 and RGT2.32 However, arguments against this putative role have also been raised.104 Besides, it was recently reported that the vacuolar low-affinity Suc transporter of A. thaliana (SUT4) is involved in signaling pathway of the Suc-induced inhibition of seed germination.105 The authors proposed that SUT4 interacts with 5 members of cytochrome b5 family (Cyb5-2) to directly sense Suc or acts as a downstream component of a Suc sensing system.105 Despite scarcity of information regarding the primary Suc sensor molecule, the components that are involved in the transduction pathway were more extensively studied. It appears that calcium as a second messenger, protein kinases (PKs)13,106-108 and protein phosphatases (PPs)13,109-111 are generally involved in intracellular Suc signaling processes.
One of the clearest processes regulated by Suc is the induction of fructan synthesis in wheat and other grasses. It has been described several years ago that in leaves and roots of a variety of plants fructans accumulate after Suc levels increase beyond a concentration threshold.5 Thereafter, it was suggested that Suc plays a double role in fructan metabolism, it is the essential substrate used in fructan synthesis and it also starts the signal transduction pathway that induces the fructosyl-sucrose synthesizing activities (FSS = 1-SST + 6-SFT; 1-SST: 1-Suc:Suc fructosyltransferase, 6-SFT: 6-Suc:fructan fructosyltransferase).112 The induction of fructan synthesis has been used as a model system to study Suc signaling by incubating detached wheat leaves in the darkness and supplemented with Suc. The advantage of this system is that fructan synthesis is strongly induced 6 h after the addition of Suc and virtually no fructans are produced in control leaves.10 Using different inhibitors and channel blockers it was demonstrated that calcium, CDPKs and PP2A activities are involved in the Suc signaling cascade which leads to the activation of fructan biosynthesis.113-116 In barley, the participation of small GTPases in Suc signaling has also been suggested.117
It has been proposed that Suc enters rapidly from the apoplast through a symporter (SUT)118 and after reaching a concentration threshold it induces the signaling pathway that regulates expression of genes, including those related to fructan synthesis. The presence of high levels of Suc triggers an increase in the concentration of cytoplasmatic calcium that in turn may activate CDPKs downstream in the signaling cascade.113,114 On the other hand, PP activity may be necessary for the expression of SUT gene and also probably to maintain the symporter in an active (unphosphorylated) form.115,119 In a later phase, Suc may inhibit PP2A enzyme activity, which could in turn lead to the inhibition of SUT gene expression and/or the inhibition of SUT activity. According to the proposed model, this negative feedback would lead to a decreasing rate of Suc uptake and ultimately stop the Suc-mediated FSS induction.115
Sucrose also appears to modulate central regulators of metabolism and development, mainly SnRK1 but possibly also the ‘target of rapamycin’.120 SnRK1 is a central integrator of stress and energy signaling in plants, causing extensive reprogramming of gene transcription and controlling plant growth.121 It has been described that Suc activates SnRK1, and that this kinase is required for the Suc signal transduction leading to starch synthesis and sucrose synthase induction in potato.16,122 However, Baena Gonzalez et al.123 showed that KIN10/KIN11 (members of SnRK1 subfamily that are the closest relatives of SNF1 and AMPK of yeast and mammals, respectively) activities are repressed by Suc in maize protoplasts. This signal transduction seems to be HK independent, although the response was also obtained with Glc.123 The reason for this apparent discrepancy is unknown, but it has been suggested that Suc effect on SnRK1 may be different in autotrophic and heterotrophic tissues and also depends on the physiological status of the cells.124 It is unclear whether SnRK regulation by Suc occurs in all Suc signaling events or not, and it is even uncertain whether SnRK1 regulation depends on the overall energy status of cells rather on Suc by itself.121,125,126 Additionally, intracellular signaling by Suc has proven to be very complex and additional components seem to be necessary in certain processes. For example, Suc increases trehalose-6P levels, and this compound in turn is regarded as a signal molecule which controls carbon metabolism and growth.127
Many genes, which have been proven to be Suc-regulated, have conserved cis elements in their promoters. Different Suc-responsive elements have been described, including SURE-box, A and B-boxes, TGGACGG element and SP8 motif.128,129 Cognate binding factors of SURE-box and SP8, which participate in Suc-signaling, have been identified as SUSIBA2 and SPF1 respectively. Both DNA-binding proteins belong to WRKY transcription factors family but they have opposite effects: while SUSIBA2 is induced by Suc,130 SPF1 is a Suc-repressed negative regulator.131 Suc affects gene expression through the regulation of other transcription factors, such as bZIP11, MYB75/PAP1 and WRKY. The A. thaliana ATB2 bZIP genes encode transcription factors that are important regulators of metabolism, and it has been reported that Suc specifically represses the translation of S-group of bZIP family.132 This repressive effect is not mediated by Glc or Fru, used separately or in combination, nor by the Suc-to-hexose ratio.133 Suc effect occurs through an upstream open reading frame (uORF) present in the 5′ leader of the bZIP transcripts.134,135 Even though bZIP translation is repressed by Suc, transcription can be induced by Suc.136 On the other hand, Suc induces the MYB75/PAP1 transcription factor gene expression that leads to anthocyanin accumulation.137 Suc also strongly induces the expression of AtWRKY20, a transcription factor that induces ApL3 transcription in A. thaliana. Other sugars and osmotic controls are either less effective or ineffective.12
As it can be envisaged from this revision, most of our knowledge about the intracellular signaling cascade initiated by Suc is mainly related to metabolic processes. Despite the importance of developmental changes for the plant life cycle, intracellular Suc signaling in meristematic cells is largely unknown. The difficulty of such a study is apparent from the fact that differentiation processes in only one or a small group of cells within the meristem may ultimately decide the fate of the whole plant.
Sucrose Cross-Talks with Environmental Signaling Pathways
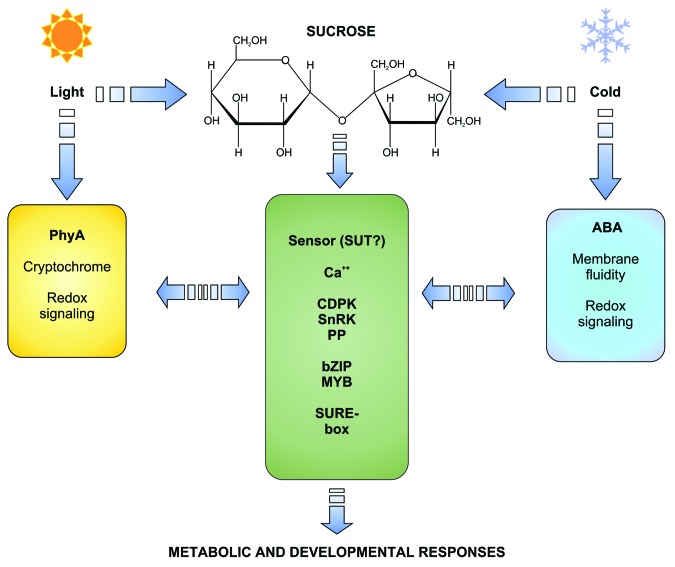
In plant tissues, Suc is constitutively present; therefore it is necessary that its concentration exceeds a certain threshold to exert a signaling role.5 In general, Suc accumulates in vivo when carbohydrate utilization is more restricted than its synthesis, such as when plants are exposed to cool temperatures under relatively high irradiances.8 In agreement with this, it has been found that Suc mimics the effect of both cold and high light intensity in many of the metabolic and developmental examples of Suc signaling cited in the present work, such as induction of fructan biosynthesis,138 anthocyanin synthesis,37 nitrate reductase regulation,44 and tuber induction.139 Moreover, Suc might be involved as a signal molecule in plant responses to elevated CO2 levels, as proposed by Coupe et al.140 These authors found that the nature of the signal which is transported from CO2-fed source leaves to the SAM where stomata development is inhibited was fully compatible with Suc. The responses of plants to increased CO2 levels, which have been the subject of thorough research during the last two decades mainly due to the prospects of climatic change, often resemble those described for high Suc levels. For example, elevated CO2 (700 ppm) resulted in a large (189%) increase in the fructan concentration in perennial ryegrass leaf blades, in parallel with increased Suc concentration in these organs.141 Also, CO2 enrichment often results in downregulation of photosyhthetic genes,142 in a similar manner than that elicited by high Suc. While in these cases Suc appears to be the signal molecule that integrates cold, high irradiance and probably high CO2 levels, in other cases a more complex picture arises from cross-talks with environmental signaling pathways. It should be recalled that cold and high irradiance are sensed independently of Suc and present their own signaling network. In the case of low temperatures, changes in the level of two plant hormones (increase in ABA and decrease in GA) are commonly observed, that in turn elicit proper responses.143 In the case of the light environment, both phytochromes and cryptochromes are the most important sensors.144 Cross-talks between Suc and these signaling pathways seem to be frequent. For example, the phytochrome-interacting factor PIF5, which integrates the response to light and time of day, was found to be upregulated by Suc, and overexpression of PIF5 led to growth dynamics similar to plants exposed to Suc.145 Regarding ABA, this hormone was found to present a synergic role with Suc on anthocyanin synthesis, while Suc-induction of this pathway was repressed by addition of GA.146 Cross-talks between Suc and another endogenous or environmental signals seem to be complex and much work is needed to shed light on this kind of interactions. For example, up to day no studies about possible interactions between cryptochrome signaling and Suc have been reported. Nevertheless, based on information available and revised in the present work, a schematic picture of the Suc signaling pathway may be drawn (Fig. 2).
Figure 2. A schematic diagram of the Suc signaling pathway and its interaction with signaling by light and cold. Components of the intracellular Suc signaling pathway include the still unknown sensor, cytosolic intermediates, transcription factors that are target of Suc and the SURE response element found in many promoters exhibiting Suc regulation. Suc mimics the effect of high light and cold on a number of metabolic and developmental responses. Horizontal arrows indicate well-known (PhyA and ABA) and putative components of light and low temperature signaling pathways with may cross-talk with Suc.
Conclusion and Perspectives
After many years of debate, the role of Suc as a signaling molecule in plants has gained wide consensus. It is generally accepted now that Suc plays an essential role in the regulation of important metabolic processes including carbon and nitrogen assimilation and transport, and responses to oxidative damage, and that its role cannot be replaced by that of other sugars such as Glc. Moreover, there is also ample evidence suggesting that Suc takes part as a signaling molecule in a large array of developmental processes, which we have attempted to review in the present work. Taken together, it appears that Suc role as a signal molecule is of uttermost importance to plant life. However, very important gaps in knowledge remain unsolved. First, the precise nature of the Suc sensor is still unknown. Second, it is uncertain how the Suc concentration threshold required for eliciting responses is monitored. Third, even though several intracellular components of the Suc signaling pathway are already known for several metabolic processes, little is known about control of developmental processes within meristems. Fourth, cross-talks between intracellular pathways elicited by Suc and those related to other environmental or endogenous signals appear to be very complex. In conclusion, Suc signaling in plants comprises a vast territory whose exploration has started not long ago and extensive research is still required to have an accurate map of its participation in the plant signaling network.
Acknowledgments
We wish to thank Dr. Leonardo Curatti and Dr. Walter Vargas for helpful criticism and comments. We also thank Ing Darío Ganem for providing illustrations in Figures 1 and 2. J.A.T., H.G.P. and G.M.A.M.N. are career investigators of CIC, FIBA and CONICET, respectively. We apologize to authors whose work could not be cited due to space limitations. This work was supported by CONICET, ANPCyT and FIBA.
Glossary
Abbreviations:
- CAB
chlorophyll a/b-binding protein
- LHCB1
light harvesting complex B1
- PEP
phosphoenolpyruvate
- PhyA
phytochrome A
- Rubisco
ribulose-1,5-bisphosphate carboxylase
- Snf-1
sucrose nonfermenting-1
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23316
References
- 1.Pontis HG. On the scent of the riddle of sucrose. Trends Biochem Sci. 1978;3:137–9. doi: 10.1016/S0968-0004(78)80034-6. [DOI] [Google Scholar]
- 2.Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol. 2004;7:235–46. doi: 10.1016/j.pbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Wind J, Smeekens S, Hanson J. Sucrose: metabolite and signaling molecule. Phytochemistry. 2010;71:1610–4. doi: 10.1016/j.phytochem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Hummel M, Rahmani F, Smeekens S, Hanson J. Sucrose-mediated translational control. Ann Bot. 2009;104:1–7. doi: 10.1093/aob/mcp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollock C, Farrar J, Tomos D, Gallagher J, Lu C, Koroleva O. Balancing supply and demand: the spatial regulation of carbon metabolism in grass and cereal leaves. J Exp Bot. 2003;54:489–94. doi: 10.1093/jxb/erg037. [DOI] [PubMed] [Google Scholar]
- 6.Wingler A, Masclaux-Daubresse C, Fischer AM. Sugars, senescence, and ageing in plants and heterotrophic organisms. J Exp Bot. 2009;60:1063–6. doi: 10.1093/jxb/erp067. [DOI] [PubMed] [Google Scholar]
- 7.Farrar J, Pollock C, Gallagher J. Sucrose and the integration of metabolism in vascular plants. Plant Sci. 2000;154:1–11. doi: 10.1016/S0168-9452(99)00260-5. [DOI] [PubMed] [Google Scholar]
- 8.Pollock CJ. The response of plants to temperature change. J Agric Sci. 1990;115:1–5. doi: 10.1017/S0021859600073834. [DOI] [Google Scholar]
- 9.Nagaraj VJ, Riedl R, Boller T, Wiemken A, Meyer AD. Light and sugar regulation of the barley sucrose: fructan 6-fructosyltransferase promoter. J Plant Physiol. 2001;158:1601–7. doi: 10.1078/0176-1617-00592. [DOI] [Google Scholar]
- 10.Noël GM, Tognetti JA, Pontis HG. Protein kinase and phosphatase activities are involved in fructan synthesis initiation mediated by sugars. Planta. 2001;213:640–6. doi: 10.1007/s004250100550. [DOI] [PubMed] [Google Scholar]
- 11.Harn CH, Bae JM, Lee SS, Min SR, Liu JR. Presence of multiple cDNAs encoding an isoform of ADP-glucose pyrophosphorylase large subunit from sweet potato and characterization of expression levels. Plant Cell Physiol. 2000;41:1235–42. doi: 10.1093/pcp/pcd049. [DOI] [PubMed] [Google Scholar]
- 12.Nagata T, Hara H, Saitou K, Kobashi A, Kojima K, Yuasa T, et al. Activation of ADP-Glucose Pyrophosphorylase Gene Promoters by a WRKY Transcription Factor, AtWRKY20, in Arabidopsis thaliana L. and Sweet Potato (Ipomoea batatas Lam.) Plant Prod Sci. 2012;15:10–8. doi: 10.1626/pps.15.10. [DOI] [Google Scholar]
- 13.Wang SJ, Yeh KW, Tsai CY. Regulation of starch granule-bound starch synthase I gene expression by circadian clock and sucrose in the source tissue of sweet potato. Plant Sci. 2001;161:635–44. doi: 10.1016/S0168-9452(01)00449-6. [DOI] [Google Scholar]
- 14.Nakamura K, Ohto MA, Yoshida N, Nakamura K. Sucrose-Induced Accumulation of Βeta-Amylase Occurs Concomitant with the Accumulation of Starch and Sporamin in Leaf-Petiole Cuttings of Sweet Potato. Plant Physiol. 1991;96:902–9. doi: 10.1104/pp.96.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, et al. Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell. 2002;14:2191–213. doi: 10.1105/tpc.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, et al. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J. 2003;35:490–500. doi: 10.1046/j.1365-313X.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- 17.Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 2003;133:838–49. doi: 10.1104/pp.103.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res. 2006;119:115–23. doi: 10.1007/s10265-005-0251-1. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd JC, Zakhleniuk OV. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot. 2004;55:1221–30. doi: 10.1093/jxb/erh143. [DOI] [PubMed] [Google Scholar]
- 20.Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–38. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Oosten JJ, Besford RT. Sugar feeding mimics effect of acclimation to high CO2-rapid down regulation of Rubisco small subunit transcripts but not of the large subunit transcripts. J Plant Physiol. 1994;143:306–12. doi: 10.1016/S0176-1617(11)81636-6. [DOI] [Google Scholar]
- 22.Flores-Pérez U, Pérez-Gil J, Closa M, Wright LP, Botella-Pavía P, Phillips MA, et al. Pleiotropic regulatory locus 1 (PRL1) integrates the regulation of sugar responses with isoprenoid metabolism in Arabidopsis. Mol Plant. 2010;3:101–12. doi: 10.1093/mp/ssp100. [DOI] [PubMed] [Google Scholar]
- 23.Yamori W, Noguchi K, Hikosaka K, Terashima I. Cold-tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol. 2009;50:203–15. doi: 10.1093/pcp/pcn189. [DOI] [PubMed] [Google Scholar]
- 24.Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta. 2001;213:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen TH, Krapp A, Röper-Schwarz U, Stitt M. The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant Cell Environ. 1998;21:443–54. doi: 10.1046/j.1365-3040.1998.00295.x. [DOI] [Google Scholar]
- 26.Harter K, Talke-Messerer C, Barz W, Schäfer E. Light- and sucrose- dependent gene expression in photomixotrophic cell suspension cultures and protoplasts of rape (Brassica napus L.) Plant J. 1993;4:507–16. doi: 10.1046/j.1365-313X.1993.04030507.x. [DOI] [Google Scholar]
- 27.Cottage A, Mott EK, Kempster JA, Gray JC. The Arabidopsis plastid-signalling mutant gun1 (genomes uncoupled1) shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. J Exp Bot. 2010;61:3773–86. doi: 10.1093/jxb/erq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckstein A, Zięba P, Gabryś H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro. J Plant Growth Regul. 2012 In press. [Google Scholar]
- 29.Ciereszko I, Johansson H, Kleczkowski LA. Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem J. 2001;354:67–72. doi: 10.1042/0264-6021:3540067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–8. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn MW, Harrington GN, Bush DR. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA. 2002;99:10876–80. doi: 10.1073/pnas.172198599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, et al. SUT2, a putative sucrose sensor in sieve elements. Plant Cell. 2000;12:1153–64. doi: 10.1105/tpc.12.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelman J, Hanson AD. Sucrose suppression of chlorophyll synthesis in carrot callus cultures. Planta. 1971;98:150–6. doi: 10.1007/BF00385347. [DOI] [PubMed] [Google Scholar]
- 34.Pamplin EJ, Chapman JM. Sucrose suppression of chlorophyll synthesis in tissue culture: changes in the activity of the enzymes of the chlorophyll biosynthetic pathway. J Exp Bot. 1975;26:212. doi: 10.1093/jxb/26.2.212. [DOI] [Google Scholar]
- 35.Kumar A, Bender L, Neumann KH. Growth regulation, plastid differentiation and the development of a photosynthetic system in cultured carrot root explants as influenced by exogenous sucrose and various phytohormones. Plant Cell Tiss Org. 1985;3:11–28. doi: 10.1007/BF00035917. [DOI] [Google Scholar]
- 36.Pirie A, Mullins MG. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol. 1976;58:468–72. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140:637–46. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legha MR, Prasad KV, Singh SK, Kaur C, Arora A, Kumar S. Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell Dev-Pl 2012:1-8. [Google Scholar]
- 39.Nishikawa F, Kato M, Hyodo H, Ikoma Y, Sugiura M, Yano M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J Exp Bot. 2005;56:65–72. doi: 10.1093/jxb/eri007. [DOI] [PubMed] [Google Scholar]
- 40.Loreti E, Poggi A, Novi G, Alpi A, Perata P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol. 2005;137:1130–8. doi: 10.1104/pp.104.057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Couée I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot. 2006;57:449–59. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- 42.Sulmon C, Gouesbet G, Amrani AE, Couée I. Sugar-induced tolerance to the herbicide atrazine in Arabidopsis seedlings involves activation of oxidative and xenobiotic stress responses. Plant Cell Rep. 2006;25:489–98. doi: 10.1007/s00299-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 43.Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009;9:28. doi: 10.1186/1471-2229-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng CL, Acedo GN, Cristinsin M, Conkling MA. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA. 1992;89:1861–4. doi: 10.1073/pnas.89.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser WM, Huber SC. Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot. 2001;52:1981–9. doi: 10.1093/jexbot/52.363.1981. [DOI] [PubMed] [Google Scholar]
- 46.Morcuende R, Krapp A, Hurry V, Stitt M. Sucrose-feeding leads to increased rates of nitrate assimilation, increased rates of α-oxoglutarate synthesis, and increased synthesis of a wide spectrum of amino acids in tobacco leaves. Planta. 1998;206:394–409. doi: 10.1007/s004250050415. [DOI] [Google Scholar]
- 47.Klein D, Morcuende R, Stitt M, Krapp A. Regulation of nitrate reductase expression in leaves by nitrate and nitrogen metabolism is completely overridden when sugars fall below a critical level. Plant Cell Environ. 2000;23:863–71. doi: 10.1046/j.1365-3040.2000.00593.x. [DOI] [Google Scholar]
- 48.Hanson J, Hanssen M, Wiese A, Hendriks MM, Smeekens S. The sucrose regulated transcription factor bZIP11 affects amino acid metabolism by regulating the expression of ASPARAGINE SYNTHETASE1 and PROLINE DEHYDROGENASE2. Plant J. 2008;53:935–49. doi: 10.1111/j.1365-313X.2007.03385.x. [DOI] [PubMed] [Google Scholar]
- 49.Roessner-Tunali U, Urbanczyk-Wochniak E, Czechowski T, Kolbe A, Willmitzer L, Fernie AR. De novo amino acid biosynthesis in potato tubers is regulated by sucrose levels. Plant Physiol. 2003;133:683–92. doi: 10.1104/pp.103.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzler HC, Mignery GA, Fisher LM, Park WD. Analysis of a chimeric class-I patatin-GUS gene in transgenic potato plants: high-level expression in tubers and sucrose-inducible expression in cultured leaf and stem explants. Plant Mol Biol. 1989;12:41–50. doi: 10.1007/BF00017446. [DOI] [PubMed] [Google Scholar]
- 51.Camañes G, Cerezo M, Primo-Millo E, Gojon A, García-Agustín P. Ammonium transport and CitAMT1 expression are regulated by light and sucrose in Citrus plants. J Exp Bot. 2007;58:2811–25. doi: 10.1093/jxb/erm135. [DOI] [PubMed] [Google Scholar]
- 52.Lejay L, Wirth J, Pervent M, Cross JM, Tillard P, Gojon A. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol. 2008;146:2036–53. doi: 10.1104/pp.107.114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uhrig RG, Ng KK, Moorhead GB. PII in higher plants: a modern role for an ancient protein. Trends Plant Sci. 2009;14:505–11. doi: 10.1016/j.tplants.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh MH, Lam HM, van de Loo FJ, Coruzzi G. A PII-like protein in Arabidopsis: putative role in nitrogen sensing. Proc Natl Acad Sci USA. 1998;95:13965–70. doi: 10.1073/pnas.95.23.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lejay L, Gansel X, Cerezo M, Tillard P, Müller C, Krapp A, et al. Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell. 2003;15:2218–32. doi: 10.1105/tpc.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Vance CP. Crucial roles of sucrose and microRNA399 in systemic signaling of P deficiency: a tale of two team players? Plant Signal Behav. 2010;5:1556–60. doi: 10.4161/psb.5.12.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammond JP, White PJ. Sugar signaling in root responses to low phosphorus availability. Plant Physiol. 2011;156:1033–40. doi: 10.1104/pp.111.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren L, Tang G, Guo A. Identification of sucrose-responsive microRNAs reveals sucrose-regulated copper accumulations in an SPL7-dependent and independent manner in Arabidopsis thaliana. Plant Sci. 2012;187:59–68. doi: 10.1016/j.plantsci.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence CH, Barker WG. A study of tuberization in the potato, Solanum tuberosum. Am J Potato Res. 1963;40:349–56. doi: 10.1007/BF02862742. [DOI] [Google Scholar]
- 60.Montaldi ER. Gibberellin-sugar interaction regulating the growth habit of bermudagrass (Cynodon dactylon (L) Pers) Cell Mol Life Sci. 1969;25:91–2. doi: 10.1007/BF01903918. [DOI] [PubMed] [Google Scholar]
- 61.Begna SH, Dwyer LM, Cloutier D, Assemat L, DiTommaso A, Zhou X, et al. Decoupling of light intensity effects on the growth and development of C3 and C4 weed species through sucrose supplementation. J Exp Bot. 2002;53:1935–40. doi: 10.1093/jxb/erf043. [DOI] [PubMed] [Google Scholar]
- 62.Van't Hof J. Experimental control of DNA synthesizing and dividing cells in excised root tips of Pisum. Am J Bot. 1966:970–6. [Google Scholar]
- 63.Menges M, Samland AK, Planchais S, Murray JAH. The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis. Plant Cell. 2006;18:893–906. doi: 10.1105/tpc.105.039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riou-Khamlichi C, Menges M, Healy JM, Murray JAH. Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol. 2000;20:4513–21. doi: 10.1128/MCB.20.13.4513-4521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kojima H, Suzuki T, Kato T, Enomoto K, Sato S, Kato T, et al. Sugar-inducible expression of the nucleolin-1 gene of Arabidopsis thaliana and its role in ribosome synthesis, growth and development. Plant J. 2007;49:1053–63. doi: 10.1111/j.1365-313X.2006.03016.x. [DOI] [PubMed] [Google Scholar]
- 66.Francis D, Halford NG. Nutrient sensing in plant meristems. Plant Mol Biol. 2006;60:981–93. doi: 10.1007/s11103-005-5749-3. [DOI] [PubMed] [Google Scholar]
- 67.Wilhelm W, McMaster GS. SYMPOSIUM ON THE PHYLLOCHRON: Importance of the Phyllochron in Studying Development and Growth in Grasses. Crop Sci. 1995;35:1–3. doi: 10.2135/cropsci1995.0011183X003500010001x. [DOI] [Google Scholar]
- 68.Assuero SG, Lorenzo M, Pérez Ramírez NM, Velázquez LM, Tognetti JA. Tillering promotion by paclobutrazol in wheat and its relationship with plant carbohydrate status. New Zeal J Agr Res. 2012;55:347–58. doi: 10.1080/00288233.2012.706223. [DOI] [Google Scholar]
- 69.Beltrano J, Montaldi ER. Further investigations on the diagravitropic effect of sucrose in Cynodon dactylan (L) Pers. Phyton. 1984;44:107–13. [Google Scholar]
- 70.Guglielmini AC, Satorre EH. Shading effects on spatial growth and biomass partitioning of Cynodon dactylon. Weed Res. 2002;42:123–34. doi: 10.1046/j.1365-3180.2002.00268.x. [DOI] [Google Scholar]
- 71.Hanson J, Johannesson H, Engström P. Sugar-dependent alterations in cotyledon and leaf development in transgenic plants expressing the HDZhdip gene ATHB13. Plant Mol Biol. 2001;45:247–62. doi: 10.1023/A:1006464907710. [DOI] [PubMed] [Google Scholar]
- 72.Assuero SG, Tognetti JA. Tillering regulation by endogenous and environmental factors and its agricultural management. American J Plant Sci Biotech. 2010;4:35–48. [Google Scholar]
- 73.Jewiss OR. Tillering in grasses-Its significance and control. Grass Forage Sci. 1972;27:65–82. doi: 10.1111/j.1365-2494.1972.tb00689.x. [DOI] [Google Scholar]
- 74.Delwiche SR, Graybosch RA. Examination of spectral pretreatments for partial least-squares calibrations for chemical and physical properties of wheat. Appl Spectrosc. 2003;57:1517–27. doi: 10.1366/000370203322640161. [DOI] [PubMed] [Google Scholar]
- 75.Willemoës JG, Beltrano J, Montaldi ER. Diagravitropic growth promoted by high sucrose contents in Paspalum vaginatum, and its reversion by gibberellic acid. Can J Bot. 1988;66:2035–7. [Google Scholar]
- 76.Digby J, Firn RD. Light modulation of the gravitropic set-point angle (GSA) J Exp Bot. 2002;53:377–81. doi: 10.1093/jexbot/53.367.377. [DOI] [PubMed] [Google Scholar]
- 77.Walter A, Nagel KA. Root growth reacts rapidly and more pronounced than shoot growth towards increasing light intensity in tobacco seedlings. Plant Signal Behav. 2006;1:225–6. doi: 10.4161/psb.1.5.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muller B, Stosser M, Tardieu F. Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots. Plant Cell Environ. 1998;21:149–58. doi: 10.1046/j.1365-3040.1998.00263.x. [DOI] [Google Scholar]
- 79.Nagel KA, Schurr U, Walter A. Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 2006;29:1936–45. doi: 10.1111/j.1365-3040.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- 80.Macgregor DR, Deak KI, Ingram PA, Malamy JE. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell. 2008;20:2643–60. doi: 10.1105/tpc.107.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeffs RA, Northcote DH. The influence of indol-3yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture. J Cell Sci. 1967;2:77–88. doi: 10.1242/jcs.2.1.77. [DOI] [PubMed] [Google Scholar]
- 82.González MV, Sadras VO, Equiza MA, Tognetti JA. Suboptimal temperature favors reserve formation in biennial carrot (Daucus carota) plants. Physiol Plant. 2009;137:10–21. doi: 10.1111/j.1399-3054.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 83.Xu X, Vermeer E, Vreugdenhil D, Vreugdenhil D, van Lammeren AA The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998;117:575–84. doi: 10.1104/pp.117.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park WD. Molecular approaches to tuberization in potato. In: (Eds) MVWP, ed. The molecular and cellular biology of the potato. Melksham, UK: Redwood Press Ltd, 1990:43-56. [Google Scholar]
- 85.Mares DJ, Marscfaner H, Krauss A. Effect of gibberellic acid on growth and carbohydrate metabolism of developing tubers of potato (Solanum tuberosum) Physiol Plant. 1981;52:267–74. doi: 10.1111/j.1399-3054.1981.tb08504.x. [DOI] [Google Scholar]
- 86.Fischer L, Lipavska H, Hausman JF, Opatrny Z. Morphological and molecular characterization of a spontaneously tuberizing potato mutant: an insight into the regulatory mechanisms of tuber induction. BMC Plant Biol. 2008;8:117. doi: 10.1186/1471-2229-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keller ERJ. Sucrose, cytokinin, and ethylene influence formation of in vitro bulblets in onion and leek. Genet Resour Crop Evol. 1993;40:113–20. doi: 10.1007/BF00052642. [DOI] [Google Scholar]
- 88.Kapoor P, Rao IU. In vitro rhizome induction and plantlet formation from multiple shoots in Bambusa bambos var. gigantea Bennet and Gaur by using growth regulators and sucrose. Plant Cell Tiss Org. 2006;85:211–7. doi: 10.1007/s11240-005-9074-y. [DOI] [Google Scholar]
- 89.Friend DJC, Bodson M, Bernier G. Promotion of flowering in Brassica campestris L. cv Ceres by sucrose. Plant Physiol. 1984;75:1085–9. doi: 10.1104/pp.75.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. Physiological signals that induce flowering. Plant Cell. 1993;5:1147–55. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corbesier L, Lejeune P, Bernier G. The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta. 1998;206:131–7. doi: 10.1007/s004250050383. [DOI] [PubMed] [Google Scholar]
- 92.Bagnall DJ, King RW. Phytochrome, photosynthesis and flowering of Arabidopsis thaliana: photophysiological studies using mutants and transgenic lines. Funct Plant Biol. 2001;28:401–8. doi: 10.1071/PP99123. [DOI] [Google Scholar]
- 93.Yang Y, Yao A, Wang J, Hu J. The effect of sucrose on the expression of the VvTFL1 and VFL genes during flower development in the “Xiangfei” grapevine. Sci Hortic (Amsterdam) 2011;129:299–305. doi: 10.1016/j.scienta.2011.04.002. [DOI] [Google Scholar]
- 94.Roldán M, Gómez-Mena C, Ruiz-García L, Salinas J, Martínez-Zapater JM. Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J. 1999;20:581–90. doi: 10.1046/j.1365-313X.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 95.Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 2001;127:252–61. doi: 10.1104/pp.127.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seo PJ, Ryu J, Kang SK, Park CM. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011;65:418–29. doi: 10.1111/j.1365-313X.2010.04432.x. [DOI] [PubMed] [Google Scholar]
- 97.Coneva V, Guevara D, Rothstein SJ, Colasanti J. Transcript and metabolite signature of maize source leaves suggests a link between transitory starch to sucrose balance and the autonomous floral transition. J Exp Bot. 2012;63:5079–92. doi: 10.1093/jxb/ers158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalchau N, Baek SJ, Briggs HM, Robertson FC, Dodd AN, Gardner MJ, et al. The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc Natl Acad Sci USA. 2011;108:5104–9. doi: 10.1073/pnas.1015452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quirino BF, Noh YS, Himelblau E, Amasino RM. Molecular aspects of leaf senescence. Trends Plant Sci. 2000;5:278–82. doi: 10.1016/S1360-1385(00)01655-1. [DOI] [PubMed] [Google Scholar]
- 100.Borochov A, Mayak S, Halevy AH. Combined effects of abscisic acid and sucrose on growth and senescence of rose flowers. Physiol Plant. 2006;36:221–4. doi: 10.1111/j.1399-3054.1976.tb04416.x. [DOI] [Google Scholar]
- 101.Park JY, Canam T, Kang KY, Unda F, Mansfield SD. Sucrose phosphate synthase expression influences poplar phenology. Tree Physiol. 2009;29:937–46. doi: 10.1093/treephys/tpp028. [DOI] [PubMed] [Google Scholar]
- 102.Weber H, Borisjuk L, Wobus U. Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J. 1996;10:823–34. doi: 10.1046/j.1365-313X.1996.10050823.x. [DOI] [Google Scholar]
- 103.Yang Z, Zhang L, Diao F, Huang M, Wu N. Sucrose regulates elongation of carrot somatic embryo radicles as a signal molecule. Plant Mol Biol. 2004;54:441–59. doi: 10.1023/B:PLAN.0000036375.40006.d3. [DOI] [PubMed] [Google Scholar]
- 104.Barth I, Meyer S, Sauer N. PmSUC3: characterization of a SUT2/SUC3-type sucrose transporter from Plantago major. Plant Cell. 2003;15:1375–85. doi: 10.1105/tpc.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Li LL, Fan RC, Peng CC, Sun HL, Zhu SY, et al. Arabidopsis sucrose transporter SUT4 interacts with cytochrome b5-2 to regulate seed germination in response to sucrose and glucose. Mol Plant. 2012;5:1029–41. doi: 10.1093/mp/sss001. [DOI] [PubMed] [Google Scholar]
- 106.Ohto M, Nakamura K. Sugar-Induced Increase of Calcium-Dependent Protein Kinases Associated with the Plasma Membrane in Leaf Tissues of Tobacco. Plant Physiol. 1995;109:973–81. doi: 10.1104/pp.109.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iwata Y, Kuriyama M, Nakakita M, Kojima H, Ohto M, Nakamura K. Characterization of a calcium-dependent protein kinase of tobacco leaves that is associated with the plasma membrane and is inducible by sucrose. Plant Cell Physiol. 1998;39:1176–83. doi: 10.1093/oxfordjournals.pcp.a029318. [DOI] [PubMed] [Google Scholar]
- 108.Raíces M, MacIntosh GC, Ulloa RM, Gargantini PR, Vozza NF, Téllez-Inón MT. Sucrose increases calcium-dependent protein kinase and phosphatase activities in potato plants. Cell Mol Biol (Noisy-le-grand) 2003;49:959–64. [PubMed] [Google Scholar]
- 109.Takeda S, Mano S, Ohto MA, Nakamura K. Inhibitors of protein phosphatases 1 and 2A block the sugar-inducible gene expression in plants. Plant Physiol. 1994;106:567–74. doi: 10.1104/pp.106.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ciereszko I, Kleczkowski LA. Effects of phosphate deficiency and sugars on expression of rab18 in Arabidopsis: hexokinase-dependent and okadaic acid-sensitive transduction of the sugar signal. Biochim Biophys Acta. 2002;1579:43–9. doi: 10.1016/S0167-4781(02)00502-X. [DOI] [PubMed] [Google Scholar]
- 111.Siedlecka A, Ciereszko I, Mellerowicz E, Martz F, Chen J, Kleczkowski LA. The small subunit ADP-glucose pyrophosphorylase ( ApS) promoter mediates okadaic acid-sensitive uidA expression in starch-synthesizing tissues and cells in Arabidopsis. Planta. 2003;217:184–92. doi: 10.1007/s00425-003-0982-y. [DOI] [PubMed] [Google Scholar]
- 112.Vijn I, Smeekens S. Fructan: more than a reserve carbohydrate? Plant Physiol. 1999;120:351–60. doi: 10.1104/pp.120.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martínez-Noël G, Tognetti J, Nagaraj V, Wiemken A, Pontis H. Calcium is essential for fructan synthesis induction mediated by sucrose in wheat. Planta. 2006;225:183–91. doi: 10.1007/s00425-006-0339-4. [DOI] [PubMed] [Google Scholar]
- 114.Martínez-Noël G, Nagaraj VJ, Caló G, Wiemken A, Pontis HG. Sucrose regulated expression of a Ca2+-dependent protein kinase (TaCDPK1) gene in excised leaves of wheat. Plant Physiol Biochem. 2007;45:410–9. doi: 10.1016/j.plaphy.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 115.Martínez-Noël GM, Tognetti JA, Salerno GL, Wiemken A, Pontis HG. Protein phosphatase activity and sucrose-mediated induction of fructan synthesis in wheat. Planta. 2009;230:1071–9. doi: 10.1007/s00425-009-1002-7. [DOI] [PubMed] [Google Scholar]
- 116.Martinez-Noël GA, Tognetti JA, Salerno GL, Pontis HG. Sugar signaling of fructan metabolism: New insights on protein phosphatases in sucrose-fed wheat leaves. Plant Signal Behav. 2010;5:311–3. doi: 10.4161/psb.5.3.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ritsema T, Brodmann D, Diks SH, Bos CL, Nagaraj V, Pieterse CM, et al. Are small GTPases signal hubs in sugar-mediated induction of fructan biosynthesis? PLoS ONE. 2009;4:e6605. doi: 10.1371/journal.pone.0006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aoki N, Scofield GN, Wang XD, Patrick JW, Offler CE, Furbank RT. Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta. 2004;219:176–84. doi: 10.1007/s00425-004-1232-7. [DOI] [PubMed] [Google Scholar]
- 119.Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett. 1998;424:165–8. doi: 10.1016/S0014-5793(98)00165-3. [DOI] [PubMed] [Google Scholar]
- 120.Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolaï M, et al. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007;8:864–70. doi: 10.1038/sj.embor.7401043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baena-González E, Sheen J. Convergent energy and stress signaling. Trends Plant Sci. 2008;13:474–82. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Purcell PC, Smith AM, Halford NG. Antisense expression of a sucrose non-fermenting-1-related protein kinase sequence in potato results in decreased expression of sucrose synthase in tubers and loss of sucrose-inducibility of sucrose synthase transcripts in leaves. Plant J. 1998;14:195–202. doi: 10.1046/j.1365-313X.1998.00108.x. [DOI] [Google Scholar]
- 123.Baena-González E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–42. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 124.Baena-González E. Energy signaling in the regulation of gene expression during stress. Mol Plant. 2010;3:300–13. doi: 10.1093/mp/ssp113. [DOI] [PubMed] [Google Scholar]
- 125.Jossier M, Bouly JP, Meimoun P, Arjmand A, Lessard P, Hawley S, et al. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 2009;59:316–28. doi: 10.1111/j.1365-313X.2009.03871.x. [DOI] [PubMed] [Google Scholar]
- 126.Halford NG, Hey SJ. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J. 2009;419:247–59. doi: 10.1042/BJ20082408. [DOI] [PubMed] [Google Scholar]
- 127.Lunn JE, Feil R, Hendriks JH, Gibon Y, Morcuende R, Osuna D, et al. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J. 2006;397:139–48. doi: 10.1042/BJ20060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grierson C, Du JS, de Torres Zabala M, Beggs K, Smith C, Holdsworth M, et al. Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J. 1994;5:815–26. doi: 10.1046/j.1365-313X.1994.5060815.x. [DOI] [PubMed] [Google Scholar]
- 129.Ishiguro S, Nakamura K. The nuclear factor SP8BF binds to the 5′-upstream regions of three different genes coding for major proteins of sweet potato tuberous roots. Plant Mol Biol. 1992;18:97–108. doi: 10.1007/BF00018460. [DOI] [PubMed] [Google Scholar]
- 130.Sun C, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15:2076–92. doi: 10.1105/tpc.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ishiguro S, Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol Gen Genet. 1994;244:563–71. doi: 10.1007/BF00282746. [DOI] [PubMed] [Google Scholar]
- 132.Rook F, Gerrits N, Kortstee A, van Kampen M, Borrias M, Weisbeek P, et al. Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J. 1998;15:253–63. doi: 10.1046/j.1365-313X.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- 133.Wiese A, Elzinga N, Wobbes B, Smeekens S. A conserved upstream open reading frame mediates sucrose-induced repression of translation. Plant Cell. 2004;16:1717–29. doi: 10.1105/tpc.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weltmeier F, Rahmani F, Ehlert A, Dietrich K, Schütze K, Wang X, et al. Expression patterns within the Arabidopsis C/S1 bZIP transcription factor network: availability of heterodimerization partners controls gene expression during stress response and development. Plant Mol Biol. 2009;69:107–19. doi: 10.1007/s11103-008-9410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rahmani F, Hummel M, Schuurmans J, Wiese-Klinkenberg A, Smeekens S, Hanson J. Sucrose control of translation mediated by an upstream open reading frame-encoded peptide. Plant Physiol. 2009;150:1356–67. doi: 10.1104/pp.109.136036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma J, Hanssen M, Lundgren K, Hernández L, Delatte T, Ehlert A, et al. The sucrose-regulated Arabidopsis transcription factor bZIP11 reprograms metabolism and regulates trehalose metabolism. New Phytol. 2011;191:733–45. doi: 10.1111/j.1469-8137.2011.03735.x. [DOI] [PubMed] [Google Scholar]
- 137.Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005;139:1840–52. doi: 10.1104/pp.105.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tognetti JA, Salerno CL, Crespi MD, Pontis HG. Sucrose and fructan metabolism of different wheat cultivars at chilling temperatures. Physiol Plant. 1990;78:554–9. doi: 10.1111/j.1399-3054.1990.tb05241.x. [DOI] [Google Scholar]
- 139.Jackson SD. Multiple signaling pathways control tuber induction in potato. Plant Physiol. 1999;119:1–8. doi: 10.1104/pp.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coupe SA, Palmer BG, Lake JA, Overy SA, Oxborough K, Woodward FI, et al. Systemic signalling of environmental cues in Arabidopsis leaves. J Exp Bot. 2006;57:329–41. doi: 10.1093/jxb/erj033. [DOI] [PubMed] [Google Scholar]
- 141.Casella E, Soussana JF. Long-term effects of CO2 enrichment and temperature increase on the carbon balance of a temperate grass sward. J Exp Bot. 1997;48:1309–21. doi: 10.1093/jxb/48.6.1309. [DOI] [Google Scholar]
- 142.Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60:2859–76. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- 143.Penfield S. Temperature perception and signal transduction in plants. New Phytol. 2008;179:615–28. doi: 10.1111/j.1469-8137.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 144.Quail PH. Photosensory perception and signalling in plant cells: new paradigms? Curr Opin Cell Biol. 2002;14:180–8. doi: 10.1016/S0955-0674(02)00309-5. [DOI] [PubMed] [Google Scholar]
- 145.Stewart JL, Maloof JN, Nemhauser JL. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS ONE. 2011;6:e19894. doi: 10.1371/journal.pone.0019894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008;179:1004–16. doi: 10.1111/j.1469-8137.2008.02511.x. [DOI] [PubMed] [Google Scholar]