Abstract
By being sessile, plants have evolved a remarkable capacity to perceive and respond to changes in environmental conditions throughout their life cycle. Light represents probably the most important environmental factor that impinge on plant development because, other than supplying the energy source for photosynthesis, it also provides seasonal and positional information that are essential for the plant survival and fitness. Changes in the light environment can dramatically alter plant morphogenesis, especially during the early phases of plant life, and a compelling amount of evidence indicates that light-mediated changes in auxin homeostasis are central in these processes. Auxin exerts its morphogenetic action through instructive hormone gradients that drive developmental programs of plants. Such gradients are formed and maintained via an accurate control on directional auxin transport. This review summarizes the recent advances in understanding the influence of the light environment on polar auxin transport.
Keywords: light, photomorphogenesis, shade avoidance, phototropism, polar auxin transport, PIN efflux carriers
The quest for light is one of the major challenges for plants since their life depends on photosynthesis. Changes in the light environment can profoundly affect the developmental program of plants and this is particularly evident during germination when seedlings have to switch from a heterotrophic existence, depending on seed reserve, to a fully photoautotrophic metabolism. When germination occurs underground, in the absence of light, seedling development is characterized by rapid hypocotyl elongation, slow root growth and unexpanded cotyledons which are folded in a structure known as the apical hook, enclosing an inactive shoot apical meristem (SAM). This developmental program, also known as etiolation or skotomorphogenesis, allows the emergence of the seedling from the soil.1,2 Etiolated seedlings are extremely sensitive to dim light and can efficiently direct their growth toward the light source, a response called phototropism. The phototropic response is mediated by the blue light-absorbing phototropin receptors, and helps the etiolated seedlings to reach the sunlight faster.3 Once the seedling shoot emerges from the soil, the elongation of the hypocotyl is arrested, the apical hook unfolds and the root growth is accelerated. The SAM starts producing leaves and active chloroplasts are developed to establish a functional photosynthetic apparatus. This developmental program called photomorphogenesis or de-etiolation is triggered by specific families of photoreceptors, the blue light-absorbing chryptochromes and the red/far-red light-absorbing phytochromes.1,2 Once the photoautotrophic lifestyle is gained, the young seedlings still have to compete with the neighboring vegetation for sunlight. The shade of plant canopies, which is depleted in photosynthetically active wavelengths, can induce in most angiosperms the so-called shade avoidance response. The shade avoidance response, which is triggered by phytochromes, is characterized by the elongation of stem-like organs at the expense of leaf and root development as an attempt to overgrow neighbors and ensure a better uptake of sunlight.4,5
Several hormones, such as gibberellins, cytokinins and brassinosteroids, have been implicated in the morphological adaptation of plants in response to changes in light environment,6-8 but a key role of auxin is clearly emerging.9 One of the most peculiar properties of auxin lies in the existence of an active intercellular polar transport throughout the plant, a mechanism that is necessary for formation of instructive auxin gradients that influence almost all developmental processes including embryogenesis, post-embryonic organogenesis, root meristem maintenance and vascular tissue differentiation.10,11 At a cellular level, polar auxin transport (PAT) is achieved through the action of a number of plasma membrane (PM) transporters that regulate the influx and efflux of auxin.10,11 In the model plant Arabidopsis thaliana, the cellular influx of auxin is mediated by the AUXIN RESISTANT1/LIKE AUX1 (AUX1/LAX) family of amino acid permease-like proteins,12 whereas the efflux from the cells is mainly controlled by members of the PIN-FORMED (PIN) family of transmembrane proteins.13 Members of the subfamily B of ATP-binding cassette/P-Glycoprotein (ABCB/PGP) transporters have also been demonstrated to mediate cellular auxin efflux and to interact with PIN transporters at different levels.14,15 Among the auxin transporters, the PIN efflux carrier proteins are polarly localized at the plasma membrane of cells and their polarity determines the direction of the auxin flux.16 PIN proteins are constitutively recycled via endocytosis and auxin has been shown to inhibit this process to enhance its own efflux, thus influencing the polarity of the PINs.17,18 Endocytic recycling and transcytosis have been shown to play a role in controlling polar PIN targeting19,20 and evidence of protein phosphorylation regulating PIN polarity have also been provided.21,22
Although several studies have revealed how the light environment can alter auxin homeostasis at different levels by modifying its biosynthesis and degradation,9 recent works indicate that the regulation of auxin fluxes plays a central role in regulating and co-ordinating plant development in response to changes in the light environment.
The apical hook is one of the hallmarks of skotomorphogenic development. The formation of the apical hook is caused by asymmetrical growth of the cells on the two opposing sides of the upper part of the hypocotyl.23,24 The differential growth response coincides with the establishment of an auxin signaling maximum on the concave side of the apical hook, dependent on transport of auxin synthesized in cotyledons.23-25 The expression and the polar localization of several PINs is dynamically regulated during the establishment of the apical hook, but only PIN3 has been demonstrated to play a key role in the early stages of apical hook formation, whereas PIN1, PIN4 and PIN7 play redundant roles in the maintenance of the hook folding.23,25 Relevantly, auxin influx carrier proteins AUX1 and LAX3 and ABCB/PGP efflux facilitators ABCB19 and ABCB1 have been shown to take part in the process of apical hook formation and maintenance.24,26 Recently, the AGC kinase WAG2 has been shown to repress apical hook unfolding and to be required for the auxin maximum at the concave side of the hook.27 WAG2 expression is regulated by light via the basic Helix-Loop-Helix transcription factor PHYTOCHROME INTERACTING FACTOR 5 (PIF5), clearly linking auxin transport to light signaling.27 WAG2 has been shown to phosphorylate several PINs in vitro, leading to the idea that it might regulate PIN polarity in vivo.27 However, its role in the regulation of auxin fluxes in the apical hook is still unclear, since no changes in PIN1 and PIN3 polarity were observed in wag2 mutants’ apical hook.27
The hypocotyl acts also as a central communication organ between the shoot and the root, the communications occurring notably through auxin transport. Recent evidence indeed demonstrated that light has a profound effect on PAT in the hypocotyl during de-etiolation.28,29 In dark-grown hypocotyls, PIN1 expression is dramatically reduced, and it is conversely induced upon exposure to light.28 The light-mediated regulation of PIN1 expression in the hypocotyl has been shown to depend on the action of CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), a RING E3 ubiquitin ligase that acts as the master regulator of photomorphogenesis.28 The repression of PIN1 expression in etiolated hypocotyls has been proposed to inhibit the PAT from the shoot to the root, in agreement with previous analyses showing impaired PAT in dark-grown hypocotyls.28,29 At a physiological level, the dark-induced inhibition of shoot-to-root PAT has been shown to strongly inhibit root growth and lateral root development,28,30 allowing for a tight co-ordination of shoot and root development during the photomorphogenetic response.28
The light-mediated regulation of PIN3 activity plays a key role in regulating phototropic hypocotyl bending.25 It was recently demonstrated that PIN3 is expressed in the endodermal cells of etiolated hypocotyls where it is localized in an apolar fashion, being present on both the outer and inner PM.31 Upon exposure to unilateral blue light PIN3 localization becomes asymmetrical, disappearing from the outer PM in the cells on the side of the hypocotyl directly exposed to the light signal.31 Polarization of PIN3 in the inner side of endodermal cells has been proposed to be required to pump away auxin from the illuminated flank of the hypocotyl and to form a hormone maximum on the side opposite to the light signal, thus promoting the asymmetric growth response that drives hypocotyl bending.25,31 This regulation of PIN3 polarity is dependent on phototropins action and it requires PIN3 phosphorylation via the PINOID kinase.31 Genetic evidence indicated that other auxin transporters, such as PIN7, AUX1 and ABCB19, may also participate in phototropic responses although their precise role is not yet clearly defined.31-33
Light does not regulate auxin transport only in the aboveground tissues. Indeed, auxin transport in the root apex is also influenced by the light environment, despite the fact that roots are not directly exposed to light. It has been shown that darkness promotes vacuolar targeting of PIN2 in the root apical meristem (RAM), a response proposed to alter local auxin transport activity, leading to reduced RAM proliferation and root growth.28,34 It was also demonstrated that, in the dark, PIN2 is targeted to the vacuole for degradation, leading to a reduction of the protein levels at the PM, similarly to what is observed on one side of the RAM during the root gravitropic responses.35,36 Dark-induced vacuolar targeting of PIN2 is not a root-autonomous process, but requires signals from the shoot.28 Recent evidence indicated that PIN2 levels in the RAM depend on the amount of shoot-derived auxin, that is itself determined by the light-mediated regulation of PIN1 expression in the hypocotyl.28 COP1 has been shown to participate in the regulation of PIN2 levels in the RAM by light and gravity.28 Moreover, a requirement of PIN2 ubiquitylation for its vacuolar targeting and degradation in the dark has also been demonstrated.32 This would suggest that PIN2 might be a direct target of the COP1 E3 ubiquitin ligase, although this remains to be demonstrated. Other auxin transporters, such as PIN1, PIN3 and PIN7 are also targeted to the vacuole in dark-grown RAM, but genetic analyses suggest that only PIN1 and PIN2 are essential for the regulation of root growth in response to light.28,34 Dark-dependent vacuolar targeting of PIN1 was also observed in organs other than the RAM, such as leaf primordia and SAM, both in adult plants37,38 and in etiolated seedlings (Fig. 1). The vacuolar targeting of PIN1 in aerial organs leads to a dramatic reduction of PIN1 PM levels that closely correlate with a decrease in the auxin signaling activity in the distal end of leaf primordia.38 This response has been associated with the reduced organogenesis and leaf growth observed in dark-grown plants,38 indicating that PIN vacuolar targeting provides a mechanism for regulating growth of both shoot and root.
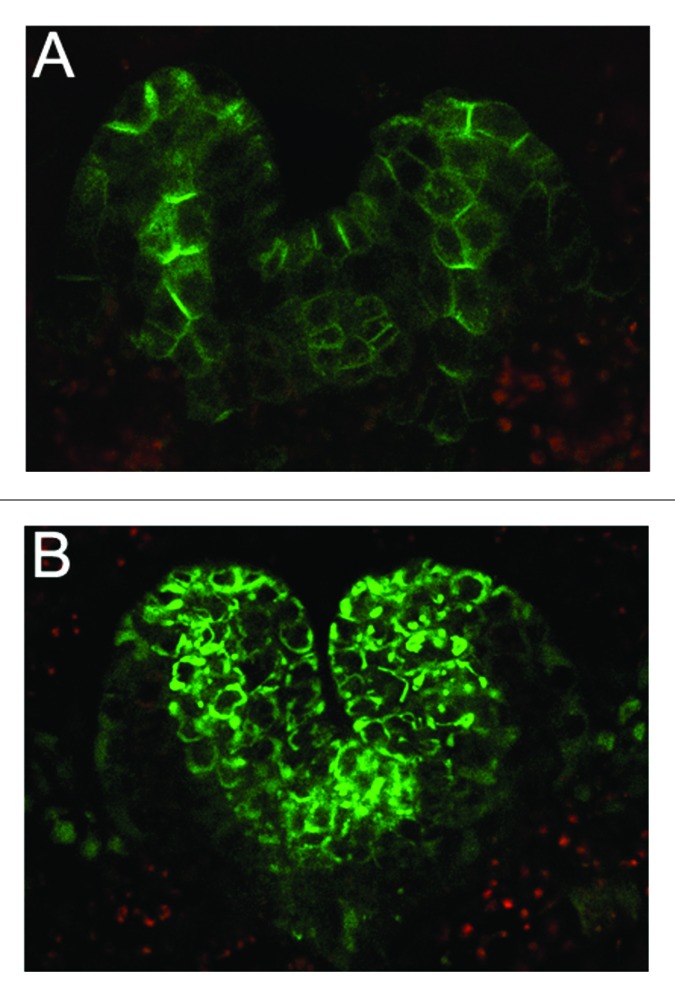
Figure 1. Confocal laser scanning microscopy images of pPIN1::PIN1 - GFP expression (green channel) in 5-d-old seedlings grown in light (A) or in the dark (B). PIN1 - GFP displays polar PM localization in the SAM and leaf primordia of light-grown seedlings (A). By contrast, PIN1 - GFP is targeted to the vacuole in the SAM and leaf primordia in etiolated seedlings (B). Red channel depicts seedling autofluorescence. The growth conditions and microscopy used for this experiment were previously described28
Alterations of plant architecture induced by canopy shade have also been associated with auxin transport.39 Indeed, canopy shade strongly increases auxin levels through the action of the auxin biosynthetic enzyme TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1/SHADE AVOIDANCE 3 (TAA1/SAV3) in a pathway that requires the action of phytochrome B and PIF7.40,41 The shade-induced increase in auxin levels has been shown to be required for the promotion of hypocotyl elongation that is central to the shade avoidance response of young seedlings.40,42 Relevantly, canopy shade induces PIN3 expression in the hypocotyl and promotes its lateral localization in the endodermal cells toward the flanks of the hypocotyl.42 This shade-induced relocalization of PIN3 has been proposed to promote auxin efflux toward the cortical cells of the hypocotyl, enhancing the cell elongation response.42 In shade conditions, a strong downregulation of PIN1 in the hypocotyl, along with a concurrent decrease in auxin levels in the RAM (as inferred by the DR5::GUS auxin signaling marker) can also be observed (Fig. 2). This suggests that shade might activate a PIN1-dependent mechanism, similar to that observed in etiolated seedlings, to partition auxin levels between shoot and root.28 Such a regulatory mechanism might inhibit growth of the root system43 while auxin-mediated responses in the shoot such as hypocotyl cell elongation and the inhibition of leaf expansion take place.42,44 Similarly to what occurs during etiolation, this shade-mediated regulation of auxin transport pathways is likely central to the re-allocation of resources allowing to fine tune plant development and architecture to light conditions.
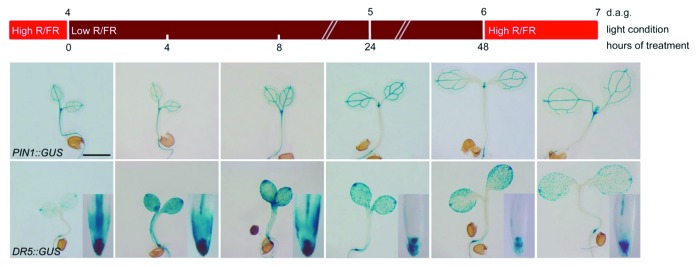
Figure 2. Time-course analysis of the histochemical localization of GUS activity in PIN1::GUS (upper row) and DR5::GUS (lower row) seedlings grown for 4 d in normal light conditions (high R/FR), and then exposed to shade (low R/FR) for 48 h. PIN1::GUS is specifically downregulated in the hypocotyl after 24 h of shade and its expression can be restored by exposing plants for 24 h to normal light conditions after the initial shade treatment. Notice that a concurrent reduction of DR5::GUS staining in the root apex of plants exposed to shade could also be observed (insets in the lower row), and could subsequently be increased by exposing plants for 24 h to normal light conditions after the initial shade treatment. The growth conditions, light settings, staining procedures and microscopy used for this experiment were previously described.28,44 d.a.g., days after germination.
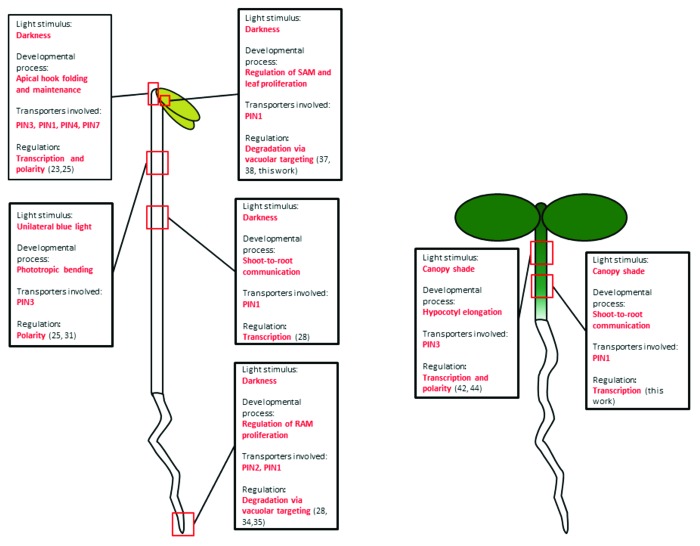
As we have discussed, it is becoming evident that light can control auxin transport in different ways, including the induction of tissue-specific PIN expression or the modulation of subcellular localization and abundance of PIN proteins28,31,35,42 (Fig. 3). In differentiated tissues, PIN expression levels and polarity appear to be preferentially targeted by light to promote ex novo formation of auxin gradients that can modify growth patterns.23,24,31,42 On the other hand, in undifferentiated tissues such as meristems and leaf primordia, light-mediated control of PIN protein abundance through the regulation of vacuolar degradation is likely to specifically and reversibly regulate auxin fluxes and as a result growth, without affecting meristem patterning.28,34,35,37,38 Another interesting aspect that is surfacing from these studies is that the environmental control of plant growth relies on common auxin transport-dependent regulatory mechanisms. For instance both phototropin- and phytochrome-mediated light response impinge on PIN3 relocalization to modify hypocotyl elongation,31,42 whereas light and gravity affect PIN2 levels to modulate root growth.28,35 These pieces of evidence indicate a surprising level of integration between environmental and developmental cues in the control of plant growth. Understanding this interplay between different environmental cues in the regulation of auxin-mediated growth response will be a major challenge for future research.
Figure 3. Schematic representation of the light-regulation of PIN auxin efflux carriers in etiolated (left) and light-grown (right) seedlings. Each square summarizes the effect of a particular light stimulus on the development of a specific tissues and the levels of regulation of PIN carriers involved (see main text for details). Numbers between parentheses indicate the corresponding references.
Acknowledgments
We apologize to all colleagues whose original works could not be cited for space constraints. Authors work is funded by the Ministry of Education of Singapore (MOE2012-T2-1-157 ; J.X.), the Human Frontier Science Program Organization (T.V.), the Agence National de la Recherche (M.S., T.V.), the iSAM ERASysBIO+ program (M.S., T.V.), the European Research Area Networks in Plant Genomics (ERA-PG) Program (I.R.) and the Italian Ministry of Agriculture and Foresty (MiPAF) Agronanotech Program (I.R.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23355
References
- 1.Chen M, Chory J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011;21:664–71. doi: 10.1016/j.tcb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arsovski AA, Galstyan A, Guseman JM, Nemhauser JL. Photomorphogenesis. Arabidopsis Book. 2012;10:e0147. doi: 10.1199/tab.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie JM. Phototropin blue-light receptors. Annu Rev Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- 4.Franklin KA. Shade avoidance. New Phytol. 2008;179:930–44. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruberti I, Sessa G, Ciolfi A, Possenti M, Carabelli M, Morelli G. Plant adaptation to dynamically changing environment: the shade avoidance response. Biotechnol Adv. 2012;30:1047–58. doi: 10.1016/j.biotechadv.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Alabadí D, Blázquez MA. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol Biol. 2009;69:409–17. doi: 10.1007/s11103-008-9400-y. [DOI] [PubMed] [Google Scholar]
- 7.Wolters H, Jürgens G. Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet. 2009;10:305–17. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- 8.Lau OS, Deng XW. Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol. 2010;13:571–7. doi: 10.1016/j.pbi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Halliday KJ, Martínez-García JF, Josse EM. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol. 2009;1:a001586. doi: 10.1101/cshperspect.a001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–8. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Friml J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur J Cell Biol. 2010;89:231–5. doi: 10.1016/j.ejcb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol. 2006;16:1123–7. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–8. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 14.Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell. 2007;19:131–47. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mravec J, Kubes M, Bielach A, Gaykova V, Petrásek J, Skůpa P, et al. Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development. 2008;135:3345–54. doi: 10.1242/dev.021071. [DOI] [PubMed] [Google Scholar]
- 16.Wisniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 17.Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–8. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 18.Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof YD, Kleine-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–6. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 19.Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wiśniewska J, Paciorek T, et al. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol. 2008;18:526–31. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc Natl Acad Sci USA. 2010;107:22344–9. doi: 10.1073/pnas.1013145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Nodzynski T, Pencík A, Rolcík J, Friml J. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci USA. 2010;107:918–22. doi: 10.1073/pnas.0909460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang F, Zago MK, Abas L, van Marion A, Galván-Ampudia CS, Offringa R. Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell. 2010;22:1129–42. doi: 10.1105/tpc.109.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zádníková P, Petrásek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development. 2010;137:607–17. doi: 10.1242/dev.041277. [DOI] [PubMed] [Google Scholar]
- 24.Vandenbussche F, Petrásek J, Zádníková P, Hoyerová K, Pesek B, Raz V, et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137:597–606. doi: 10.1242/dev.040790. [DOI] [PubMed] [Google Scholar]
- 25.Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–9. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 26.Wu G, Cameron JN, Ljung K, Spalding EP. A role for ABCB19-mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B. Plant J. 2010;62:179–91. doi: 10.1111/j.1365-313X.2010.04137.x. [DOI] [PubMed] [Google Scholar]
- 27.Willige BC, Ogiso-Tanaka E, Zourelidou M, Schwechheimer C. WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5. Development. 2012;139:4020–8. doi: 10.1242/dev.081240. [DOI] [PubMed] [Google Scholar]
- 28.Sassi M, Lu Y, Zhang Y, Wang J, Dhonukshe P, Blilou I, et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development. 2012;139:3402–12. doi: 10.1242/dev.078212. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Cohen JD, Gardner G. Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiol. 2011;157:891–904. doi: 10.1104/pp.111.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–32. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- 31.Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol. 2011;13:447–52. doi: 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- 32.Stone BB, Stowe-Evans EL, Harper RM, Celaya RB, Ljung K, Sandberg G, et al. Disruptions in AUX1-dependent auxin influx alter hypocotyl phototropism in Arabidopsis. Mol Plant. 2008;1:129–44. doi: 10.1093/mp/ssm013. [DOI] [PubMed] [Google Scholar]
- 33.Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, et al. phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 2011;9:e1001076. doi: 10.1371/journal.pbio.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laxmi A, Pan J, Morsy M, Chen R. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS One. 2008;3:e1510. doi: 10.1371/journal.pone.0001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci USA. 2008;105:17812–7. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leitner J, Petrášek J, Tomanov K, Retzer K, Pařezová M, Korbei B, et al. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci USA. 2012;109:8322–7. doi: 10.1073/pnas.1200824109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirakawa M, Ueda H, Shimada T, Nishiyama C, Hara-Nishimura I. Vacuolar SNAREs function in the formation of the leaf vascular network by regulating auxin distribution. Plant Cell Physiol. 2009;50:1319–28. doi: 10.1093/pcp/pcp076. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida S, Mandel T, Kuhlemeier C. Stem cell activation by light guides plant organogenesis. Genes Dev. 2011;25:1439–50. doi: 10.1101/gad.631211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morelli G, Ruberti I. Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci. 2002;7:399–404. doi: 10.1016/S1360-1385(02)02314-2. [DOI] [PubMed] [Google Scholar]
- 40.Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–76. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012;26:785–90. doi: 10.1101/gad.187849.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salisbury FJ, Hall A, Grierson CS, Halliday KJ. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007;50:429–38. doi: 10.1111/j.1365-313X.2007.03059.x. [DOI] [PubMed] [Google Scholar]
- 44.Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, et al. Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev. 2007;21:1863–8. doi: 10.1101/gad.432607. [DOI] [PMC free article] [PubMed] [Google Scholar]