Abstract
In leguminous plants, rhizobial infection of the epidermis triggers proliferation of cortical cells to form a nodule primordium. Recent studies have demonstrated that two classic phytohormones, cytokinin and auxin, have important functions in nodulation. The identification of these functions in Lotus japonicus was facilitated by use of the spontaneous nodule formation 2 (snf2) mutation of the putative cytokinin receptor LOTUS HISTIDINE KINASE 1 (LHK1). Analyses using snf2 demonstrated that constitutive activation of cytokinin signaling causes formation of spontaneous nodule-like structures in the absence of rhizobia and that auxin responses are induced in proliferating cortical cells during such spontaneous nodule development. Thus, cytokinin signaling positively regulates the auxin response. In the present study, we further investigated the induction of the auxin response using a gain-of-function mutation of Ca2+/calmodulin-dependent protein kinase (CCaMK) that causes spontaneous nodule formation. We demonstrate that CCaMKT265D-mediated spontaneous nodule development is accompanied by a localized auxin response. Thus, a localized auxin response at the site of an incipient nodule primordium is essential for nodule organogenesis.
Keywords: auxin, CCaMK, Lotus japonicus, nodulation, spontaneous nodule formation
Leguminous species have the ability to form nodular structures on their roots in a symbiotic relationship with soil bacteria (termed rhizobia). The formation of nodules is initiated by rhizobial infection of the plant root epidermis, which induces an infection signaling cascade that ultimately activates a Ca2+/calmodulin-dependent protein kinase (CCaMK).1,2 This kinase is believed to be a key regulator for decoding Ca2+ signals. Loss-of-function mutations of CCaMK cause a nodulation-deficient phenotype in plants.3 By contrast, spontaneous formation of nodule-like structures in the absence of rhizobia occurs in plants with a gain-of-function mutation that confers a constitutively active CcaMK,3-7 e.g., spontaneous nodule formation 1 (snf1) or CCaMKT265D.
In Lotus japonicus, spontaneous nodule formation is also mediated by snf2, a gain-of-function mutation of LOTUS HISTIDINE KINASE 1 (LHK1), which encodes a putative cytokinin receptor.8,9 Phenotypic analysis of snf2 mutant plants indicated that cytokinin signaling is constitutively activated. Confirmation of the important role of cytokinin comes from the observation that spontaneous nodule formation is induced by exogenous application of the phytohormone in L. japonicus.10 Moreover, some cytokinin response regulators are reported to be involved in nodulation.11 The findings from these studies suggest that activation of cytokinin signaling is crucial for the initiation of nodule organogenesis.
Although there is now strong evidence of the role of cytokinin in nodule formation, much less is known about how a second phytohormone, such as auxin, functions to regulate nodule development. We recently investigated auxin reporter lines in L. japonicus to determine auxin response patterns and the interaction between auxin response and key factors in nodule development.12 These analyses showed that induction of auxin response predominantly occurs during cortical cell proliferation. In addition, auxin response appears to occur in a downstream part of the cytokinin signaling pathway since it is induced during snf2-dependent spontaneous nodule formation.
In this study, we sought to elucidate the potential interaction between auxin and CCaMK by analyzing auxin response patterns during spontaneous nodule development resulting from the introduction of the CCaMKT265D mutation into DR5::GFP-NLS L. japonicus plants. To identify transgenic hairy roots in these plants, the GFP of the original binary vector containing the CCaMKT265D construct was replaced by mKO2.6,12 We used the Agrobacterium rhizogenes mediated hairy root transformation method to introduce the recombinant plasmid into the DR5::GFP-NLS plants. In the CCaMKT265D /DR5::GFP-NLS plants, GFP expression was observed in cortical cells that were proliferating to form the primordia of spontaneous nodules (Fig. 1A). GFP continued to be expressed after the proliferating cortical cells had formed an obvious protuberance at the site of a spontaneous nodule (Fig. 1B). At this early stage of spontaneous nodule development following the initial rounds of cell division, the morphology of the spontaneous nodule primordium resembled that of a developing lateral root; this similarity made it difficult to identify spontaneous nodules on the basis of their physical appearance. However, differences in GFP expression patterns enabled us to unambiguously distinguish these two types of structure: the developing lateral root had a more strongly localized GFP signal, corresponding to the position of the future root apex (Fig. 1C and D) than the spontaneous nodule. The induced GFP expression during CCaMKT265D-dependent spontaneous nodule formation indicates that an auxin response takes place in a downstream part of the calcium signaling pathway mediated by CCaMK. This conclusion is consistent with the suggestion that activation of cytokinin signaling occurs in a downstream part of the CCaMK signaling pathway as the ccamk mutation does not affect snf2-dependent spontaneous nodulation.8
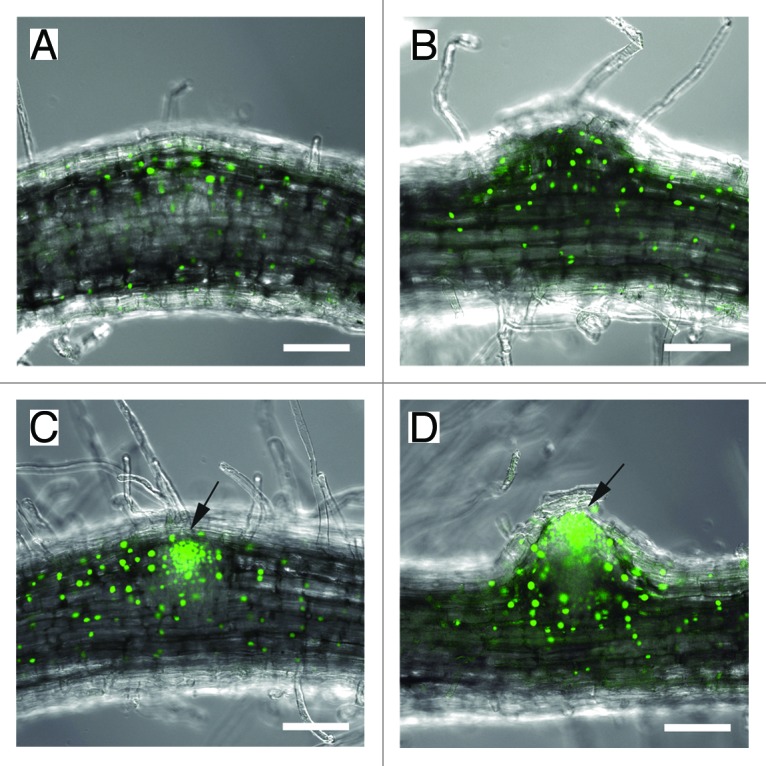
Figure 1. Auxin response patterns during spontaneous nodule formation and lateral root development in Lotus japonicus. Auxin responses were monitored indirectly by GFP expression patterns during spontaneous nodule formation (A and B) and lateral root development (C and D) in DR5::GFP-NLS stable transgenic plants; these plants have transgenic hairy roots in which CCaMKT265D and mKO2 are constitutively expressed. Transgenic hairy roots were identified by the mKO2 signal (data not shown). Arrows indicate the strongly localized GFP expression, which is observed specifically during lateral root development. Scale bars = 100 μm.
The present study demonstrates that an auxin response is induced during CCaMKT265D-dependent spontaneous nodule development. This result provides further support for the hypothesis that a localized auxin response at the site of an incipient nodule primordium is essential for nodule organogenesis. Interestingly, in an examination of normal nodule development, we showed previously that after colonization by rhizobia the auxin response was restricted to the regions surrounding the sites of rhizobial colonization.12 This observation suggests that there might be a mechanism to exclude auxin from sites of rhizobial colonization. Since nodulation requires a collaborative interaction between rhizobia and the plant host, it is possible that rhizobial factors are also involved in the regulation of auxin exclusion as well as those of the host. Further investigation of the detailed auxin response patterns, such as through use of host and rhizobial mutants that are deficient in rhizobial colonization in nodules should aid elucidation of the genetic factors involved in nodulation.
Acknowledgments
We thank Haruko Imaizumi-Anraku for providing original CCaMKT265D construct. This research was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22870035 and 23012038 to T.S.; 22128006 to M.K.). Confocal images were acquired at Spectrography and Bioimaging Facility, NIBB Core Research Facilities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23359
References
- 1.Kouchi H, Imaizumi-Anraku H, Hayashi M, Hakoyama T, Nakagawa T, Umehara Y, et al. How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant Cell Physiol. 2010;51:1381–97. doi: 10.1093/pcp/pcq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S, Parniske M. Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr Opin Plant Biol. 2012;15:444–53. doi: 10.1016/j.pbi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, et al. Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature. 2006;441:1153–6. doi: 10.1038/nature04862. [DOI] [PubMed] [Google Scholar]
- 4.Gleason C, Chaudhuri S, Yang T, Muñoz A, Poovaiah BW, Oldroyd GE. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature. 2006;441:1149–52. doi: 10.1038/nature04812. [DOI] [PubMed] [Google Scholar]
- 5.Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, et al. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat Commun. 2010;1:10. doi: 10.1038/ncomms1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi T, Banba M, Shimoda Y, Kouchi H, Hayashi M, Imaizumi-Anraku H. A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 2010;63:141–54. doi: 10.1111/j.1365-313X.2010.04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoda Y, Han L, Yamazaki T, Suzuki R, Hayashi M, Imaizumi-Anraku H. Rhizobial and fungal symbioses show different requirements for calmodulin binding to calcium calmodulin-dependent protein kinase in Lotus japonicus. Plant Cell. 2012;24:304–21. doi: 10.1105/tpc.111.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–7. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 9.Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–4. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- 10.Heckmann AB, Sandal N, Bek AS, Madsen LH, Jurkiewicz A, Nielsen MW, et al. Cytokinin induction of root nodule primordia in Lotus japonicus is regulated by a mechanism operating in the root cortex. Mol Plant Microbe Interact. 2011;24:1385–95. doi: 10.1094/MPMI-05-11-0142. [DOI] [PubMed] [Google Scholar]
- 11.Op den Camp RH, De Mita S, Lillo A, Cao Q, Limpens E, Bisseling T, et al. A phylogenetic strategy based on a legume-specific whole genome duplication yields symbiotic cytokinin type-A response regulators. Plant Physiol. 2011;157:2013–22. doi: 10.1104/pp.111.187526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M. Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development. 2012;139:3997–4006. doi: 10.1242/dev.084079. [DOI] [PubMed] [Google Scholar]


