Abstract
Plant circadian clock controls a wide variety of physiological and developmental events, which include the short-days (SDs)-specific promotion of the elongation of hypocotyls during de-etiolation and also the elongation of petioles during vegetative growth. In A. thaliana, the PIF4 gene encoding a phytochrome-interacting basic helix-loop-helix (bHLH) transcription factor plays crucial roles in this photoperiodic control of plant growth. According to the proposed external coincidence model, the PIF4 gene is transcribed precociously at the end of night specifically in SDs, under which conditions the protein product is stably accumulated, while PIF4 is expressed exclusively during the daytime in long days (LDs), under which conditions the protein product is degraded by the light-activated phyB and also the residual proteins are inactivated by the DELLA family of proteins. A number of previous reports provided solid evidence to support this coincidence model mainly at the transcriptional level of the PIF4 and PIF4-traget genes. Nevertheless, the diurnal oscillation profiles of PIF4 proteins, which were postulated to be dependent on photoperiod and ambient temperature, have not yet been demonstrated. Here we present such crucial evidence on PIF4 protein level to further support the external coincidence model underlying the temperature-adaptive photoperiodic control of plant growth in A. thaliana.
Keywords: arabidopsis thaliana, circadian clock, external coincidence model, light signaling, photomorphogenesis
Plant circadian clock generates biological rhythms with a period close to 24 h, and it controls a wide variety of physiological and developmental events.1,2 In Arabidopsis thaliana, the best-characterized clock-controlled output pathway is the photoperiodic control of flowering time, in which the clock regulates the long-days (LDs)-specific promotion of reproductive transition.3,4 The second one is the photoperiodic control of vegetative growth, including the short-days (SDs)-specific promotion of the elongation of hypocotyls during de-ethiolation.5,6 The underlying molecular mechanisms of these cock-controlled (or photoperiod-dependent) output pathways are commonly explained by external coincidence models. With regard to the photoperiodic control of plant growth, we recently proposed the clock and PHYTOCHROME-INTERACTING FACTOR 4 (PIF4)-mediated external coincidence model, as briefly introduced below.6–10
The circadian clock regulates the photoperiodic plant growth in an SDs-specific manner, in which the clock-controlled PIF4 gene encoding a phyB-interacting basic helix-loop-helix (bHLH) transcription factor plays crucial roles (Fig. 1A).11–16 As schematically shown in Figure 1B, PIF4 is transcribed precociously at the end of night in SDs, under which conditions the active protein product is stably accumulated,6,8 while PIF4 is expressed exclusively during daytime in long days (LDs), under which conditions the protein product is degraded by the light-activated phyB,15 and also the residual proteins are inactivated through binding with the DELLA family of proteins,16 which redundantly serve as repressors in the gibberellin (GA) signaling pathway.17 Based on the fact that PIF4 functions as a positive regulator for the elongation of hypocotyls, the SDs-specific elongation of hypocotyls is explained by the coincident accumulation of the active PIF4 proteins before dawn specifically in SDs.6,7 This event is followed by the coordinate induction of a set of PIF4-target genes, such as ARABIDOPSIS THALIANA HOMEOBOX PROTEIN 2 (ATHB2) encoding a homeodomain-leucine zipper protein, and INDOLE-3-ACETIC ACID INDUCIBLE 29 (IAA29) encoding an auxin signaling-associated factor.7,8 As will be discussed later, the circadian clock and PIF4-mediated external coincidence mechanism coordinately integrates both of the cues from seasonal changes in photoperiod and temperature to regulate plant growth in Arabidopsis thaliana.9,10

Figure 1. Essence of the external coincidence model underlying the clock and PIF4-mediated photoperiodic control of the elongation of hypocotyls. (A) PIF4-dependent and SDs-specific elongation of hypocotyls. Wild-type (Col-0) and pif4–101 mutant seedlings were grown for 8 d in either LDs (16 h light/8 h dark) or SDs (8 h light/16h dark) on gellan gum plates containing MS salts with 1% sucrose, as described previously.6 Pictures were taken for representatives to compare the lengths of hypocotyls, as indicated. (B) A schematic representation of the proposed external coincidence model, by which the mechanism underlying the clock and PIF4-mediated photoperiodic control of the elongation of hypocotyls is explained. Details were given in the text.6–8 (C) Evaluation of the PIF4-tag/pif4 transgenic line with special reference to the photoperiodic control of hypocotyl elongation. The experiments were performed, as described in (A). Pictures were taken for representatives to compare the lengths of hypocotyls, as indicated. Length of hypocotyls was also shown as mean values ± SD (n ≥ 10).
The Issue Addressed in this Study
A number of reports from our and other groups have provided evidence to support this coincidence model mainly at the transcriptional level of the PIF4 and PIF4-target genes.5–10,18–20 Nevertheless, the postulated photoperiod-dependent alteration of the PIF4 protein-profile has not yet been demonstrated, because of the lack of proper means to detect the photoperiod-dependent diurnal oscillation profile of PIF4 proteins. Here we employed an Arabidopsis transgenic line, which carries a transgene consisting of the PIF4 promoter followed by the PIF4-citrine-hemagglutinin epitope tag (HA) coding sequence. The transgenic line was established by introducing the PIF4 promoter-PIF4-citrine-HA composite gene into pif4–101 null mutant background (the detailed procedures of construction will be described elsewhere, Lorrain and Fankhauser), and was named PIF4pro::PIF4-citrine-HA/pif4–101 (line #2). Hereafter, the name will be abbreviated as PIF4-tag/pif4 for clarity in this text. The new transgenic line was designed to properly detect cellular content of PIF4 proteins. Taking advantage of this material, here we would like to verify the proposed circadian clock and PIF4-dependent external coincidence model at the level of PIF4 proteins.
Phenotypic Evaluation of the Transgenic Line with Regard to the Photoperiodic Control of Plant Growth
For the first step, the phenotype of the transgenic line was examined with reference to the elongation of hypocotyls to make sure that the transgenic plants display the proper nature with regard to the photoperiodic control of plant growth (Fig. 1C). Seedlings (Col-0 and PIF4-tag/pif4) were grown under both the LDs and SDs conditions, as described previously.6–8 The transgenic seedlings displayed the SDs-promoted elongation of hypocotyls, although the transgene appeared to over-complement the pif4 mutation, suggesting that the transgenic line might produce a slightly excess amount of PIF4-tag proteins as compared with the reference strain (Col-0). However, the transgenic line is suitable enough for our purpose of this study in the sense that the phenomenon of the photoperiodic control of hypocotyls elongation is clearly reproduced (Fig. 1C).
Molecular Essence of the Clock and PIF4-Mediated Photoperiodic Control of Plant Growth and Critical Preconditioning of this Study
As the next step, the transgenic line was examined more critically with regard to the external coincidence model at the molecular level. The PIF4-tag/pif4 transgenic plants together with Col-0 were grown in LDs and SDs for 8 d, and RNA samples were prepared at every 3 h interval, as described previously.6–8 The diurnal expression profiles were examined for PIF4, together with ATHB2 and IAA29 (i.e., the representatives of PIF4-targets) (Fig. 2). As introduced in the earlier section (see Fig. 1B), the SDs-specific precocious expression of PIF4 at the end of night was observed for Col-0 (Fig. 2, left upper panel). This resulted in the SDs- and dawn-specific inductions of ATHB2 and IAA29 (Fig. 2, middle and lower panels, respectively). The transcriptions of ATHB2 and IAA29 were hardly detected in the daytime, albeit a significant amount of PIF4 transcripts were present. These are consistent with the proposed scenario that the PIF4 protein-products are degraded by the light-activated phyB, and also the remaining PIF4 proteins are inactivated by the DELLA family of proteins. These are the molecular essence of the external coincidence model, which explain the mechanism underlying the photoperiodic control of plant growth (see Fig. 1B). Essentially the same molecular events were observed for PIF4-tag/pif4 (Fig. 2, right panels). A greatly enhanced expression of PIF4, together with those of ATHB2 and IAA29, was observed before dawn in an SDs-specific manner. In PIF4-tag/pif4 plants, however, a significant level of PIF4 expression was observed at the end of night even in LDs, most likely due to a slightly enhanced basal level expression of the transgene, which is consistent with the phenotype of the transgenic line (Fig. 1C). Taken these results together (Figs. 1 and 2), we concluded that the transgenic line is proper enough to study the diurnal expression level of PIF4 proteins with reference to the external coincidence mechanism, although we need to pay attention to the fact that the transgenic line tends to produce a slightly excess amount of PIF4-tag proteins as compared with the wild-type.

Figure 2. Characterization of diurnal expression profiles of PIF4, ATHB2 and IAA29. Wild-type (Col-0) and PIF4-tag/pif4 transgenic seedlings were grown in LDs and SDs for 8 d, and mRNA samples were prepared at every 3 h interval, as described previously.6–8 The resultant diurnal expression profiles were examined by means of quantitative real-time PCR (qRT-PCR) analyses. The experimental conditions and primers used were described in the previous papers.6–8 Note that we could not detect any PIF4-derived PCR products in pif4–101 plants with the primers used, indicating that the PIF4-tag-derived PCR products were specifically detected. Relative expression levels were shown as mean values ± SD (n = 3), for which the maximum value was taken as 1.0. The dark periods were indicated with shadings.
Verification of the Proposed External Coincidence Model at the Level of PIF4 Proteins
Then, we examined the diurnal expression profiles of PIF4-tag proteins in the transgenic plants grown in both LDs and SDs (Fig. 3). The results pointed out at least two events; (i) a large amount of PIF4-tag proteins was stably accumulated predominantly at the end of night in SDs, as compared with LDs (see the red arrows); (ii) a significant amount of PIF4-tag proteins was detected even in daytime in both SDs and LDs, although they were degraded along with the decrease in the PIF4 transcripts. These events at the level of PIF4-tag proteins were essentially consistent with the proposed external coincidence model. However, they also pointed out the following new aspect. The light-activated phyB could not completely wipe off the PIF4-tag proteins during the daytime, although the proteins were eventually degraded to a basal level. This might be partly due to the fact that basal level expression of the PIF4-tag transgene is enhanced slightly, or might be due to the possibility that the addition of the citrine protein to the C-terminus of PIF4 results in stabilization of proteins. In any case, it was revealed that the remaining PIF4-tag proteins in daytime are largely inactivated so that they are unable to induce the transcriptions of the target-genes ATHB2 and IAA29 throughout daytime (see Fig. 2). The fact is indeed in good agreement with the idea in the proposed model that the DELLA family proteins do play a prominent role in the inactivation of PIF4 proteins during daytime, under which conditions the level of GA (antagonist of DELLA) is greatly reduced.16,17
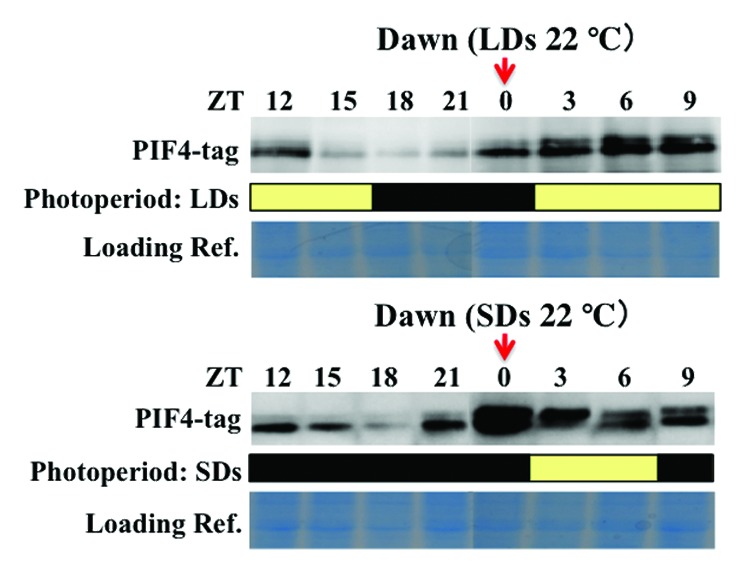
Figure 3. Characterization of diurnal expression profiles of PIF4-tag proteins. PIF4-tag/pif4 transgenic seedlings were grown in LDs and SDs for 10 d, and whole plant samples (50 mg) were prepared at every 3 h interval, as indicated. Proteins were extracted from homogenized frozen powder of each sample with 100 mM TRIS-HCl (pH 8), 50 mM EDTA, 0.25 M NaCl, 0.7% SDS and 1mM DTT and immediately heated at 65°C for 10 min. 15 µl of the extract was added to SDS-PAGE sample buffer, and the total proteins were separated on 10% SDS-PAGE gels and transferred onto nitrocellulose membrane. The PIF4-tag products were detected by the Anti-HA monoclonal antibody (3F10) conjugated with peroxidase (Roche). The yellow and black rectangles correspond to light and dark periods, respectively, indicating the photoperiodic conditions schematically. Red arrows point out samples of ZT = 0 in order to demonstrate that a large amount of PIF4-tag proteins was detected at the end of night (denoted by Dawn) in the seedlings grown in SDs, as compared with those grown in LDs.
Further Verification of the Proposed External Coincidence Model Underlying the PIF4-Mediated Temperature-Adaptive Photoperiodic Control of Plant Growth
The original coincidence model was challenged with the recent finding that the elongation of hypocotyls is markedly promoted at high growth temperature (i.e., 28°C) even in LDs in a PIF4-dependent manner (Fig. 4A).21,22 As reported previously,9,10 however, we found that the clock and PIF4-mediated external coincidence mechanism integrates external cues not only from photoperiod but also from a wide range of ambient temperature (16°C to 28°C) into the regulation of plant architecture.9,10 In other words, we have extended the model by showing that the transcription of PIF4 occurs precociously at the end of nighttime at 28°C even in LDs, similarly at 22°C in SDs, as schematically shown in Figure 4B (compare with Fig. 1B). Furthermore, we showed that both the conditions (i.e., at 22°C in SDs and at 28°C in LDs) result in the same consequence that a set of PIF4-target genes (i.e., ATHB2 and IAA29) is induced accordingly in a time-of-day-specific manner.9,10 Taken together, we previously proposed an extended double coincidence mechanism, by which the two environmental cues (i.e., photoperiod and temperature) are integrated into the common clock and PIF4-mediated output pathway that regulates a hormone-signaling network to fit plant architectures properly to domestic habitats, where both photoperiod and temperature are ever-changing. To further address the issue with special reference to this temperature-adaptive external coincidence model, the PIF4-tag/pif4 seedlings were grown at both 22°C and 28°C in LDs, as described previously (Fig. 4A).9 Phenotypic observation showed that the transgenic line displayed high temperature induced elongation of hypocotyls in LDs. Then, we examined the diurnal expression profiles of PIF4-tag proteins in the transgenic plants grown under the same conditions (Fig. 4C). As observed at 22°C in SDs (Fig. 3C), a large amount of PIF4-tag proteins was stably accumulated predominantly at the end of night specifically at 28°C in LDs, but not at 22°C in LDs (see the red arrows). The results also strongly support the external coincidence mechanism underlying the temperature-adaptive photoperiodic control of plant growth.

Figure 4. Characterization of diurnal expression profiles of PIF4-tag proteins with special reference to the temperature-adaptive photoperiodic control of hypocotyl elongation. (A) Wild-type (Col-0) and PIF4-tag/pif4 transgenic seedlings were grown respectively at either 22°C or 28°C in LDs for 8 d. Pictures were taken for representatives to compare the lengths of hypocotyls, as indicated. Length of hypocotyls was also shown as mean values ± SD (n ≥ 10). (B) A schematic representation of the proposed external coincidence model, by which the mechanism underlying the temperature-adaptive photoperiodic control of the elongation of hypocotyls is explained. Details were given in the text.6–8 (C) PIF4-tag/pif4 transgenic seedlings were grown at either 22°C or 28°C in LDs for 10 d, respectively, and protein samples were prepared at every 3 h interval, as indicated. The PIF4-tag products were detected as Figure 3. Diurnal PIF4-tag protein expressions at 22°C (upper) and at 28°C (lower) were shown. Note that PIF4-tag proteins were detected on the same membrane for the samples of both 22°C and 28°C, and thus the detected amounts are compared relatively. Note also that the samples prepared at 22°C were biologically independent ones from those analyzed in Figure 3. The colored and black rectangles correspond to light and dark periods, respectively, indicating the photoperiodic conditions schematically. Red arrows point out samples of ZT = 0 in order to demonstrate that a large amount of PIF4-tag proteins was detected at the end of night (denoted by Dawn) in the seedlings grown at 28°C, as compared with those grown at 22°C.
Implication and Future Problems
As emphasized above, we had not pursued one of the crucial experiments in the series of our earlier studies on the clock and PIF4-medited photoperiodic control of plant growth, mainly because of lack of an appropriate way to detect the diurnal oscillation profile of PIF4 proteins. A widely-used transgenic line carrying a 35S-promoter::PIF4-tag coding sequence is not proper for the evaluation of the diurnal expression profile of PIF4 proteins. Taking advantage of a new transgenic line carrying a PIF4 native promoter::PIF4-tag coding sequence, here we provided experimental evidence of photoperiod-dependent accumulation of PIF4 proteins at the end of night, which is postulated in the proposed external coincidence mechanism underlying the photoperiodic control of plant growth (Fig. 3). We also previously showed that this model holds under a wide range of ambient temperature conditions (16°C to 28°C).9,10 To support this notion, we provided further evidence for the high temperature-dependent accumulation of PIF4 proteins at the end of night even in LDs (Fig. 4). Taken together, the results of this study strongly support that the circadian clock and PIF4-mediated external coincidence mechanism coordinately integrates both of the cues from seasonal changes in photoperiod and temperature to regulate plant growth in natural habitats.
The results of this study in turn raised a number of future problems. How is the diurnal oscillation profile of the PIF4-transcription regulated in a manner dependent on photoperiod? Is any known clock component (e.g., the ELF4-ELF3-LUX evening complex that functions as a transcriptional repressor for PIF4) implicated in this regulation?23 Similarly, how is the diurnal oscillation profile of the PIF4-transcription regulated in a manner dependent on ambient temperature? Can the circadian clock integrate both the cues of photoperiod and temperature coordinately in order to regulate PIF4 at the level of transcription? The answers for these questions should shed new light on not only the specific aspect of photoperiodic control of plant growth, but also on the general aspects of plant circadian clock that has the fundamental abilities (referred to as “entrainment on temperature cycles” and “temperature compensation of period”) to integrate both the environmental cues from light and temperature.
Acknowledgments
This work was supported by Japan Society of the Promotion of Science (no. 23580133 and no. 23012018 to T.Y., no. 20370018 to T.M.).
Glossary
Abbreviations:
- ATHP2
Arabidopsis thaliana homeobox protein 2
- bHLH
basic helix-loop-helix
- GA
gibberellin
- HA
Human influenza hemagglutinin epitope-tag
- IAA29
Indole-3-Acetic acid inducible 29
- LDs
long days
- PIF4
Phytochrom-interchanging factor 4
- qRT-PCR
quantitative real-time PCR
- SDs
short days
Disclosure of Potential Conflicts of Interests
There were no potential conflicts of interests to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23390
References
- 1.McClung CR. The genetics of plant clocks. Adv Genet. 2011;74:105–39. doi: 10.1016/B978-0-12-387690-4.00004-0. [DOI] [PubMed] [Google Scholar]
- 2.Nakamichi N. Molecular mechanisms underlying the Arabidopsis circadian clock. Plant Cell Physiol. 2011;52:1709–18. doi: 10.1093/pcp/pcr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fornara F, de Montaigu A, Coupland G. SnapShot: Control of flowering in Arabidopsis. Cell. 2010;141:550–, 550, e1-2. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 2006;11:550–8. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, et al. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–61. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 6.Niwa Y, Yamashino T, Mizuno T. The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol. 2009;50:838–54. doi: 10.1093/pcp/pcp028. [DOI] [PubMed] [Google Scholar]
- 7.Kunihiro A, Yamashino T, Nakamichi N, Niwa Y, Nakanishi H, Mizuno T. Phytochrome-interacting factor 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol. 2011;52:1315–29. doi: 10.1093/pcp/pcr076. [DOI] [PubMed] [Google Scholar]
- 8.Nomoto Y, Kubozono S, Yamashino T, Nakamichi N, Mizuno T. Circadian clock and PIF4-controlled plant growth: A coincidence mechanism directly integrates a hormones-signaling network into the photoperiodic control of plant architectures in Arabidopsis thaliana. Plant Cell Physiol. 2012 doi: 10.1093/pcp/pcs137. [DOI] [PubMed] [Google Scholar]
- 9.Nomoto Y, Kubozono S, Miyachi M, Yamashino T, Nakamichi N, Mizuno T. A circadian clock and PIF4-mediated double coincidence mechanism is implicated in the thermo-sensitive photoperiodic control of plant architectures in Arabidopsis thaliana. Plant Cell Physiol. 2012 doi: 10.1093/pcp/pcs141. [DOI] [PubMed] [Google Scholar]
- 10.Nomoto Y, Kubozono S, Miyachi M, Nakamichi N, Mizuno T, Yamashino T. Circadian clock and PIF4-mediated external coincidence mechanism coordinately integrates both of the cues from seasonal changes in photoperiod and temperature to regulate plant growth in Arabidopsis thaliana. Plant Signal Behav. 2012;8 doi: 10.4161/psb.22863. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanna R, Huq E, Kikis EA, Al-Sady B, Lanzatella C, Quail PH. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell. 2004;16:3033–44. doi: 10.1105/tpc.104.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duek PD, Fankhauser C. bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 2005;10:51–4. doi: 10.1016/j.tplants.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Leivar P, Monte E, Cohn MM, Quail PH. Phytochrome signaling in green Arabidopsis seedlings: impact assessment of a mutually negative phyB-PIF feedback loop. Mol Plant. 2012;5:734–49. doi: 10.1093/mp/sss031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–23. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 16.de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–4. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 17.Sun TP. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr Biol. 2011;21:R338–45. doi: 10.1016/j.cub.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 18.Michael TP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, et al. A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol. 2008;6:e225. doi: 10.1371/journal.pbio.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, et al. Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet. 2008;4:e14. doi: 10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nozue K, Harmer SL, Maloof JN. Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis. Plant Physiol. 2011;156:357–72. doi: 10.1104/pp.111.172684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, et al. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19:408–13. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA. 2011;108:20231–5. doi: 10.1073/pnas.1110682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, et al. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475:398–402. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]


