Abstract
In somatic cell division, cytokinesis is the final step of the cell cycle and physically divides the mother cytoplasm into two daughter cells. In the meiotic cell division, however, pollen mother cells (PMCs) undergo two successive nuclear divisions without an intervening S-phase and consequently generate four haploid daughter nuclei out of one parental cell. In line with this, the physical separation of meiotic nuclei does not follow the conventional cytokinesis pathway, but instead is mediated by alternative processes, including polar-based phragmoplast outgrowth and RMA-mediated cell wall positioning. In this review, we outline the different cytological mechanisms of cell plate formation operating in different types of PMCs and additionally focus on some important features associated with male meiotic cytokinesis, including cytoskeletal dynamics and callose deposition. We also provide an up-to-date overview of the main molecular actors involved in PMC wall formation and additionally highlight some recent advances on the effect of cold stress on meiotic cytokinesis in plants.
Keywords: cytokinesis, plant meiosis, phragmoplast, RMA, callose
Cell Plate Formation in Plants
In mitotically dividing cells, separation of chromosomes (e.g., nuclear division) is followed by a physical separation of the two daughter nuclei by the establishment of a cell plate and/or cell wall (e.g., cell division). This process is generally termed cytokinesis and is considered an essential part of the mitotic cell cycle, more specifically as the final step of the mitotic M-phase. Indeed, loss of cell plate formation in most organisms typically leads to severe defects in cell proliferation and cell differentiation, mostly causing a premature abortion of the tissue or organ involved.1-3
Based on the different cell structure and morphology of various biological systems (e.g., rigid cell wall in plants does not occur in animal cells), the process of cytokinesis shows a large variability between the different kingdoms, with a strong difference between plants and other eukaryotic organisms.4,5 In animals and yeast, following nuclear division, cytokinesis is initiated at the periphery of the division plane with the specification of the cleavage plane and subsequent rearrangement of microtubule (MT) structures that form a structural basis for the formation of the actin-myosin-based contractile ring.6-9 This actomyosin ring contracts centripetally and mediates the ingression of cleavage furrows, eventually forming a small intercellular bridge (1−1.5 µm) that contains a microtubular midbody. In the final step of cytokinesis, MT-mediated vesicle trafficking completes midzone assembly and forms an internuclear membrane, physically separating the two newly formed daughter cells.7,10-12
In contrast to the centripetal-directed furrow ingression observed in animals and yeast, cell plate formation in plant cells typically involves the establishment of a centrally initiated MT structure; the phragmoplast.13-16 The phragmoplast is composed of short, overlapping microtubules of opposite polarity formed at the center of the cell and centrifugally expands to mediate the deposition of membrane vesicles along a plane marked earlier by a cortical microtubule array; the preprophase band (PPB).5,17 Subsequent fusion of Golgi-derived vesicles and endosomes at the equatorial midzone of the phragmoplast generates a transient tubule-vesicular membrane, also called the cell plate, which grows out in a centrifugal direction to fuse with the parental plasma membrane at the cell periphery.18-20 In the final step, the intermediate cell plate matures and transforms into a rigid cell wall; a process which involves different actions, including the closure of plate fenestrae, incorporation of pectins and xyloglucans, removal of excess membrane and replacement of callose by cellulose.15,21,22
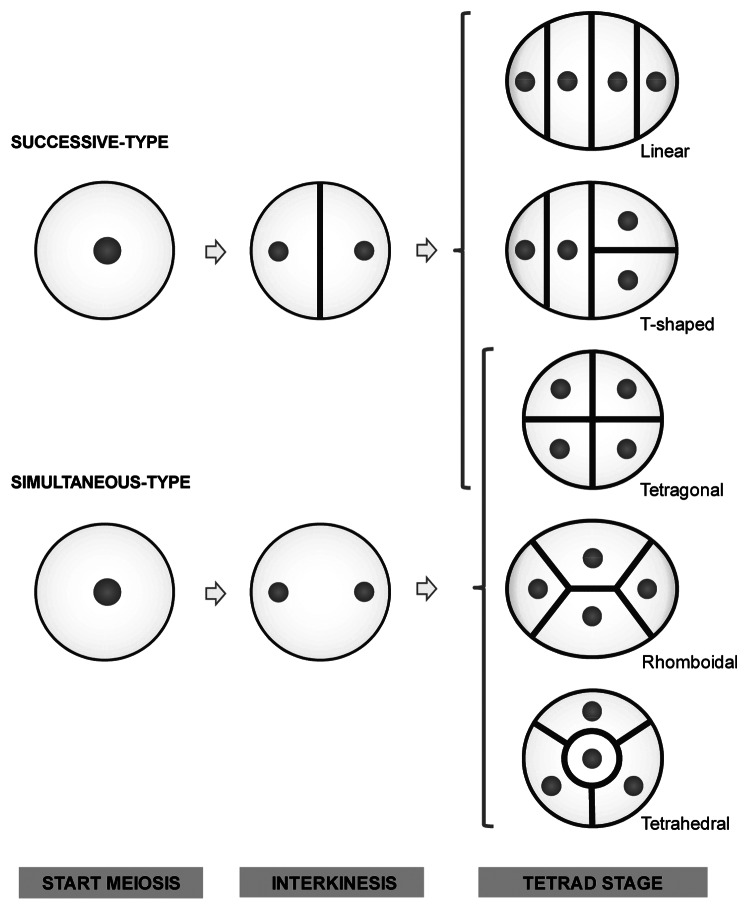
In the meiotic cell division, similar to the mitotic one, segregation of chromosomes (nuclear division) is always accompanied by the formation of a cell plate (cytokinesis). In plant male meiosis, however, two different types of cell plate formation are documented; namely successive and simultaneous cytokinesis. In the former mechanism each meiotic cell division is directly followed by a cytokinetic event. As such, a transitory dyad is generated after meiosis I and a tetrad is formed after meiosis II (Fig. 1). Inherent to this way of division, the tetrad figure in successive-type PMCs is typically constrained to a tetragonal (isobilateral) shape and sometimes to a T-shaped or linear shape,23,24 as for example observed in the Asparagales clade.25 In contrast, in the process of simultaneous cytokinesis, cell plate formation is uncoupled from chromosomal segregation and cytokinesis occurs when both meiotic divisions are finalized. So, in this mechanism, a double ‘perpendicular’ cell plate structure is generated at the end of meiosis II.26,27 Resulting tetrads are hereby much more variable in morphology (Fig. 1), displaying both tetragonal, rhomboidal and tetrahedral arrangements.25 In general, the successive-type of cytokinesis is typically observed in monocotyledonous PMCs, whereas the simultaneous-type is characteristic for dicotyledonous male meiocytes.28-30
Figure 1. Schematic overview of successive and simultaneous type of cytokinesis in plant male meiosis and the corresponding morphological variation of the resulting tetrads.
Cytological Mechanisms of Male Meiotic Cell Plate Formation
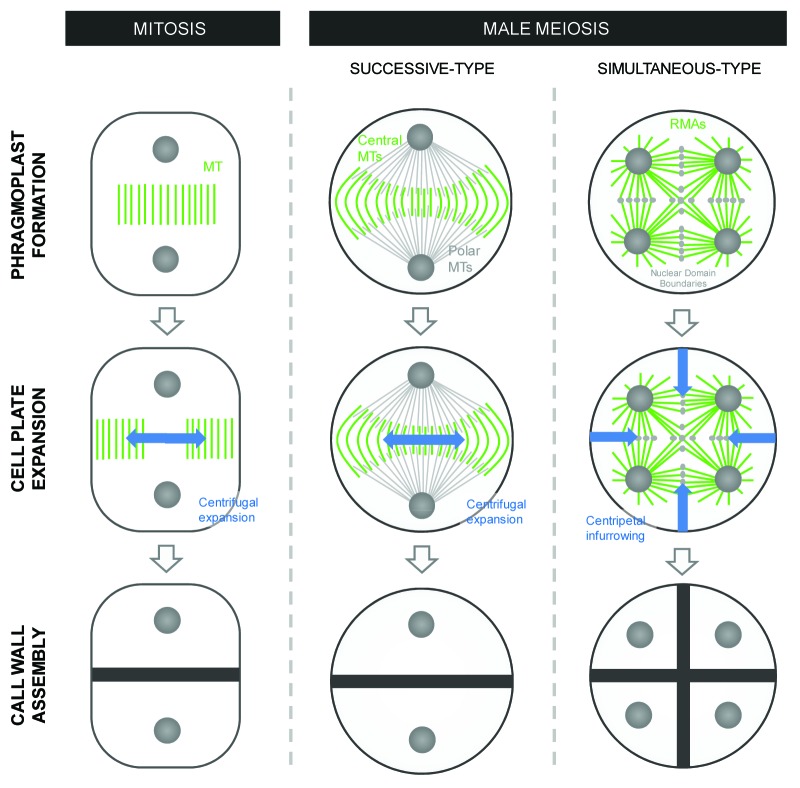
From a mechanistic point of view, cell plate formation in successive-type male meiocytes follows a similar pattern as observed in ‘conventional’ cytokinesis.31 Indeed, by studying cytoskeletal rearrangements during meiotic telophase I in a number of monocotyledonous plant species, Shamina et al. (2007) demonstrated that the successive type of meiotic cytokinesis is directed by a centrifugally expanding phragmoplast and that this mobile phragmoplast directly guides vesicle-mediated deposition of cell wall components to generate the cell plate.32 However, compared with normal “conventional” cell plate formation, meiotic cytokinesis displays some alterations, more specifically in the establishment and dynamics of the MT structures.32 First, the position of the meiotic cell plate is not pre-defined by a pre-prophase band,33 but instead is determined by the MI spindle structure, more specifically by the central spindle fibers. These MT fibers are installed at mid-prometaphase and, upon separation of homologous chromosomes, are not removed, but instead remain positioned at the meiocyte’s midzone. During MI cytokinesis these central spindle fibers form a phragmoplast-like MT structure that, supplemented with and spatially guided by polar MT bundles, surrounds the growing cell plate and mediates centrifugal cell wall expansion (Fig. 2). As such, Shamina et al. (2007) stated that the position of the MI cell plate depends on the spatial organization of the MI spindle apparatus and the localization of the MI nuclear poles.32 In support of this, the absence of a central bipolar spindle in maize dv PMCs (e.g., chaotic, monopolar and non-polar spindle figures) often results in a complete loss of meiotic cell plate formation, eventually yielding multinuclear monads that occasionally display randomly positioned cell wall fragments.34 Similarly, other defects in MI spindle formation, such as the unequal distribution of free MT bundles between the two MI half-spindles and alterations in anti-parallel spindle connection (e.g., the “disconnected” spindle phenotype in wide F1 hybrids) typically leads to MI cytokinetic abnormalities, including loss of cell wall establishment and the formation of short daughter membranes (e.g., incisions).32
Figure 2. Microtubule array formation and direction of cell wall formation in “conventional” cytokinesis and in male meiosis I and II of respectively successive and simultaneous type PMC cytokinesis. The newly formed cell plate and associated deposition of transient callose is presented in blue. Phragmoplast and RMA microtubule structures are indicated in green and polar MT bundles are shown in gray.
A second feature of successive PMC cytokinesis, not observed in conventional somatic cytokinesis, is the increased circumference (increased number of MTs) and progressive curvature of the phragmoplast MT fibers during centrifugal movement.32 This increased circumference and associated MT accumulation is not attributed to MT recycling within the phragmoplast, as observed in mitotic cells,18 but instead is caused by the polymerization of new MTs emanating from the polar regions of the telophase spindle structure. These newly formed MTs grow toward the equator, closely associating with the pre-existing central phragmoplast fibers, and connect with the opposite polar MTs to replicate the midzonal phragmoplast structure. This polar-based MT supply not only increases the cytoskeletal MT amount of the expanding phragmoplast, but also constitutes the basis for the C-curved shape of the phragmoplast edge fibers (Fig. 2). The antiparallel connection at the equator region combined with the progressive expansion of the polar MT fibers is hereby suggested to be the driving force inducing the enhanced curvature of the phragmoplast MT fibers.32
Based on these unique features, the cytological mechanism underlying centrifugal movement of the phragmoplast in the successive-type PMCs is thought to be differentially regulated as compared with mitotic cell division. In ‘conventional’ somatic cytokinesis, lateral phragmoplast expansion is mediated through dynamic MT fiber processing; e.g., by degrading centrally located MTs and emergence of new MTs at the external side (Fig. 2). This MT-driven lateral expansion is most likely guided by a pre-established cytoskeletal array, composed of actin fibers that connect the phragmoplast midzone with the cortical division plane.35 In meiotic cell division, however, no such actin structures are present36,37 and phragmoplast MTs do not show an outward polymerization-depolymerization process. Based on these findings, Shamina et al. (2007) speculated that centrifugal phragmoplast movement in successive-type PMCs is analogous to that of anaphase-B in animal cell division.32
In contrast to the successive-type of cytokinesis, the simultaneous-type strongly differs from somatic cytokinesis as neither a preprophase band (PPB)33 nor a classical phragmoplast structure is formed.35,38-41 Moreover, in contrast to the centrifugally directed outgrowth of the cell plate in somatic and successive-type PMC cells, cell plates in these PMCs typically show an inward-oriented infurrowing of the callosic parental wall to partition the meiotic cytoplasm.30,31,39,42 This centripetal ingrowth process is mediated by and directed along phragmoplast-like MT structures, called radial microtubule arrays (RMAs), which physically demarcate the meiocyte’s nuclear cytoplasmic domains (NCDs) and define the position of the future cell wall.38,43 RMAs are formed by the interaction of actin filaments and microtubuli that emanate from the microtubule organizing centers (MTOCs) localized on the outer surface of the telophase II nuclei.41,44 Hence, subsequent formation of the meiotic cell plate is not fixed to pre-defined positions on the parental wall, but rather is imposed by the spatial positioning of the nuclei at the end of MII.16,37,45,46 This is well demonstrated by several Arabidopsis meiotic mutants, in which post-meiotic cleavage plane formation is not constituted by a pre-defined conformation pattern, but instead always occurs in between the gametophytic nuclei formed,47 generating polyads instead of the normal tetrads. As such, the simultaneous-type of meiotic cytokinesis constitutes a special type of cell plate formation, in which the position of the cell plate is mediated by the location of the newly formed daughter nuclei and not through a predefined cytoskeletal imprinting (e.g., PPB). A similar mechanism of cell plate formation also occurs in the nuclear endosperm, more specifically at the moment of cellularization.35,48 Indeed, in nuclear endosperms, initial development is comprised of several successive cycles of nuclear division without cytokinesis, generating a syncytial group of nuclei that reposition to the cortical cytoplasm. Next, similar as in simultaneous-type PMCs, MT arrays radiate from MTOCs on the nuclear envelope and interdigitate at the equator regions, defining the cellular boundaries and NCDs of the syncytial nuclei.49 Cell plate formation mediated by these RMA structures then finally leads to the assembly of cell walls between both sister and non-sister nuclei (e.g., cellularization). Strikingly, in another syncytial plant organ, namely the female gametophyte (embryo sac with eight nuclei), ‘non-conventional’ RMA-mediated cell plate formation only occurs between non-sister nuclei, whereas a conventional cytokinesis event (spindle-derived interzonal MTs) occurs between sister chromatids.35,50 Thus, in a more general perspective, RMA-based cell plate formation in simultaneous-type PMCs is not a unique biological process, but instead can be considered as a general type of cytokinesis, specifically occurring in multinuclear coenocytic cells.
Callose Deposition at the Meiotic Cell Plate
A characteristic feature of meiotic cytokinesis is the abundant presence of callose. Although deposition of callose is also observed in mitotic cell division, more specifically as a transient intermediate in de novo cell plate formation,19,51 in meiotically dividing cells callose is placed both at the division site as well as at the outer cell wall.52 Outer PMC callose deposition initiates at prophase I and progressively continues during the meiotic cell cycle, resulting in a thick callosic cell wall at the end of MII.53 A similar callose deposition has also been observed in female meiocytes,54 suggesting that the callosic cell wall functions as a molecular filter to isolate developing PMCs and MMCs from the surrounding tissue, enabling the specific differentiation and programmed development of the enclosed meiocytes.53 In addition, meiotic deposition of callose is also essential for subsequent spore development, and more specifically for the establishment of a proper pollen cell wall.55 In male sporogenesis, the outer callose wall of newly formed microspores constitutes a basic framework for the deposition of new cell wall components (e.g., sporopollenin) and, upon degradation, provides components essential for the synthesis of the outer pollen wall layer; e.g., the exine. In line with this, loss of meiotic callose deposition typically leads to an abortion of microspores and associated gametophytic sterility.56-58
Besides its functional role in microspore development, callose deposition at PMC cytokinesis also strongly influences pollen morphology and aperture pattern ontogeny.59,60 Albert et al. (2011) hereby found that PMC cytokinetic callose deposition is a two-step process, in which transient callosic cell plates are formed first, as an intermediate structure for cell wall formation, whereas callosic plugs are deposited later at specific places on the outer tetrad wall to specify the position and the pattern of the pollen apertures.61
Molecular Regulation of PMC Cytokinesis
In contrast to somatic cytokinesis, little is yet known about the molecular mechanism(s) underlying meiotic cell plate formation. Most cytokinesis-defective mutants (e.g., hyd1, knolle, kor, keule) are seedling-lethal, impeding the analysis of putative meiosis-specific functionalities.62,63 It is suggested that the construction of the cell wall in both mitotic and meiotic cells is dependent on or shares similar molecular mechanisms, however, both cytological and genetic studies in Arabidopsis have revealed some distinct alterations in molecular regulation. For example, immunocytological examination demonstrated that KNOLLE, a cytokinesis-specific syntaxin protein63 that normally localizes to the newly formed cell plate in mitotically dividing cells, is not required for male meiotic cell plate formation.64 As such, it was suggested that -at least partially- different cell plate forming mechanisms operate in both types of cell division.
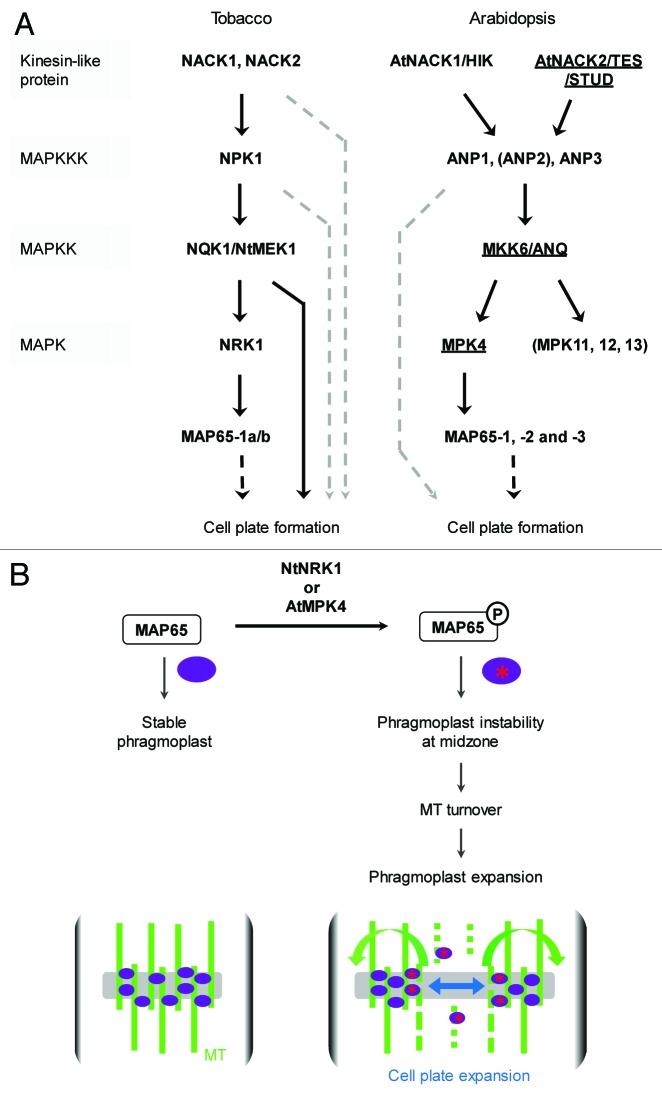
Forward genetic screens in Arabidopsis have resulted in the isolation and characterization of several mutants showing clear defects in male meiotic cytokinesis; e.g., tes, stud, anq1/mkk6 and mpk4.65-68 All these mutants show normal meiotic chromosome segregation but exhibit a complete failure of cytokinesis at the end of male meiosis and consequently generate large tetraspores harboring four haploid nuclei.26 Molecular identification of tes and stud revealed that they are two allelic variants of the same gene, namely TES/STUD/AtNACK2. The corresponding protein has an N-terminal domain homologous to kinesin motor proteins and shares sequence similarity with a small number of plant kinesins, including the Arabidopsis HINKEL (HIK) protein and NACK1 and NACK2 from tobacco.43,69-71 Since all these proteins are involved in cytoskeletal MT organization during mitotic cytokinesis, TES was suggested to function as a PMC-specific MT-associated motor protein, playing an essential role in the regulation of meiotic RMA formation and/or stability.43
Similar to HIK/AtNACK1, ANQ1/MKK6 and MPK4 are both components of the MAPK signaling cascade that regulates somatic cytokinesis in Arabidopsis. Indeed, using in vitro expression assays in yeast, Takahashi et al. (2010) demonstrated that cytokinesis is controlled by a pathway that consists of ANP MAPKKKs, that can be activated by HIK, and which in their turn activate downstream ANQ1/MKK6 MAPKK, with MPK4 MAPK being the presumed target of ANQ1/MKK672 (Fig. 3A). Consequently, functional loss of one of these proteins, such as MPK4 and ANQ1, causes severe defects in somatic cell plate formation and leads to dwarfism and stunted growth.73-75 In addition, loss of MPK4 and ANQ1 in Arabidopsis also induces defects in male meiotic cell plate formation, similar as observed in tes.67 As such, it is now generally conceived that TES, as a functional homolog of HIK, together with MPK4 and ANQ1/MKK6 constitute a similar MAPK signaling pathway that mediates post-meiotic RMA and cell plate formation in Arabidopsis74 (Fig. 3A).
Figure 3. Cytokinesis in plants is regulated by a distinct MAPK signaling pathway. (A) Schematic overview of all the components involved in the MAPK signaling cascade that mediates de novo cell plate formation in tobacco and Arabidopsis cells. Proteins with a proven function in male meiotic cytokinesis are underlined. Full black arrows represent phosphorylation steps, dotted arrows indicate for an indirect or not fully known regulatory pathway. Figure based on previously published network figures.67,72 (B) Putative model for the downstream regulatory cascade mediating MAPK-activated cell plate expansion in both “conventional” and PMC cytokinesis.
The exact mechanism by which this MAPK signaling cascade initiates and regulates cell plate formation in PMCs is yet unknown. However, recent studies have demonstrated that the downstream MAPK actor MPK4 is able to phosphorylate three members of the microtubule-associated protein MAP65 family; e.g., MAP65-1, -2 and -3.68,76 Similarly, in the orthologous tobacco NACK-PQR pathway, the MPK4 ortholog NRK1/NTF6 was found to phosphorylate MAP65-1.77 MAP65s are MT-associated proteins that are involved in the dynamic organization and structural positioning of MTs at distinct sites during the cell cycle.78 More specifically, AtMAP65-1 promotes tubulin polymerization and microtubule nucleation79 and additionally drives microtubule cross-bridging between parallel or antiparallel aligned MT to induce the formation of large MT bundles, making them more resistant to cold and MT-destabilizing drugs.78,80-82 Similarly, MAP65-3 controls MT stabilization by selectively cross-linking antiparallel interdigitating microtubules (IMTs) toward their plus ends.83,84 For MAP65-2, less is known about the specific function in MT dynamics. GFP expression studies and genetic analysis in somatic cells demonstrated that both MAP65-1, -2 and -3 localize at the phragmoplast76,81,85 and are essential for phragmoplast formation and cell plate assembly.86 Although subcellular localization and functional assessment of these MAP65s has not been performed in developing PMCs, the strong homology with somatic cytokinesis suggests that MPK4 most likely controls meiotic cell plate formation through the phosphorylation of MAP65s. A more detailed mechanistic insight into MAPK-mediated cytokinesis comes from Sasabe et al. (2006) who found that phosphorylated NtMAP65-1 accumulates during the late M phase of the cell cycle (at the phragmoplast) and that phosphorylation of NtMAP65-1 by NRK1/NTF6 reduces its MT-bundling activity in vitro.77 Thus, in contrast to its presumed promotive function in MT bundling, it is now assumed that MAPK-mediated phosphorylation of MAP65-1 and other MAP65s enhances the destabilization and turnover of MTs at the equator of the phragmoplast, thereby mediating or facilitating the expansion of the phragmoplast at the end of the cell cycle (Fig. 3B).
Another protein involved in male meiotic RMA formation in Arabidopsis is SEPARASE (AESP). AESP is a caspase family protease that is required for the proteolytic cleavage of the cohesin complex at the metaphase-to-anaphase transition and the release of sister chromatid cohesion during meiosis and mitosis.87 Interestingly, besides a persisted chromatid cohesion and aberrant chromosome segregation, aesp meiocytes (e.g., RNAi and the conditional rsw4 mutant) also show clear alterations in telophase II RMA formation, including reduced MT extension, partial phragmoplast formation and complete loss of RMA establishment.88,89 In addition, aesp PMCs often generate microspores with multiple nuclei, clearly indicating for a defect in meiotic cytokinesis. Since these alterations are not in observed other chromosome segregation-defective mutants (e.g., Arabidopsis syn1 and ask1 and maize dv),47,90,91 AESP is thought to play a pivotal role in PMC cytokinesis, and more specifically in the formation of the RMA. In addition,89 speculated that AESP regulates the maintenance of PMC cell polarity, most presumably through the control of cyclin levels. However, a clear connection between AESP activity and meiotic RMA formation remains to be established.
Two proteins have been found to be specifically required for the deposition of callose at the developing cell plate in male meiotic cytokinesis: glucan synthase-like GSL1 and GSL5. Indeed, cytological analysis of double gsl1/gsl1 gsl5/+ and gsl1/+ gsl5/gsl5 mutants not only revealed defects in pollen grain development, but additionally displayed loss of callose at newly formed PMC cell plates, without affecting the deposition of callose at the outer wall. Moreover, since the resulting microspores were occasionally larger and multinucleate, similar as observed in the cytokinesis-defective tes and mpk4, the deposition of callose at the male meiotic cell plate appears to be essential for normal PMC cytokinesis and haploid spore formation. Single gsl1 or gsl5 mutants, on the other hand, do not show any defect in meiotic callose deposition or microspore development. As such, Enns et al. (2005) concluded that GSL1 and GSL5 are both redundantly required for the deposition of callose at PMC cell plates and for the proper separation of the four daughter cytoplasms.57
Glucan synthase-like proteins, also termed callose synthases (CalSs) have been shown to interact with phragmoplastin, annexins, Rop1, sucrose synthase (SuSy) and UGT1 to form a functional complex that mediates callose deposition in a wide set of cell or organ types.92,93 Proteomics studies revealed that annexin-like proteins may modulate CalS activity94 whereas SuSy could be involved in providing primer substrate for callose synthesis.95 The Arabidopsis genome encodes 12 putative callose synthases (CalS1-12) and most have been found to mediate the deposition of callose in one or more specific organs or cell types; e.g., the cell plate, pollen tube, plasma membrane, plasmodesmata, etc. As such, GSL5 is not only required for the callosic cell wall in dividing PMCs but also mediates deposition of callose in developing pollen and wounded tissues (e.g., papillae).96 Moreover, GSL5 expression is highly induced in leaves subjected to wounding, pathogen infection and SA treatment.97
Impact of Temperature Stress on PMC Cell Plate Formation
Recent publications have demonstrated that meiotic cell plate formation, both in successive- and simultaneous-type PMCs, is extremely sensitive to temperature stress, and more particularly to cold stress. Tang et al. (2011) for example found that cold-induced pollen lethality in a wheat thermo-sensitive genic male sterile (TGMS) line is caused by an abnormal separation of dyads during male meiosis I, indicating for a specific defect in male cytokinesis.98 Histological studies hereby revealed that low temperatures alter the formation of the MI phragmoplast, leading to severe defects in cell plate assembly and meiocyte development. A more in depth cytological examination demonstrated that cold stress specifically affects the typical Chinese lantern-shaped actin MI phragmoplast structure.99 In addition, transcriptome studies revealed that cold stress dramatically represses the expression of many structural cytoskeleton-associated genes, including profilin, ADF (actin-depolymerizing factor), myosin and formins.98 Hence, sensitivity of meiotic cytokinesis to low temperature stress appears to be mediated by transcriptomic alterations of genes that play key roles in the dynamic organization of the actin cytoskeleton. Similar to TGMS wheat, cold also induces defects in meiotic cell plate formation in the simultaneous-type PMCs of Arabidopsis.100 Cytological examination hereby revealed that short periods of low temperature stress specifically alter the formation the microtubular RMAs at telophase II and hence cause defects in meiotic cytokinesis and cell wall formation, yielding restituted meiocytes that contain bi- and polynuclear spores. However, in contrast to the TGMS wheat line, the Arabidopsis cold-induced multinuclear spores do not abort, but instead display nuclear fusion before pollen mitosis I (PMI), eventually generating diploid and polyploid pollen grains. Thus, in plants showing simultaneous type of meiotic cytokinesis, cold stress may lead to sexual polyploidization, yielding polyploid offspring with higher genetic plasticity and phenotypic adaptability to cope with adverse climate conditions. In support of this hypothesis, also in Brassica, a substantial increase in 2n male gamete formation was observed under low temperature conditions, but the mechanism by which these cold-induced gametes are formed is not yet known.101
In contrast to temperature-affected MT figures in somatic cells, cold-affected RMA structures in Arabidopsis PMCs are not reassembled upon transfer to normal conditions, but instead persist and lead to defects in cell plate formation, similar as observed in the Arabidopsis mutants tes and mpk4. As such, one could presume that the cold-sensitivity of meiotic RMAs is not based upon physical or structural features, but instead is regulated on the molecular level, for example, through alterations in MAPK signaling. Initial studies hereby did not show any regulatory involvement of TES, ANQ, MPK4 or MKK2; a cold-induced kinase that positively regulates MPK4 activity.100 However, more in depth studies are needed to fully examine the molecular mechanism underlying the cold sensitivity of PMC cytokinesis.
Conclusions and Future Perspectives
The formation of the cell plate in plant male meiocytes is a unique biological process that shares both cytological and molecular features with somatic cytokinesis. The large variability in meiotic tetrad forms together with the different cytoskeletal dynamics observed in successive- and simultaneous-type PMCs demonstrates that male meiotic cytokinesis has adopted many forms and may be regulated differently between different plant species and/or clades. Although during the last years some regulatory cues have been revealed (e.g., MAPK signaling cascade), many essential components are still unknown, withholding us from a complete insight into the molecular regulation of PMC cytokinesis. As such, future research needs to be focused on the identification and elucidation of both molecular and environmental factors underlying the specificity and variability in male meiotic cell plate formation in plants.
Acknowledgments
This work was supported by the Fund for Scientific Research, Flanders (PhD fellowship to N.D.S. and grant no. G.0067.09N).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23394
References
- 1.Söllner R, Glässer G, Wanner G, Somerville CR, Jürgens G, Assaad FF. Cytokinesis-defective mutants of Arabidopsis. Plant Physiol. 2002;129:678–90. doi: 10.1104/pp.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–77. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- 3.Wu JQ, Bähler J, Pringle JR. Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol Biol Cell. 2001;12:1061–77. doi: 10.1091/mbc.12.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliferenko S, Chew TG, Balasubramanian MK. Positioning cytokinesis. Genes Dev. 2009;23:660–74. doi: 10.1101/gad.1772009. [DOI] [PubMed] [Google Scholar]
- 5.Dhonukshe P, Samaj J, Baluska F, Friml J. A unifying new model of cytokinesis for the dividing plant and animal cells. Bioessays. 2007;29:371–81. doi: 10.1002/bies.20559. [DOI] [PubMed] [Google Scholar]
- 6.Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annu Rev Cell Dev Biol. 2012;28:29–58. doi: 10.1146/annurev-cellbio-101011-155718. [DOI] [PubMed] [Google Scholar]
- 7.Balasubramanian MK, Bi E, Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol. 2004;14:R806–18. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Wu JQ, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell. 2003;5:723–34. doi: 10.1016/S1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 9.Bringmann H, Hyman AA. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–4. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- 10.Bi E. Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct Funct. 2001;26:529–37. doi: 10.1247/csf.26.529. [DOI] [PubMed] [Google Scholar]
- 11.Müller S, Wright AJ, Smith LG. Division plane control in plants: new players in the band. Trends Cell Biol. 2009;19:180–8. doi: 10.1016/j.tcb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–66. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 13.Granger CL, Cyr RJ. Microtubule reorganization in tobacco BY-2 cells stably expressing GFP-MBD. Planta. 2000;210:502–9. doi: 10.1007/s004250050037. [DOI] [PubMed] [Google Scholar]
- 14.Assaad FF. Plant cytokinesis. Exploring the links. Plant Physiol. 2001;126:509–16. doi: 10.1104/pp.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seguí-Simarro JM, Austin JR, 2nd, White EA, Staehelin LA. Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell. 2004;16:836–56. doi: 10.1105/tpc.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RC, Lemmon BE. The cytoskeleton and spatial control of cytokinesis in the plant life cycle. Protoplasma. 2001;215:35–49. doi: 10.1007/BF01280302. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme D, Inzé D, Russinova E. Vesicle trafficking during somatic cytokinesis. Plant Physiol. 2008;147:1544–52. doi: 10.1104/pp.108.120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jürgens G. Cytokinesis in higher plants. Annu Rev Plant Biol. 2005;56:281–99. doi: 10.1146/annurev.arplant.55.031903.141636. [DOI] [PubMed] [Google Scholar]
- 19.Verma DPS. Cytokinesis and building of the cell plate in plants. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:751–84. doi: 10.1146/annurev.arplant.52.1.751. [DOI] [PubMed] [Google Scholar]
- 20.Baluška F, Menzel D, Barlow PW. Cytokinesis in plant and animal cells: endosomes ‘shut the door’. Dev Biol. 2006;294:1–10. doi: 10.1016/j.ydbio.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Samuels AL, Giddings TH, Jr., Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol. 1995;130:1345–57. doi: 10.1083/jcb.130.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baluska F, Liners F, Hlavacka A, Schlicht M, Van Cutsem P, McCurdy DW, et al. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma. 2005;225:141–55. doi: 10.1007/s00709-005-0095-5. [DOI] [PubMed] [Google Scholar]
- 23.Nadot S, Furness CA, Sannier J, Penet L, Triki-Teurtroy S, Albert B, et al. Phylogenetic comparative analysis of microsporogenesis in angiosperms with a focus on monocots. Am J Bot. 2008;95:1426–36. doi: 10.3732/ajb.0800110. [DOI] [PubMed] [Google Scholar]
- 24.Nunes ELP, Bona C, Moço MC, Coan AI. Release of developmental constraints on tetrad shape is confirmed in inaperturate pollen of Potamogeton. Ann Bot. 2009;104:1011–5. doi: 10.1093/aob/mcp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penet L, Nadot S, Ressayre A, Forchioni A, Dreyer L, Gouyon PH. Multiple developmental pathways leading to a single morph: monosulcate pollen (examples from the Asparagales) Ann Bot. 2005;95:331–43. doi: 10.1093/aob/mci030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YN, et al. Microtubule organization during successive microsporogenesis in Allium cepa and simultaneous cytokinesis in Nicotiana tabacum. Biol Plant. 2011;55:752–6. doi: 10.1007/s10535-011-0181-9. [DOI] [Google Scholar]
- 27.Bhatt AM, Canales C, Dickinson HG. Plant meiosis: the means to 1N. Trends Plant Sci. 2001;6:114–21. doi: 10.1016/S1360-1385(00)01861-6. [DOI] [PubMed] [Google Scholar]
- 28.Rudall PJ, et al. Microsporogenesis and pollen sulcus type in Asparagales (Lilianae) Canadian Journal of Botany-Revue Canadienne De Botanique. 1997;75:408–30. doi: 10.1139/b97-044. [DOI] [Google Scholar]
- 29.Furness CA, Rudall PJ. Microsporogenesis in monocotyledons. Ann Bot (Lond) 1999;84:475–99. doi: 10.1006/anbo.1999.0942. [DOI] [Google Scholar]
- 30.Owen HA, Makaroff CA. Ultrastructure of Microsporogenesis and Microgametogenesis in Arabidopsis-Thaliana (L) Heynh Ecotype Wassilewskija (Brassicaceae) Protoplasma. 1995;185:7–21. doi: 10.1007/BF01272749. [DOI] [Google Scholar]
- 31.Ressayre A, Dreyer L, Triki-Teurtroy S, Forchioni A, Nadot S. Post-meiotic cytokinesis and pollen aperture pattern ontogeny: comparison of development in four species differing in aperture pattern. Am J Bot. 2005;92:576–83. doi: 10.3732/ajb.92.4.576. [DOI] [PubMed] [Google Scholar]
- 32.Shamina NV, Gordeeva EI, Kovaleva NM, Seriukova EG, Dorogova NV. Formation and function of phragmoplast during successive cytokinesis stages in higher plant meiosis. Cell Biol Int. 2007;31:626–35. doi: 10.1016/j.cellbi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Brown RC. (1991) The cytokinetic apparatus in meiosis: control of division plane in the absence of a preprophase band of microtubules. In The cytoskeletal basis of plant growth and form (Lloyd, C.W., ed), pp. 269–273, Academic Press. [Google Scholar]
- 34.Shamina NV, Dorogova N, Trunova S. Radial spindle and the phenotype of the maize meiotic mutant, dv. Cell Biol Int. 2000;24:729–36. doi: 10.1006/cbir.2000.0533. [DOI] [PubMed] [Google Scholar]
- 35.Otegui M, Staehelin LA. Cytokinesis in flowering plants: more than one way to divide a cell. Curr Opin Plant Biol. 2000;3:493–502. doi: 10.1016/S1369-5266(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 36.Staiger CJ, Cande WZ. Microfilament Distribution in Maize Meiotic Mutants Correlates with Microtubule Organization. Plant Cell. 1991;3:637–44. doi: 10.1105/tpc.3.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traas JA, et al. The Organization of the Cytoskeleton during Meiosis in Eggplant (Solanum-Melongena (L)) - Microtubules and F-Actin Are Both Necessary for Coordinated Meiotic Division. J Cell Sci. 1989;92:541–50. [Google Scholar]
- 38.Otegui MS, Staehelin LA. Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta. 2004;218:501–15. doi: 10.1007/s00425-003-1125-1. [DOI] [PubMed] [Google Scholar]
- 39.Heese M, Mayer U, Jürgens G. Cytokinesis in flowering plants: cellular process and developmental integration. Curr Opin Plant Biol. 1998;1:486–91. doi: 10.1016/S1369-5266(98)80040-X. [DOI] [PubMed] [Google Scholar]
- 40.Wick SM. Spatial aspects of cytokinesis in plant cells. Curr Opin Cell Biol. 1991;3:253–60. doi: 10.1016/0955-0674(91)90149-S. [DOI] [PubMed] [Google Scholar]
- 41.Brown RC, Lemmon BE. Nuclear cytoplasmic domains, microtubules and organelles in microsporocytes of the slipper orchid Cypripedium californicum A Gray dividing by simultaneous cytokinesis. Sex Plant Reprod. 1996;9:145–52. doi: 10.1007/BF02221394. [DOI] [Google Scholar]
- 42.Brown RC, Lemmon BE. Microtubules Associated with Simultaneous Cytokinesis of Coenocytic Microsporocytes. Am J Bot. 1988;75:1848–56. doi: 10.2307/2444739. [DOI] [Google Scholar]
- 43.Yang CY, Spielman M, Coles JP, Li Y, Ghelani S, Bourdon V, et al. TETRASPORE encodes a kinesin required for male meiotic cytokinesis in Arabidopsis. Plant J. 2003;34:229–40. doi: 10.1046/j.1365-313X.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 44.Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N, et al. Gamma-tubulin in basal land plants: characterization, localization, and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell. 2004;16:45–59. doi: 10.1105/tpc.016501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown RC, Lemmon BE. Minispindles and Cytoplasmic Domains in Microsporogenesis of Orchids. Protoplasma. 1989;148:26–32. doi: 10.1007/BF01403988. [DOI] [Google Scholar]
- 46.Brown RC, Lemmon BE. Control of Division Plane in Normal and Griseofulvin-Treated Microsporocytes of Magnolia. J Cell Sci. 1992;103:1031–8. [Google Scholar]
- 47.Peirson BN, Bowling SE, Makaroff CA. A defect in synapsis causes male sterility in a T-DNA-tagged Arabidopsis thaliana mutant. Plant J. 1997;11:659–69. doi: 10.1046/j.1365-313X.1997.11040659.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown RC, Lemmon BE, Nguyen H, Olsen O-A. Development of endosperm in Arabidopsis thaliana. Sex Plant Reprod. 1999;12:32–42. doi: 10.1007/s004970050169. [DOI] [Google Scholar]
- 49.Otegui M, Staehelin LA. Syncytial-type cell plates: a novel kind of cell plate involved in endosperm cellularization of Arabidopsis. Plant Cell. 2000;12:933–47. doi: 10.1105/tpc.12.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell SD. The Egg Cell: Development and Role in Fertilization and Early Embryogenesis. Plant Cell. 1993;5:1349–59. doi: 10.1105/tpc.5.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen XY, Liu L, Lee E, Han X, Rim Y, Chu H, et al. The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol. 2009;150:105–13. doi: 10.1104/pp.108.133918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teng NJ, et al. Microsporogenesis and pollen development in Leymus chinensis with emphasis on dynamic changes in callose deposition. Flora. 2005;200:256–63. doi: 10.1016/j.flora.2004.12.001. [DOI] [Google Scholar]
- 53.Abramova LI, Avalkina NA, Golubeva EA, Pyzhenkova ZS, Golubovskaya IN. Synthesis and deposition of callose in anthers and ovules of meiotic mutants of maize (Zea mays) Russ J Plant Physiol. 2003;50:324–9. doi: 10.1023/A:1023866019102. [DOI] [Google Scholar]
- 54.Kuran N. Callose Localization in the Walls of Megasporocytes and Megaspores in the Course of Development of Monospore Embryo Sacs. Acta Societatis Botanicorum Poloniae. 1972;41:520–34. [Google Scholar]
- 55.Dong XY, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005;42:315–28. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 56.Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R. Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell. 1992;4:759–71. doi: 10.1105/tpc.4.7.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE. Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol. 2005;58:333–49. doi: 10.1007/s11103-005-4526-7. [DOI] [PubMed] [Google Scholar]
- 58.Chen RZ, Zhao X, Shao Z, Wei Z, Wang Y, Zhu L, et al. Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell. 2007;19:847–61. doi: 10.1105/tpc.106.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ressayre A, Raquin C, Mignot A, Godelle B, Gouyon PH. Correlated variation in microtubule distribution, callose deposition during male post-meiotic cytokinesis, and pollen aperture number across Nicotiana species (Solanaceae) Am J Bot. 2002;89:393–400. doi: 10.3732/ajb.89.3.393. [DOI] [PubMed] [Google Scholar]
- 60.Albert B, Nadot S, Dreyer L, Ressayre A. The influence of tetrad shape and intersporal callose wall formation on pollen aperture pattern ontogeny in two eudicot species. Ann Bot. 2010;106:557–64. doi: 10.1093/aob/mcq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albert B, Ressayre A, Nadot S. Correlation between pollen aperture pattern and callose deposition in late tetrad stage in three species producing atypical pollen grains. Am J Bot. 2011;98:189–96. doi: 10.3732/ajb.1000195. [DOI] [PubMed] [Google Scholar]
- 62.Zuo JR, Niu QW, Nishizawa N, Wu Y, Kost B, Chua NH. KORRIGAN, an Arabidopsis endo-1,4-beta-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell. 2000;12:1137–52. doi: 10.1105/tpc.12.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lukowitz W, Mayer U, Jürgens G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell. 1996;84:61–71. doi: 10.1016/S0092-8674(00)80993-9. [DOI] [PubMed] [Google Scholar]
- 64.Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol. 1997;139:1485–93. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hulskamp M, Parekh NS, Grini P, Schneitz K, Zimmermann I, Lolle SJ, et al. The STUD gene is required for male-specific cytokinesis after telophase II of meiosis in Arabidopsis thaliana. Dev Biol. 1997;187:114–24. doi: 10.1006/dbio.1997.8554. [DOI] [PubMed] [Google Scholar]
- 66.Spielman M, Preuss D, Li FL, Browne WE, Scott RJ, Dickinson HG. TETRASPORE is required for male meiotic cytokinesis in Arabidopsis thaliana. Development. 1997;124:2645–57. doi: 10.1242/dev.124.13.2645. [DOI] [PubMed] [Google Scholar]
- 67.Soyano T, Nishihama R, Morikiyo K, Ishikawa M, Machida Y. NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis. Genes Dev. 2003;17:1055–67. doi: 10.1101/gad.1071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosetsu K, Matsunaga S, Nakagami H, Colcombet J, Sasabe M, Soyano T, et al. The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell. 2010;22:3778–90. doi: 10.1105/tpc.110.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim AJ, Endow SA. A kinesin family tree. J Cell Sci. 2000;113:3681–2. doi: 10.1242/jcs.113.21.3681. [DOI] [PubMed] [Google Scholar]
- 70.Strompen G, El Kasmi F, Richter S, Lukowitz W, Assaad FF, Jürgens G, et al. The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle-dependent manner. Curr Biol. 2002;12:153–8. doi: 10.1016/S0960-9822(01)00655-8. [DOI] [PubMed] [Google Scholar]
- 71.Nishihama R, Soyano T, Ishikawa M, Araki S, Tanaka H, Asada T, et al. Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell. 2002;109:87–99. doi: 10.1016/S0092-8674(02)00691-8. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi Y, Soyano T, Kosetsu K, Sasabe M, Machida Y. HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:1766–76. doi: 10.1093/pcp/pcq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–20. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 74.Zeng QN, Chen JG, Ellis BE. AtMPK4 is required for male-specific meiotic cytokinesis in Arabidopsis. Plant J. 2011;67:895–906. doi: 10.1111/j.1365-313X.2011.04642.x. [DOI] [PubMed] [Google Scholar]
- 75.Krysan PJ, Jester PJ, Gottwald JR, Sussman MR. An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell. 2002;14:1109–20. doi: 10.1105/tpc.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sasabe M, Kosetsu K, Hidaka M, Murase A, Machida Y. Arabidopsis thaliana MAP65-1 and MAP65-2 function redundantly with MAP65-3/PLEIADE in cytokinesis downstream of MPK4. Plant Signal Behav. 2011;6:743–7. doi: 10.4161/psb.6.5.15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, et al. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–14. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mao TL, Jin L, Li H, Liu B, Yuan M. Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiol. 2005;138:654–62. doi: 10.1104/pp.104.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smertenko AP, Chang HY, Wagner V, Kaloriti D, Fenyk S, Sonobe S, et al. The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell. 2004;16:2035–47. doi: 10.1105/tpc.104.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Damme D, Van Poucke K, Boutant E, Ritzenthaler C, Inzé D, Geelen D. In vivo dynamics and differential microtubule-binding activities of MAP65 proteins. Plant Physiol. 2004;136:3956–67. doi: 10.1104/pp.104.051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaillard J, Neumann E, Van Damme D, Stoppin-Mellet V, Ebel C, Barbier E, et al. Two microtubule-associated proteins of Arabidopsis MAP65s promote antiparallel microtubule bundling. Mol Biol Cell. 2008;19:4534–44. doi: 10.1091/mbc.E08-04-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H, Mao T, Zhang Z, Yuan M. The AtMAP65-1 cross-bridge between microtubules is formed by one dimer. Plant Cell Physiol. 2007;48:866–74. doi: 10.1093/pcp/pcm059. [DOI] [PubMed] [Google Scholar]
- 83.Ho CMK, Hotta T, Guo F, Roberson RW, Lee YR, Liu B. Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis. Plant Cell. 2011;23:2909–23. doi: 10.1105/tpc.110.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho CMK, Lee YR, Kiyama LD, Dinesh-Kumar SP, Liu B. Arabidopsis microtubule-associated protein MAP65-3 cross-links antiparallel microtubules toward their plus ends in the phragmoplast via its distinct C-terminal microtubule binding domain. Plant Cell. 2012;24:2071–85. doi: 10.1105/tpc.111.092569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mao GJ, Chan J, Calder G, Doonan JH, Lloyd CW. Modulated targeting of GFP-AtMAP65-1 to central spindle microtubules during division. Plant J. 2005;43:469–78. doi: 10.1111/j.1365-313X.2005.02464.x. [DOI] [PubMed] [Google Scholar]
- 86.Müller S, Smertenko A, Wagner V, Heinrich M, Hussey PJ, Hauser MT. The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function. Curr Biol. 2004;14:412–7. doi: 10.1016/j.cub.2004.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Z, Makaroff CA. Arabidopsis separase AESP is essential for embryo development and the release of cohesin during meiosis. Plant Cell. 2006;18:1213–25. doi: 10.1105/tpc.105.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang XH, Boateng KA, Strittmatter L, Burgess R, Makaroff CA. Arabidopsis separase functions beyond the removal of sister chromatid cohesion during meiosis. Plant Physiol. 2009;151:323–33. doi: 10.1104/pp.109.140699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang XH, Boateng KA, Yuan L, Wu S, Baskin TI, Makaroff CA. The radially swollen 4 separase mutation of Arabidopsis thaliana blocks chromosome disjunction and disrupts the radial microtubule system in meiocytes. PLoS ONE. 2011;6:e19459. doi: 10.1371/journal.pone.0019459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang M, Ma H. Male meiotic spindle lengths in normal and mutant arabidopsis cells. Plant Physiol. 2001;126:622–30. doi: 10.1104/pp.126.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Staiger CJ, Cande WZ. Microtubule distribution in dv, a maize meiotic mutant defective in the prophase to metaphase transition. Dev Biol. 1990;138:231–42. doi: 10.1016/0012-1606(90)90193-M. [DOI] [PubMed] [Google Scholar]
- 92.Verma DPS, Hong ZL. Plant callose synthase complexes. Plant Mol Biol. 2001;47:693–701. doi: 10.1023/A:1013679111111. [DOI] [PubMed] [Google Scholar]
- 93.Hong ZL, Delauney AJ, Verma DP. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell. 2001;13:755–68. doi: 10.1105/tpc.13.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrawis A, Solomon M, Delmer DP. Cotton fiber annexins: a potential role in the regulation of callose synthase. Plant J. 1993;3:763–72. doi: 10.1111/j.1365-313X.1993.00763.x. [DOI] [PubMed] [Google Scholar]
- 95.Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–7. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, et al. An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell. 2003;15:2503–13. doi: 10.1105/tpc.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ostergaard L, Petersen M, Mattsson O, Mundy J. An Arabidopsis callose synthase. Plant Mol Biol. 2002;49:559–66. doi: 10.1023/A:1015558231400. [DOI] [PubMed] [Google Scholar]
- 98.Tang ZH, Zhang L, Yang D, Zhao C, Zheng Y. Cold stress contributes to aberrant cytokinesis during male meiosis I in a wheat thermosensitive genic male sterile line. Plant Cell Environ. 2011;34:389–405. doi: 10.1111/j.1365-3040.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 99.Xu C, Liu Z, Zhang L, Zhao C, Yuan S, Zhang F. Organization of actin cytoskeleton during meiosis I in a wheat thermo-sensitive genic male sterile line. Protoplasma. 2012 doi: 10.1007/s00709-012-0386-6. [DOI] [PubMed] [Google Scholar]
- 100.De Storme N, Copenhaver GP, Geelen D. Production of diploid male gametes in Arabidopsis by cold-induced destabilization of postmeiotic radial microtubule arrays. Plant Physiol. 2012;160:1808–26. doi: 10.1104/pp.112.208611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mason AS, Nelson MN, Yan G, Cowling WA. Production of viable male unreduced gametes in Brassica interspecific hybrids is genotype specific and stimulated by cold temperatures. BMC Plant Biol. 2011;11:103. doi: 10.1186/1471-2229-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]