Abstract
Transition metals such as Iron (Fe) and Copper (Cu) are essential for plant cell development. At the same time, due their capability to generate hydroxyl radicals they can be potentially toxic to plant metabolism. Recent works on hydroxyl-radical activation of ion transporters suggest that hydroxyl radicals generated by transition metals could play an important role in plant growth and adaptation to imbalanced environments. In this mini-review, the relation between transition metals uptake and utilization and oxidative stress-activated ion transport in plant cells is analyzed, and a new model depicting both apoplastic and cytosolic mode of ROS signaling to plasma membrane transporters is suggested.
Keywords: copper, iron, membrane transport, potassium, calcium, hydroxyl radicals, oxidative stress, toxicity, adaptation, development
Recently, reactive oxygen species (ROS) have emerged as important signaling molecules mediating a broad range of plant adaptive and developmental responses. In plant cells, both photosynthetic and respiratory electron-transport chains, as well as the NADPH oxidases and peroxidases are involved in ROS generation.1 ROS have been shown to regulate gene expression and signaling transduction pathways and, as such, can control numerous processes, like root gravitropism, hypersensitive response to pathogens, stomatal closure and cell expansion and development.1-5 During pathogen attack, programmed cell death (PCD) is induced in order to isolate cells and therefore avoiding pathogen spread. ROS play a crucial role in this signaling network.6 In many cases, ROS production is genetically programmed and is induced during development. Generation of singlet oxygen induces controlled PCD in aleurone cells, leaf senescence, tracheary elements maturation or trichome development.4
While ROS control over numerous adaptive and developmental responses is absolutely essential, a controlled balance of ROS-producing and ROS-scavenging systems must be kept to ensure an accurate execution of signaling without provoking toxicity. Several types of ROS may be formed in plant cells. These forms can be poorly reactive (non-radicals, such as H2O2 or O3) or can react extremely quickly with other free radicals such as superoxide (O2-) or hydroxyl radicals (OH·)7; see also Figure 1. The detrimental effects of ROS are a result of their ability to cause lipid peroxidation in cellular membranes, DNA damage, protein denaturation, carbohydrate oxidation, pigment breakdown and an impairment of enzymatic activity.8,9 To protect cells against oxidative injury plants use various antioxidant components a number of enzymes and low molecular weight compounds capable of quenching ROS without themselves undergoing conversion to a destructive radical.8 Antioxidant defense includes enzymatic and non-enzymatic mechanisms. The first group includes the enzymes superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione peroxidase. The second group includes cellular redox buffers such as ascorbate, glutathione (GSH), tocopherol, flavonoids, alkaloids and carotenoids.1
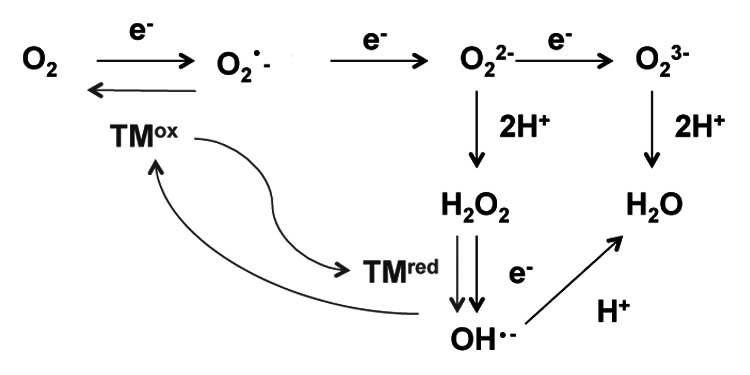
Figure 1. ROS production by multistep reduction of oxygen (adapted from refs.1 and 4). Grey lines show the Haber-Weiss reactions. TM, transition metal.
Transition metals such as iron (Fe), manganese (Mn), zinc (Zn) or copper (Cu) are classified as essential micronutrients and act as cofactors of many fundamental proteins in plant cells. Among many roles, each of them may be included as a metal component of the prosthetic group in superoxide dismutases (SOD) and, thus, plays central role in detoxification of the superoxide anion free radicals.10,11 SODs are present in all subcellular compartments: FeSODs are localized in chloroplasts, MnSODs are present in both mitochondrion and peroxisomes and Cu/ZnSODs, the type of SOD predominant in plant cells, are localized in chloroplasts, peroxisomes, cysosol and extracellular space (reviewed in ref.12). As a result, deficiency in one of these micronutrients can alter the activities of antioxidant enzymes resulting in increased susceptibility to oxidative stress.13
The standard electrode potential is an excellent predictor of both the prooxidant activity and the toxicity of metal ions.14 While Zn and Mn have no or very little reducing potential under biological conditions,15 Fe and Cu are highly redox active (and, hence, potentially toxic) metals. If they are not chelated, at every concentration they can mediate ROS production by getting oxidized in the Fenton reaction and further getting reduced in the net Haber-Weiss reactions (Fig. 1). The production of ROS by Cu and Fe ions by the Fenton reaction can occur in plant cells in presence of ascorbate or H2O2,16 leading to the production of hydroxyl radicals.7 To deal with Fe- or Cu- induced toxicity, plants need to enhance antioxidant defenses; this phenomenon has been reported for a wide range of species such as Nicotiana plumbagnifolia,17 Phaseolus vulgaris,18Ulva compressa,19 Zea mays20 or Arabidopsis thaliana.21
This essentiality/toxic duality of transition metals has resulted in the development of a complex homeostatic network for their acquisition and use in aerobic organisms.22-25 In Arabidopsis thaliana, primary root uptake of the transition metals includes members of different families: NRAMP, ZIP and COPT (Table 1). In all cases, the transport is induced under limiting conditions of the transition metals and in all cases, these transporters can mediate the influx of more than one transition metal. Interestingly, β-glucuronidase (GUS) expression pattern of these transporters reveals a possible specialization of the different root zones in the acquisition of the different essential micronutrients: AtCOPT1, responsible of high affinity copper transport, is located at root tip;26 AtNRAMP1, that mediates Mn transport, is highly expressed at the elongation zone.27 In relation to ZIP family transporters (IRT1-3 and ZIP1-4), responsible of both Fe and Zn transport, AtIRT1, responsible of Fe transport, and AtZIP4, that mediates Zn transport, are mainly located at the mature zone level.28,29
Table 1. Transporters implicated in primary uptake of TM in Arabidopsis.
| High affinity transporter | Expression under limiting conditions? | Expression pattern in roots | Main ion transported | Also transports | References | |
|---|---|---|---|---|---|---|
| AtNRAMP1 |
+ |
Plasma membrane.Higher expression at the elongation zone level |
Mn |
Fe, Co |
27,49 |
|
| AtIRT1 |
+ |
Plasma membrane. External cell layers of the mature zone. |
Fe |
Zn, Mn, Cd, Co |
28,50–51 |
|
| AtIRT2 |
+ |
Epidermis, root hairs and cortex. Absent in root apex |
Fe* |
Zn* |
52 |
|
| AtIRT3 |
+ |
Plasma membrane. Vascular tissues. Absent at the elongation zone |
Zn* |
Fe* |
53 |
|
| AtZIP1-3 |
+ |
|
Zn |
Cu (ZIP2) |
54 |
|
| AtZIP4 |
+ |
Whole roots? |
Zn* |
Cu* |
29,54 |
|
| COPT1 | + | Plasma membrane. Root apex | Cu | Ag, Mn | 26,55–56 | |
Based on expression in yeast data.
While membrane transporters are essential for acquisition of transition metals, they may also represent a downstream target of ROS signaling. The activation of plasma membrane calcium influx by ROS in plant cells has been a hot topic during the last decade resulting in multiple publications.30-37 This activation shows high spatial- and dose-dependence, and varies with the type of ROS.33-37
Of specific interest is ROS-induced K+ efflux. The latter has been reported in most of the abiotic stresses that imply ROS generation, such as copper37,38 or Al toxicity,39,40 salinity,33,42 and waterlogging.43,44 The first evidence of ROS-induced activation of K+ -permeable conductance was reported in combined patch-clamped and MIFE experiments by Demidchik et al.35 The underlying molecular mechanisms were studied later in more details, by comparing ROS-induced activation of K+ currents and fluxes between Arabidopsis wild type and gork1-1 mutants. This study revealed that, similarly to animal cells, hydroxyl radicals-activated K+ channels are involved in programmed cell death in plant cells.33 Recently, Lahoavist et al.36 showed that the hydroxyl-radical activated Ca2+ and K+ conductance in Arabidopsis is mediated by annexin1 at the mature and elongation zone levels. In all these studies, the hydroxyl radical generation occurred at the external side of the plasma membrane, and under non-physiological (1mM of the hydroxyl-radical generation mixture Cu/Asc) conditions.
Using a range of Arabidopsis loss- or gain- of Cu transport function mutants,37 we have recently showed that copper transport into cytosol in root apex results in generation of hydroxyl radical at the cytosolic side, with a consequent regulation of plasma membrane OH•-sensitive Ca2+ and K+ transport systems. Based on stoichiometry between Ca2+ and K+ fluxes and pharmacological experiments evidence, non-selective cation channels (NSCC) have been suggested as a possible target. Such cytosolic activation of NSCC by hydroxyl radicals has been previously shown for animal cells45 but not in plant cells. Keeping in mind high tissue-specificity of expression patterns of transporters mediating uptake of transition metals into plant roots (see Table 1), this finding may explain specificity or ROS effects in different root tissues, both in adaptive5,46,47 and developmental4,48 context.
While much more work is needed to completely understand the catalytic role of these transient metals in ROS-mediated responses in plants, and their impact on intracellular ionic homeostasis, there is no doubt that our current models depicting ROS generation under stress conditions in plant roots must be updated to include both apoplastic and cytosolic modes of action (Fig. 2). This model also highlights a complex role transition metals play in ROS generation and signaling in plants. On one hand, they are essential components of plant antioxidant defense system and, as such, are involved in ROS scavenging (e.g., as a part of SOD). On the other hand, both Cu and Fe may be directly involved in ROS production, both in the apoplast and the cytosol (Fig. 2). This “double sward” action should be finely balanced to optimize plant adaptive and developmental performance. The fine print of this balancing process must be a subject of dedicated research in the future. (Ref. 41; Fig. 3)
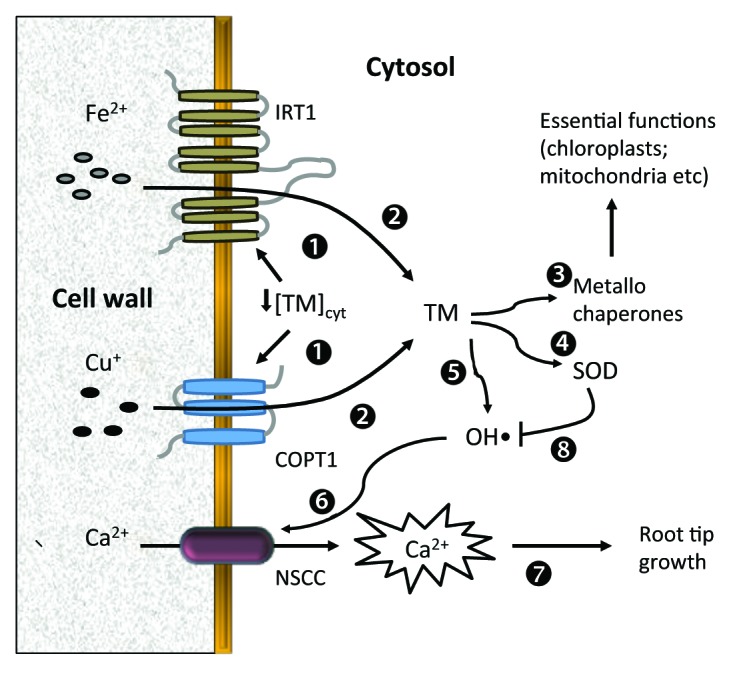
Figure 2. Suggested model for hydroxyl radical generation by transition metals and activation of ion transport systems under low copper or iron conditions. Low transition metal (TM) concentrations in the cytosol induce activity of the plasma membrane high affinity COPT1 and IRT1 transporters.1 These transporters mediate Cu and Fe transport into the cytosol.2 Cytosolic TM are then transported to metallochaperones for essential functions in major plant organelles.3 as well as to cytosolic-located SOD enzymes.4 A small part of the TM generates moderate OH• in the cytosol5 that will activate Ca2+ entry through NSCC6 at the root tip level.37 This cytosolic calcium increase is essential for root tip growth.7,48 Abbreviations: COPT1, high affinity copper transporter 1; IRT-3, iron-regulated transporter 1; NSCC, non-selective cation channel; SOD, superoxide dismutase.
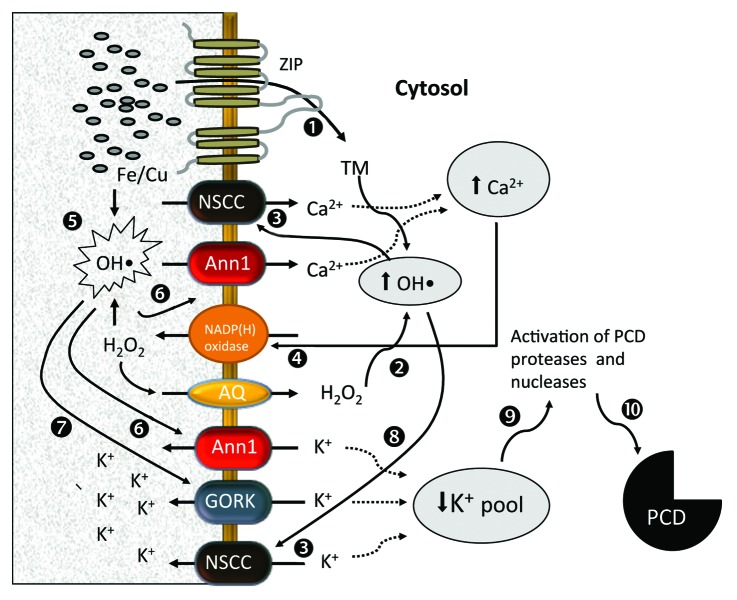
Figure 3. Hydroxyl radical generation and activation of ion transport systems under high copper or iron conditions. When TM are present in excessive quantities in soil solution, they are transported into cytosol through ZIP transporters1 where they will interact with cytosolic H2O2 to generate OH•.2 The latter will activate NSCC-mediated Ca2+ uptake3 in root tips.37 The resultant increase in cytosolic Ca2+ pool will further increase net Ca2+ uptake into cytosol via positive feedback regulation of NADP(H)oxidase57 and production of extracellular H2O2.4 The latter may interact with TM in the cell walls in the presence of ascorbic acid, to produce substantial quantities of OH• in apoplast.5 This apoplastic OH• could activate ANN1-mediated Ca2+ and K+ conductances6 at both mature and elongation zones36 resulting in further increase in cytosolic Ca2+ (hence, a positive feedback loop) and decline in cytosolic K+ pool. Apoplastic peroxide produced as a result of NADP(H) oxidase stimulation will be also transported in the cytosol via aquaporins, further increasing cytosolic OH• levels by interacting with TM.2 Apoplastic OH• generation will also stimulate K+ leak via outward-rectifying K+ GORK channel,7,33 further reducing cytosolic K+ pool. OH• generated in the cytosol will also contribute to this process by activating NSCC from cytosolic site.8,37 The massive decrease in cytosolic K+ pool (mediated by Ann1, GORK and NSCC channels) will result in activation of various proteases and nucleases,9,33,42,58 leading to programmed cell death.10 Abbreviations: ZIP, Zrt-, Irt-like protein; NSCC, non-selective cation channel; ANN1, Annexin 1; NADP(H), oxidase (Nicotinamide Adenine Dinucleotide Phosphate-Oxidase); PCD, programmed cell death; AQ, aquaporin.
Acknowledgments
This work has been supported by the Spanish MICINN (Projects BFU2007-60332 and BFU2010-14873). A.R-M. acknowledges her PhD fellowship from Ministerio de Ciencia e Innovación (BES-2008-005096). S.S. acknowledges financial support from the Australian Research Council. The authors would like to thank Lola Peñarrubia for their critical revision of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23425
References
- 1.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 2.Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld JP. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 2004;134:1006–16. doi: 10.1104/pp.103.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006;28:1091–101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- 5.De Pinto MC, Locato V, De Gara L. Redox regulation in plant programmed cell death. Plant Cell Environ. 2012;35:234–44. doi: 10.1111/j.1365-3040.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 6.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–22. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–79. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 10.Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F. Function of nutrients: Micronutrients In: Marschner H ed, Mineral Nutrition of Higher Plants. 3rd ed. London: Academic Press., 2012: 191-248. [Google Scholar]
- 11.Shabala S. Metal cations in CO2 assimilation and conversion by plants. J Met. 2009;61:28–34. [Google Scholar]
- 12.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331–41. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- 13.Yu Q, Osborne L, Rengel Z. Micronutrient deficiency changes activities of superoxide dismutase and ascorbate peroxidase in tobacco plants. J Plant Nutr. 1998;21:1427–37. doi: 10.1080/01904169809365493. [DOI] [Google Scholar]
- 14.Kinraide TB, Poschenrieder C, Kopittke PM. The standard electrode potential (Eθ) predicts the prooxidant activity and the acute toxicity of metal ions. J Inorg Biochem. 2011;105:1438–45. doi: 10.1016/j.jinorgbio.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Crichton RR. An overview of metals and selected nonmetals in Biology. In: Crichton ed, Biological Inorganic Chemistry. 2nd ed. Oxford: Elsevier, 2012:1-19. [Google Scholar]
- 16.Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J. 1998;332:507–15. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kampfenkel K, Van Montagu M, Inzé D. Effects of iron excess on Nicotiana plumbagnifolia Plants (Implications to Oxidative Stress) Plant Physiol. 1995;107:725–35. doi: 10.1104/pp.107.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pekker I, Tel-Or E, Mittler R. Reactive oxygen intermediates and glutathione regulate the expression of cytosolic ascorbate peroxidase during iron-mediated oxidative stress in bean. Plant Mol Biol. 2002;49:429–38. doi: 10.1023/A:1015554616358. [DOI] [PubMed] [Google Scholar]
- 19.González A, Vera J, Castro J, Dennett G, Mellado M, Morales B, et al. Co-occurring increases of calcium and organellar reactive oxygen species determine differential activation of antioxidant and defense enzymes in Ulva compressa (Chlorophyta) exposed to copper excess. Plant Cell Environ. 2010;33:1627–40. doi: 10.1111/j.1365-3040.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- 20.Madejón P, Ramírez-Benítez JE, Corrales I, Barceló J, Poschenrieder C. Copper-induced oxidative damage and enhanced antioxidant defenses in theroot apex of maize cultivars differing in Cu tolerance. Environ Exp Bot. 2009;67:415–20. doi: 10.1016/j.envexpbot.2009.08.006. [DOI] [Google Scholar]
- 21.Drazkiewicz M, Skórzyńska-Polit E, Krupa Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals. 2004;17:379–87. doi: 10.1023/B:BIOM.0000029417.18154.22. [DOI] [PubMed] [Google Scholar]
- 22.Hall JL, Williams LE. Transition metal transporters in plants. J Exp Bot. 2003;54:2601–13. doi: 10.1093/jxb/erg303. [DOI] [PubMed] [Google Scholar]
- 23.Krämer U, Talke IN, Hanikenne M. Transition metal transport. FEBS Lett. 2007;581:2263–72. doi: 10.1016/j.febslet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Pilon M, Cohu CM, Ravet K, Abdel-Ghany SE, Gaymard F. Essential transition metal homeostasis in plants. Curr Opin Plant Biol. 2009;12:347–57. doi: 10.1016/j.pbi.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Puig S, Peñarrubia L. Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol. 2009;12:299–306. doi: 10.1016/j.pbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Sancenón V, Puig S, Mateu-Andrés I, Dorcey E, Thiele DJ, Peñarrubia L. The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J Biol Chem. 2004;279:15348–55. doi: 10.1074/jbc.M313321200. [DOI] [PubMed] [Google Scholar]
- 27.Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell. 2010;22:904–17. doi: 10.1105/tpc.109.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–33. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assunção AGL, Herrero E, Lin YF, Huettel B, Talukdar S, Smaczniak C, et al. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc Natl Acad Sci U S A. 2010;107:10296–301. doi: 10.1073/pnas.1004788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–4. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 31.Köhler B, Hills A, Blatt MR. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol. 2003;131:385–8. doi: 10.1104/pp.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rentel MC, Knight MR. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 2004;135:1471–9. doi: 10.1104/pp.104.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, et al. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci. 2010;123:1468–79. doi: 10.1242/jcs.064352. [DOI] [PubMed] [Google Scholar]
- 34.Demidchik V, Shabala SN, Davies JM. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 2007;49:377–86. doi: 10.1111/j.1365-313X.2006.02971.x. [DOI] [PubMed] [Google Scholar]
- 35.Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci. 2003;116:81–8. doi: 10.1242/jcs.00201. [DOI] [PubMed] [Google Scholar]
- 36.Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, et al. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca²+- and K+-permeable conductance in root cells. Plant Cell. 2012;24:1522–33. doi: 10.1105/tpc.112.097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigo-Moreno A, Andrés-Colás N, Poschenrieder C, Gunsé B, Peñarrubia L, Shabala S. Calcium- and potassium-permeable plasma membrane transporters are activated by copper in Arabidopsis root tips: linking copper transport with cytosolic hydroxyl radical production. Plant Cell Environ. 2012 doi: 10.1111/pce.12020. [DOI] [PubMed] [Google Scholar]
- 38.De Vos CHR, Vonk MJ, Vooijs R, Schat H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992;98:853–8. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delhaize EP, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–21. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wherrett T, Shabala S, Pottosin I. Different properties of SV channels in root vacuoles from near isogenic Al-tolerant and Al-sensitive wheat cultivars. FEBS Lett. 2005;579:6890–4. doi: 10.1016/j.febslet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 41.Madejón P, Corrales I, Barcelo J, Poschenrieder C. Copper- induced oxidative damage and enhanced antioxidant defenses in the root apex of maize cultivars differing in Cu tolerance. Environ Exp Bot. 2009;67:415–20. doi: 10.1016/j.envexpbot.2009.08.006. [DOI] [Google Scholar]
- 42.Shabala S. Salinity and programmed cell death: unravelling mechanisms for ion specific signalling. J Exp Bot. 2009;60:709–12. doi: 10.1093/jxb/erp013. [DOI] [PubMed] [Google Scholar]
- 43.Pang J, Cuin T, Shabala L, Zhou M, Mendham N, Shabala S. Effect of secondary metabolites associated with anaerobic soil conditions on ion fluxes and electrophysiology in barley roots. Plant Physiol. 2007;145:266–76. doi: 10.1104/pp.107.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shabala S. Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 2011;190:289–98. doi: 10.1111/j.1469-8137.2010.03575.x. [DOI] [PubMed] [Google Scholar]
- 45.Simon F, Varela D, Eguiguren AE, Díaz LF, Sala F, Stutzin A. Hydroxyl radical activation of a Ca2+-sensitive nonselective cation channel involved in epithelial cell necrosis. Am J Physiol Cell Physiol. 2004;287:963–70. doi: 10.1152/ajpcell.00041.2004. [DOI] [PubMed] [Google Scholar]
- 46.Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–6. doi: 10.1016/S1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- 47.Doke N. Miura. Y, Sanchez L, Kawakita K. Involvement of superoxide in signal transduction: Responses to attack by pathogens, physical and chemical shocks, and UV irradiation. In: Foyer CH, Mullineaux PM eds, Causes of photooxidative stresses and amelioration of defense systems in plants. Boca raton:CRC Press, 1994:177-198. [Google Scholar]
- 48.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci U S A. 2007;104:20996–1001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J. 2000;347:749–55. doi: 10.1042/0264-6021:3470749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henriques R, Jásik J, Klein M, Martinoia E, Feller U, Schell J, et al. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol Biol. 2002;50:587–97. doi: 10.1023/A:1019942200164. [DOI] [PubMed] [Google Scholar]
- 51.Varotto C, Maiwald D, Pesaresi P, Jahns P, Salamini F, Leister D. The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J. 2002;31:589–99. doi: 10.1046/j.1365-313X.2002.01381.x. [DOI] [PubMed] [Google Scholar]
- 52.Vert G, Briat JF, Curie C. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J. 2001;26:181–9. doi: 10.1046/j.1365-313x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- 53.Lin YF, Liang HM, Yang SY, Boch A, Clemens S, Chen CC, et al. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 2009;182:392–404. doi: 10.1111/j.1469-8137.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 54.Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, et al. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem. 2003;278:47644–53. doi: 10.1074/jbc.M309338200. [DOI] [PubMed] [Google Scholar]
- 55.Sancenón V, Puig S, Mira H, Thiele DJ, Peñarrubia L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol. 2003;51:577–87. doi: 10.1023/A:1022345507112. [DOI] [PubMed] [Google Scholar]
- 56.Andrés-Colás N, Perea-García A, Puig S, Peñarrubia L. Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiol. 2010;153:170–84. doi: 10.1104/pp.110.153676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 58.Shabala S, Cuin TA, Prismall L, Nemchinov LG. Expression of animal CED-9 anti-apoptotic gene in tobacco modifies plasma membrane ion fluxes in response to salinity and oxidative stress. Planta. 2007;227:189–97. doi: 10.1007/s00425-007-0606-z. [DOI] [PubMed] [Google Scholar]