Abstract
Abscisic acid (ABA) signal transduction during Arabidopsis seed development and germination requires a Group A bZIP transcription factor encoded by ABA INSENSITIVE5 (ABI5). In addition to the basic leucine zipper DNA binding domain, Group A bZIPs are characterized by three N-terminal conserved regions (C1, C2 and C3) and one C-terminal conserved region (C4). These conserved regions are considered to play roles in ABI5 functions; however, except for the phosphorylation site, the importance of the highly conserved amino acids is unclear. Here, we report a novel abi5 recessive allele (abi5-9) that encodes an intact ABI5 protein with one amino acid substitution (A214G) in the C3 domain. The abi5-9 plants showed ABA insensitivity during germination and could germinate on medium containing 175 mM NaCl or 500 mM mannitol. Em1 and Em6—both encoding late embryogenesis abundant (LEA) proteins and directly targeted by ABI5 regulation—were expressed at very low levels in abi5-9 plants compared with the wild type. In yeast, the abi5-9 protein exhibited greatly reduced interaction with ABI3 compared with ABI5. These data suggest that Ala214 in ABI5 contributes to the function of ABI5 via its interaction with ABI3.
Keywords: ABA, abiotic stress tolerance, abscisic acid, gene expression, growth control, hormone signaling, plant signaling, transcriptional regulation
Introduction
The sesquiterpene abscisic acid (ABA) is produced in organisms across all kingdoms.1 In land plants, it plays essential roles in adaptation to environmental stresses, such as drought,2 and in the developmental regulation of maturation3 and desiccation tolerance of seeds.4 Exogenously applied ABA is known to prevent seed germination.5,6 Genetic screening of mutagenized Arabidopsis has identified mutants that can germinate on media containing ABA.7 These ABA-insensitive (abi) mutants exhibit defects in molecular components of the ABA signal transduction machinery. Subsequent isolation of causal genes revealed that ABI1 and ABI2 encode Group A protein phosphatases type 2C (PP2Cs),8,9 while ABI3,10 ABI411 and ABI512 encode transcription factors. ABI5 encodes a bZIP transcription factor, and is dominantly expressed in seeds but not in vegetative tissues, indicating that these transcription factors specifically function in seed maturation and germination.12,13 Like drought and high-salt stress, exogenous application of ABA induces ABI5 expression in germinating embryos.14 In turn, ABI5 in concert with ABI3 regulates ABA-inducible expression of Em1 and Em6,15,16 which encode late embryogenesis abundant (LEA) proteins17 that are suggested to protect cells from desiccation.18 These ABA-induced events are greatly reduced in abi5 mutant plants,14 indicating that ABI5 is a key factor in monitoring environmental conditions upon seed germination.
ABI5 is a Group A bZIP. This group includes 13 genes present in Arabidopsis,19 and can be further divided into two sub-groups based on the conserved N-terminal domains. Nine bZIPs form the ABI5/AREB/ABF family, which each contain three N-terminal conserved regions (C1, C2 and C3) and one C-terminal conserved region (C4); the other sub-group includes four bZIPs that lack the C1 domain.20 The ABI5/AREB/ABF family is characterized by involvement in ABA signaling during seed development and germination (ABI5, EEL, DPBF2/AtbZIP67, DPBF4 and AREB3) or in vegetative tissues (AREB1/ABF2, AREB2/ABF4, ABF1 and ABF3).21-23 These bZIPs bind to ABA-responsive elements (ABREs: PyACGTGG/TC)24 of the ABA-regulated genes via the bZIP DNA-binding domain.17,25,26
Compared with the bZIP domain, less is known about the functional significance of the conserved regions of the ABI5/AREB/ABF family. The C2 and C3 domains of ABI5 reportedly interact with ABI3.27 The R-X-X-S/T motifs distributed in the conserved domains are the preferred recognition motifs for subclass III SnRK2s (SRK2D/SnRK2.2, OST1/SRK2E/SnRK2.6 and SRK2I/SnRK2.3),28,29 which are activated in response to ABA30 and are essential for ABA signal transduction in Arabidopsis.31-34 In plants, OST1/SRK2E/SnRK2.6 phosphorylates T451 in the C4 domain of ABF3, and this phosphorylation appears to be important for ABF3 stabilization.35 In contrast, the R-X-X-T motif in the C3 domain is apparently not essential for ABI5 function; expression of mutated abi5 protein, in which T201 within the R-X-X-T motif of the C3 domain was changed to Ala, can complement phenotypes of abi5-4 plants,15 indicating that the C3 domain does not mediate ABI5 function through phosphorylation.
All abi5 alleles reported to date encode premature proteins terminated in front of the bZIP domain, or are obtained from T-DNA insertion lines—except abi5-3, which has a small rearrangement adjacent to the 5′ splice site of the final exon.12,17,36,37 In the present study, we report the first abi5 recessive allele (abi5-9) that encodes a full-length ABI5 protein with one amino acid substitution in the conserved alanine (A214G) of the C3 domain. The mutant showed insensitivity to ABA and salinity, comparable to the abi5-1 null mutant. In yeast, the abi5-9 protein showed reduced ability to interact with ABI3 compared with intact ABI5. Our data demonstrate the importance of the conserved Ala in the C3 domain for the in vivo function of ABI5.
Results
Physiological characterization of a salt-tolerant mutant
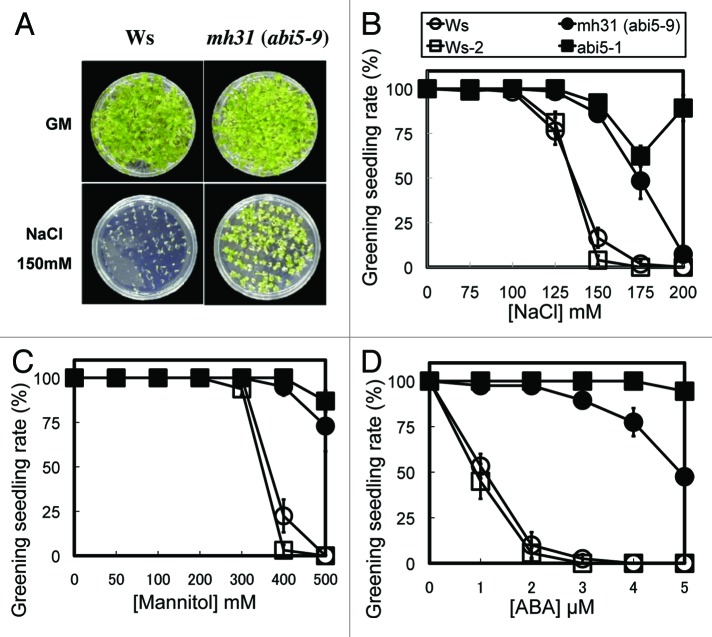
A salt-tolerant mutant (originally designated as mh31) was isolated from activation-tagged lines of Arabidopsis thaliana (Ws) that can develop green seedlings on medium supplemented with 150 mM NaCl (Fig. 1A and B). The mh31 mutant could also grow on medium containing up to 500 mM mannitol, which arrested growth of wild-type germinated embryos (Fig. 1C). Since post-germinative developmental arrest involves ABA signaling,14 we also tested the ABA sensitivity of the mh31 mutant. As expected, the germination and subsequent seedling development of the mh31 mutant was less inhibited by exogenous ABA compared with the wild type (Fig. 1D), suggesting that the mh31 mutant was also ABA insensitive. In the absence of stress treatments or exogenous ABA, we observed no significant growth difference between the mh31 mutant and the wild type (Fig. 1A).
Figure 1. Physiological analysis of the salt-tolerant mutant mh31. (A) Phenotypes of Ws and mh31 (Ws background) ; after stratification, seeds were sown on the medium, grown and then photographed. Upper left and upper right panels, respectively, show Ws and mh31 grown on GM. Lower left and lower right panels, respectively, show Ws and mh31 grown on GM supplemented with 150 mM NaCl. (B−D) Greening rates of the plants grown on GM supplemented with the indicated concentration of NaCl (B), mannitol (C) or ABA (D). Stratified seeds were sown on the plate and incubated at 23°C for two weeks under continuous light (2,000 lx). After incubation, plates were photographed. The numbers of seedlings and greening seedlings were counted, and greening seedling rates were calculated. Data represent the average of values from quadruplicate plates of 20 seeds. Bars indicate standard errors. abi5-1 is in a Ws-2 background. Open circles, closed circles, open squares and closed square indicate Ws, mh31 (abi5-9), Ws-2 and abi5-1, respectively. Greening seedling rate is expressed as the percentage of seedlings with green cotyledons (n = 20). Mean ± SE; n = 4.
mh31 is a novel abi5 allele with one amino acid substitution in the C3 domain
We performed genetic analyses to identify the causal mutated gene of the mh31 mutant. The mh31 mutant was crossed with Col-0, and the resulting F1 plants were self-pollinated to produce the next generation. This F2 population was tested for ABA sensitivity and osmotolerance (Table S1). The F2 population included 196 ABA-sensitive plants and 74 ABA-insensitive plants, suggesting the ABA-insensitive phenotype to be a recessive trait. On the other hand, an osmotolerance assay of the F2 population revealed 61 osmotolerant plants and 14 osmosensitive plants. Moreover, F1 seeds obtained by crossing mh31 (male) with Ws (female) are NaCl tolerant (Table S2), suggesting that NaCl tolerance was a dominant trait of the mh31 plants. Since the mh31 plants were not tolerant to LiCl (data not shown) but were tolerant to NaCl- and osomo-stresses (Fig. 1B and C), their NaCl tolerance was probably caused by osomotolerance. Because of the genetic nature of the mh31 mutation, the ABA-insensitive plants and osmosensitive plants were rescued from F2 seedlings and used for genetic mapping.
The mh31 mutant was originally isolated from activation-tagged lines with the single-copy T-DNA inserted in the upper arm of chromosome 1 (Fig. S1). However, rough mapping of F2 plants showed that both ABA insensitivity and osmo-intolerance had linkage to the SSLP markers CZSOD2 and NGA168 that are located on the bottom arm of chromosome 2 (Fig. S1). The chromosome region around the two markers contained ABI4 and ABI5, two important loci for ABA signal transduction during seed germination. We amplified cDNAs of both genes. Sequencing revealed that compared with wild type, the ABI4 sequence was the same in the mh31 mutant, whereas the ABI5 sequence in mh31 contained one base substitution (C to G) that resulted in an amino acid substitution (A214G) in the conserved C3 region (Fig. 2A and B). This mutation was strongly associated with the mutant phenotype (Fig. S1).
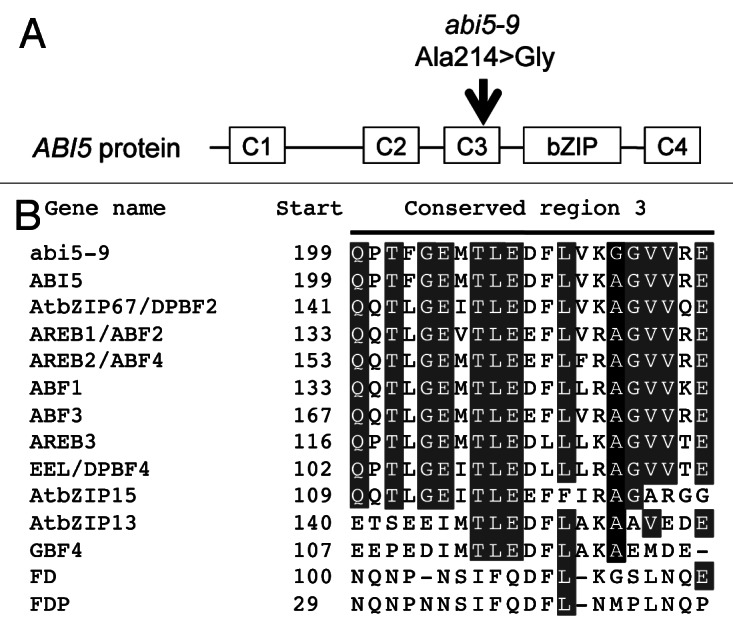
Figure 2. New abi5 allele abi5-9 and the conserved property of alanine 214 in the Arabidopsis Group A bZIPs. (A) Position of the abi5-9 mutation. Schematic structure of the ABI5 protein is presented with boxes. C1, C2, C3, C4 and bZIP indicate conserved region 1, conserved region 2, conserved region 3, conserved region 4 and basic DNA binding domain, respectively. The abi5-9 mutation, in which alanine 214 is substituted with a glycine, is located on C3. (B) Alignment of the Arabidopsis Group A bZIPs. The sequence of conserved region 3 is indicated. Gray shading indicates conserved amino acids, black shading indicates alanine 214. Arabidopsis Genome Institutive (AGI) code for the genes as follows: ABI5 (At2g36270), AREB1/ABF2 (At1g45249), AREB2/ABF4 (At3g19290), AREB3 (At3g56850), ABF1 (At1g49720), ABF3 (At4g34000), AtbZIP67/DPBF2 (At3g44460), EEL/DPBF4 (At2g41070), GBF4 (At1g03970), AtbZIP13 (At5g44080), AtbZIP15 (At5g42910), FD (At4g35900) and FDP (At2g17770).
To verify that ABI5 was the causal gene of the mh31 mutant, we performed an allelism test, crossing mh31 plants (male) with abi5-1 plants (female) to obtain F1 progeny. The F1 plants were tested for ABA sensitivity during germination. As shown in Table 1, the F1 progeny showed ABA insensitivity comparable to the parental mh31 and abi5-1 plants, indicating that mh31 and abi5-1 did not complement each other. We concluded that mh31 and abi5-1 affect the same locus; therefore, the mh31 mutant was re-named as abi5-9 since abi5-1 to abi5-8 have been previously described.12,17,36,38,39
Table 1. Allelism test.
| Genotype | ABA sensitive | ABA insensitive |
|---|---|---|
| Ws |
28 |
0 |
|
mh31 |
0 |
32 |
|
abi5-1 |
0 |
32 |
| F1abi5-1 x mh31 | 5 | 26 |
After stratification, seeds were sown on the plate with 3 μM ABA and incubated at 23°C under continuous light (2,000 lx). After incubation for 2 weeks, the numbers of growth-arrested seedling and greening seedling were counted. “ABA sensitive” and “ABA insensitive” indicate the growth-arrested seedling and the greening seedling, respectively.
Molecular characterization of abi5-9 protein in yeasts
Regulation of Em1 and Em6 reportedly requires both ABI5 and ABI3,16,27,40 which physically interact with each other through the ABI5 region containing the C2 and C3 domains in yeast.27 We performed a yeast two-hybrid assay to test the physical interaction between abi5-9 and ABI3. The yeasts harboring the Gal4 DNA-binding domain (BD)-abi5-9 but not BD-ABI5 could grow on medium lacking histidine and adenine, suggesting stronger autoactivation of the reporter genes by abi5-9 (Fig. 3A). The full-length ABI3 fused to Gal4-BD showed strong autoactivation of the reporter genes (data not shown), as previously reported;41 therefore, truncated ABI3 proteins were fused to Gal4-BD and used to evaluate the interaction with abi5-9 and ABI5 (Fig. 3B). As shown previously,27 ABI5 interacted with truncated ABI3 proteins, including the B1 domain alone (B1S and B1L) and the B1 and B2 domains together (B1B2). On the other hand, abi5-9 showed weak interaction only with B1B2, as judged by the growth of yeasts harboring both AD-abi5-9 and BD-B1B2 constructs, and growth was completely inhibited by addition of 10 mM 3-AT, a competitive inhibitor of the HIS3 used to titrate the expression level of the HIS3 reporter gene.42 These results suggested that the conserved Ala in the C3 domain is important for the physical interaction of ABI5 with ABI3 in yeast.
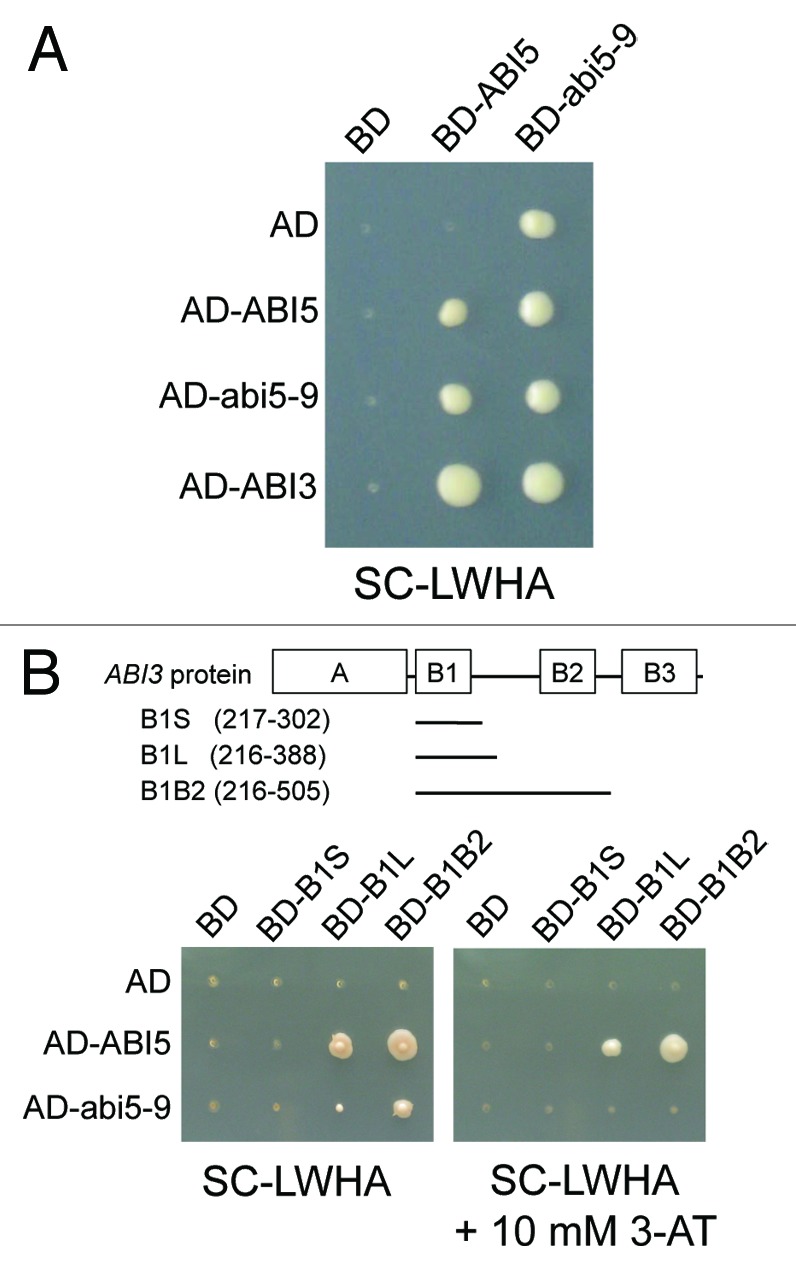
Figure 3. Interaction of abi5-9 with ABI3 in yeast two-hybrid system. Yeast strain PJ69-4 harboring constructs of the Gal4-Activation domain (AD) fusion and Gal4-DNA binding domain (BD) fusion, as indicated to the left and above, were grown for 4 d at 30°C on Synthetic Complete dropout medium lacking the amino acids leucine and tryptophan (SC-LW). Yeast cultures were replicated on the synthetic complete dropout medium lacking the amino acids leucine, tryptophan, histidine and adenine (SC-LWHA) with or without 3-amino-1,2,4-triazole (3-AT), and were grown for an additional 5 d (A) or 10 d (B) at 30°C. B1S, B1L and B1B2 are the partial regions of the ABI3, which interact with ABI5, as described by Nakamura et al.27 Schematic representation of the full-length and truncated regions of ABI3 are indicated (B). A, B1, B2 and B3 are acidic domain, basic domain 1, basic domain 2 and basic domain 3, respectively. Numbers in parentheses indicate the position of amino acid residues from the first methionine of ABI3.
Functional analysis of abi5-9 in plants
Experiments using yeast suggested that abi5-9 retained transactivation ability but lacked the ability to interact with ABI3, suggesting that failure of abi5-9 to interact with ABI3 affected the regulation of downstream genes. We investigated the expression profiles of abi5-9, ABI5 and the downstream genes Em1 and Em6 in dry seeds and in imbibed seeds with or without ABA treatment (Fig. 4). We did not observe any significant changes in the expression profiles between abi5-9 and ABI5, indicating that the abi5-9 mutation affects ABI5 function at the post-transcription level (Fig. S2). On the other hand, expressions of Em1 and Em6 were reduced in dry seeds and in ABA-treated imbibed seeds of abi5-9 plants compared with wild type, and the reduction level was comparable to that seen in abi5-1 plants (Fig. 4). These data clearly demonstrated that abi5-9 mutation severely affected ABI5 function.

Figure 4. RNA expressions of Em1 and Em6 in abi5-9 mutant. RNA expression levels determined by northern blot. After stratification, seeds were sown on the plate with or without 3 μM ABA and incubated at 23°C for the indicated duration under 24-h continuous light (2,000 lx). Total RNA was extracted and 2 μg total RNA was used per lane. The rRNA bands stained with ethidium bromide in the gel were used to verify equal loading.
Discussion
In this study, we report a novel allele of abi5 that encodes a full-length protein with one amino acid substitution in the conserved C3 region, and we prove that this domain is essential for ABI5 function. In the abi5-9 mutant, the C to G mutation of a single nucleotide resulted in the substitution of an evolutionarily conserved alanine with a glycine at residue 214. This conserved alanine was not located in the C3 phosphorylation site and it has been previously shown that mutation of the C3 phosphorylation site does not affect ABI5 function in plants,15 indicating that the A214G mutation affected ABI5 regulation mechanisms other than phosphorylation. Interactions have been previously shown between members of the ABI5/AREB/ABF and various proteins, including kinases (SnRK2s and CPKs),43-46 DREB47,48 and the Arm repeat protein interacting with ABF3 (ARIA).49 Our present data clearly demonstrated that interaction between ABI5 and ABI3 was mediated through the C3 domain and that A214 had an important role in this interaction. The small plant-specific protein AFP1 interacts with the C3 domain of ABI5, promoting ABI5 degradation,50 and the C3 domain might also be important in regulating ABI5 activity through interaction with other regulatory proteins.
Previous reports have suggested the autoregulation of ABI5 transcription.12,14 Notably, the abi5-1 and abi5-9 mutations greatly affected the mRNA accumulation of Em1 and Em6 (Fig. 4); however, reduction of ABI5 mRNA was only observed in the abi5-1 plants, not the abi5-9 plants (Fig. S2). We demonstrated that the abi5-9 mutation, which produces the full-length of ABI5 protein with one amino acid substitution, did not affect ABI5 mRNA accumulation. These results suggest that autoregulation of ABI5 transcription may not require interaction with ABI3. Future studies, such as microarray analysis of the abi5-9 plants, will enable differentiation between ABI3-dependent and ABI3-independent ABI5 targets.
We observed several effects of A214G mutation on ABI5 function; however, these differences could not explain why the abi5-9 allele results in loss of function. The A214G mutation in the C3 domain could affect ABI5 protein stability, resulting in a variety of phenotypes; however, we do not think that this is likely because we observed GFP expression in the nucleus of the transgenic Arabidopsis plants in which abi5-9 cDNA translationally fused to the 3′ end of the GFP gene was introduced under control of the CaMV 35S promoter (data not shown). Moreover, in a transient assay using mesophyll protoplasts, the Em6 promoter was significantly activated by abi5-9, comparable to that by ABI5 (Fig. S3). In contrast, Em6 transcript accumulation was drastically reduced in abi5-9 seedlings (Fig. 4). These differences may be partly attributed to the heterologous assay systems we employed: a yeast system and a transient assay system using mesophyll protoplasts, which do not express ABI5. We also speculate that chromatin modification of the Em6 promoter is important for the regulation by ABI5 and ABI3. The seed-specific phaseolin promoter is repressed by histone modification, and PvALF, a Phaseolus vulgaris ABI3 ortholog, is involved in the chromatin remodeling to bring the phaseolin promoter to the potentiated state, which is followed by activation by ABA.37,51,52 Since ABI3 and ABI5 interact with each other and the A214G mutation reduced this interaction, ABI5 may also participate in chromatin remodeling with ABI3. It is possible that abi5-9 affects the regulation of Em6 at the chromatin remodeling level, but is not defective in ABRE binding and/or transactivation. This hypothesis may explain why abi5-9 can activate the Em6 promoter in a transient assay.
The Ala214 in the C3 domain is highly conserved among AREB/ABF proteins (Fig. 2B). It is possible that Ala214 is also involved in the regulation of AREB/ABF activity in vegetative tissues. Our data demonstrated that abi5-9 increased autoactivation ability in yeasts compared with ABI5 (Fig. 3A). This improved transactivation ability was also observed in the mesophyll protoplasts gene expression assay, in which the Em6 promoter was activated more strongly by abi5-9 than by ABI5 (Fig. S3). Tang et al.53 recently demonstrated that the OsbZIP46 lacking the C3 domain shows constitutive transactivation activity in yeasts and plants, and proposed that the C3 domain might have a role in transactivation repression. These observations are in good agreement with our data in yeasts and mesophyll protoplasts. In contrast to these results, we found that in seedlings and dry seeds—in which the ABI5 gene is expressed—Em6 transcript accumulation was drastically reduced in the abi5-9 mutant compared with wild type. These data suggested that the C3-mediated transactivation activity is also important for the expression of ABI5-regulated genes such as Em6 in germinating seeds.
Our findings highlight the importance of the Ala, which is strictly conserved in the C3 domains but not the direct target motif for phosphorylation of AREB/ABF proteins. It would be interesting to see whether substitutions of the alanine to various types of amino acids affect activities of AREB/ABF proteins, which may eventually lead to the genetic engineering of drought-tolerant crops, since these proteins play critical roles in regulating ABA responses of vegetative tissues.
Materials and Methods
Plant materials and growing conditions
The Arabidopsis thaliana ecotypes Col-0 (Columbia-0), Ws (Wassilewskija) and Ws-2 and the ABA-insensitive Arabidopsis mutant abi5-1 (Ws-2 background) were obtained from the Arabidopsis Biological Resource Center (ABRC). Arabidopsis plants were grown at 23°C under light conditions of 2,000 lx for 16 h/day. For the greening assay, after stratification at 4°C for 3–4 d, seeds were sown on germination medium (GM)54 containing 0.8% (w/v) agar supplemented with different concentrations of NaCl, mannitol, or ABA. The plates were incubated for two weeks at 23°C under 24-h continuous light (2,000 lx). For RNA expression assays, stratified seeds were sown on filter paper placed on top of 0.8% agar, and grown at 23°C under continuous light (2,000 lx). For 2-d-old seedlings, the seedlings were transferred to a 0.8%-agar plate containing ABA or dimethylsulfoxide (DMSO) and incubated for indicated periods.
Isolation of a salt-tolerant mutant
Calluses were produced from the hypocotyl of Ws. Generation of callus, transformation of callus using Agrobacterium tumefaciens strain GV3101::pMP90RK containing pPCVICEn4HPT, and plant regeneration were performed according to the methods described by Kakimoto.54 After transformation by A. tumefaciens, calluses derived from Ws hypocotyl were grown on shoot-inducing media (SIM) supplemented with 20 mg/L hygromycin and 150 mM NaCl. The mh31 mutant was isolated as salt-tolerant shoots regenerated from callus.
Genetic mapping
The F1 generation was obtained by crossing the mh31 mutant with the wild-type Col-0; it was then self-fertilized to establish the F2 generation. The F2 mapping populations were screened based on the ABA-insensitive phenotype or mannitol-sensitive phenotype, and these plants were used for genetic linkage analysis with simple sequence length polymorphism (SSLP) DNA markers. SSLP mapping for Arabidopsis was performed as described by Bell and Ecker.55 SSLP DNA markers were amplified by polymerase chain reaction (PCR) using oligonucleotide sequences registered as specific primers at the Arabidopsis information resource (TAIR; www.arabidopsis.org/). DNA extraction was performed as previously described.56
Sequencing analysis of ABI4 and ABI5.
The cDNAs of ABI5 and ABI4 were amplified by PCR and sequenced. The oligonucleotide sequences of the primer pairs were as follows: ABI5 cDNA forward primer, 5′-TCTCTTTCTCAAAACCTTTCAGTC-3′, reverse primer, 5′-TTCTATAACCTCATTCCTCAAAGACA-3′; and ABI4 cDNA forward primer, 5′-AAGTGAGTGAGAAGAGAGTGTAAGT-3′, reverse primer, 5′-ACCGTAATCTCTTTTACGAATTCC-3′.
Yeast strain and construction
Yeast strains PJ69-4A and PJ69-4α57 were used for the yeast two-hybrid assay. These strains have nutritional requirements for histidine, adenine, tryptophan and leucine and harbor the reporter genes GAL1-HIS3 and GAL2-ADE2. Plasmids pGBTK58 and pGAD424 (Clontech) were used to construction the Gal4 DNA-binding domain (BD) fusion and Gal4-activation domain (AD) fusion, respectively. The plasmid pGBTK contained the TRP1 nutritional marker and Gal4-BD. The plasmid pGAD424 contained the LEU2 nutritional marker and Gal4-AD.
ABI5 was amplified by PCR with the following primer pair: forward primer, 5′-ATCATCgaattcATGGTAACTAGAGAAACGAAG-3′ and reverse primer, 5′- ATCATCggatccTTAGAGTGGACAACTCGG-3′. The amplified ABI5 ORF was cloned into the EcoRI and BamHI sites of pGBTK or pGAD424. ABI3 was amplified by PCR with the following pair of primers: forward primer, 5′-ATCATCggatccGTATGAAAAGCTTGCATG-3′ and reverse primer, 5′-ATCATCgtcgacTCATTTAACAGTTTGAGAAG-3′. The ABI3 ORF was cloned into the BamHI and SalI sites of pGBTK and pGAD424. The coding regions of the B1S, the B1L and the B1B2, which have been indicated to physically interact with ABI5,27 were amplified by PCR with following pair of primers: B1S forward, 5′-ATCATCggatccGTGAAGACCAGGTCGTTG-3′ and reverse, 5′-ATCATCgtcgacTCATTGGACCCATTCAAGAA-3′; B1L forward, 5′- ATCATCggatccGTCAAGAAGACCAGGTCGT-3′ and reverse, 5′-ATCATCgtcgacTCATTCAAGTAAAGGAAGGA-3′; and B1B2 forward, 5′-ATCATCggatccGTCAAGAAGACCAGGTCGT-3′ and reverse, 5′-ATCATCgtcgacTCAGTTAAGTTGTGGAGCCA-3′. These amplified fragments were cloned into BamHI and SalI sites of pGBTK. Insertions of the resultant plasmids were verified by sequencing.
Yeast two-hybrid analysis
The Gal4-BD fusion constructs based on the plasmid pGBTK and the Gal4-AD fusion constructs based on pGAD424 were used for transformation of yeast strains PJ69-4A and PJ69-4α, respectively, using the LiCl/ssDNA/PEG method.59 Methods described by Yoshimura et al.58 were used to acquire diploid yeast strains harboring the Gal4-BD fusion construct with the Gal4-AD fusion construct by mating PJ69-4A with PJ69-4α, as well as to perform the plate assay on synthetic complete (SC) dropout medium lacking the amino acids leucine (L), tryptophan (W), histidine (H) and adenine (A) (SC-LWHA). 3-Amino-1,2,4-triazole (3-AT) was used as a histidine synthesis inhibitor.
Constructions for transient assay
The overexpression vector pGHX—which is basically the same as pGKX60 but with the resistance marker changed to hygromycin—was used for construction of pGHX-ABI5 and pGHX-abi5-9. ABI5 cDNA was amplified by PCR with the following pair of primers: forward primer, 5′-ATCATCggatccATGGTAACTAGAGAAACGAA-3′ and reverse primer, 5′-ATCATCgcggccgcCCAAAGATTGATGATGTTGA-3′. Amplified ABI5 was cloned into the BamHI and NotI sites of pGHX. For the pGHX-ABI3 plasmid, pGBTK-ABI3 was digested with BamHI and SalI. After blunting using the DNA Blunting kit (Takara, Japan), the DNA fragment containing the ABI3 ORF was subcloned into the SmaI site of pGHX. For construction of the Em6 promoter-GUS plasmid, the pGreen0029-GUS-NosT plasmid was constructed by subcloning GUS-NosT from pBI221 into pGreen0029.61 The promoter region of Em6 was amplified by PCR with the following pair of primers: forward, 5′-ATCATCtctagaTAATAATGATGTATAGATGATTGGAG-3′ and reverse, 5′-ATCATCcagctgAGCTGCTTCTTCTCTTGTTG-3′. The XbaI-PvuII fragment of the amplified Em6 promoter was cloned into the XbaI and SmaI sites of pGreen0029-GUS-NosT. The cDNAs and Em6 promoter of the constructed plasmids were verified by sequencing analysis. p35S-Emerald luciferase (Eluc) was created by digesting pBI221 with SmaI and SacI to release the GUS gene. The vector was ligated to the 1896-base EcoRV-SacI fragment of pEluc-test (Toyobo, Japan).
Isolation of protoplasts and transient gene expression
The transient gene expression assay was performed as previously described.62 GUS and LUC activities were determined as described by Komatsu et al.63 Pica Gene luciferase assay reagent (Toyo-Ink, Japan) was used for the luciferase-luciferin reaction.
RNA gel blot analysis
Total RNA extractions from dry seeds and seedlings were performed using methods described by Vicient et al.64 and Shirzadegan et al.,65 respectively. RNA gel blot analysis was performed as previously described.63 Probe DNAs for Em1 and Em6 were acquired by RT-PCR. The probe DNA for ABI5 was PCR-amplified from pGHX-ABI5. The oligonucleotide sequences for the primer pairs were as follows: Em1 forward, 5′-CAGAGAAGAGCTTGATGAGA-3′ and reverse, 5′-GCTACATTAGACCCTAGTTA-3′; Em6 forward, 5′-CTCAACAAGAGAAGAAGCAGCTGG-3′ and reverse, 5′-GGTCTTGGTCCTGAATTTGGATT-3′; ABI5 forward, 5′-ATCATCggatccATGGTAACTAGAGAAACGAA-3′ and reverse, 5′- ATCATCgcggccgcCCAAAGATTGATGATGTTGA-3′.
Supplementary Material
Acknowledgments
We thank R. Walden for providing the agrobacterium strain GV3101::pMP90RK harboring pPCVICEn4HPT, M. Fujita for providing the binary vector pGHX-SfiI and T. Yoshida and Y. Fujita for technical support with the reporter assay by the transient gene expression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/psb/article/23455
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23455
References
- 1.Takezawa D, Komatsu K, Sakata Y. ABA in bryophytes: how a universal growth regulator in life became a plant hormone? J Plant Res. 2011;124:437–53. doi: 10.1007/s10265-011-0410-5. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res. 2011;124:509–25. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 3.Santos-Mendoza M, Dubreucq B, Baud S, Parcy F, Caboche M, Lepiniec L. Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J. 2008;54:608–20. doi: 10.1111/j.1365-313X.2008.03461.x. [DOI] [PubMed] [Google Scholar]
- 4.Meurs C, Basra AS, Karssen CM, van Loon LC. Role of Abscisic Acid in the Induction of Desiccation Tolerance in Developing Seeds of Arabidopsis thaliana. Plant Physiol. 1992;98:1484–93. doi: 10.1104/pp.98.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L. The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell. 2008;20:2729–45. doi: 10.1105/tpc.108.061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Curr Opin Plant Biol. 2002;5:33–6. doi: 10.1016/S1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- 7.Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–83. doi: 10.1111/j.1399-3054.1984.tb06343.x. [DOI] [Google Scholar]
- 8.Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–5. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 9.Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–71. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–61. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10:1043–54. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6:1567–82. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Molina L, Mongrand S, Chua NH. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA. 2001;98:4782–7. doi: 10.1073/pnas.081594298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 2002;32:317–28. doi: 10.1046/j.1365-313X.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 16.Gampala SSL, Finkelstein RR, Sun SSM, Rock CD. ABI5 interacts with abscisic acid signaling effectors in rice protoplasts. J Biol Chem. 2002;277:1689–94. doi: 10.1074/jbc.M109980200. [DOI] [PubMed] [Google Scholar]
- 17.Carles C, Bies-Etheve N, Aspart L, Léon-Kloosterziel KM, Koornneef M, Echeverria M, et al. Regulation of Arabidopsis thaliana Em genes: role of ABI5. Plant J. 2002;30:373–83. doi: 10.1046/j.1365-313X.2002.01295.x. [DOI] [PubMed] [Google Scholar]
- 18.Manfre AJ, Lanni LM, Marcotte WR., Jr. The Arabidopsis group 1 LATE EMBRYOGENESIS ABUNDANT protein ATEM6 is required for normal seed development. Plant Physiol. 2006;140:140–9. doi: 10.1104/pp.105.072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakoby M, Weisshaar B, Dröge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, et al. bZIP Research Group bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002;7:106–11. doi: 10.1016/S1360-1385(01)02223-3. [DOI] [PubMed] [Google Scholar]
- 20.Bensmihen S, Rippa S, Lambert G, Jublot D, Pautot V, Granier F, et al. The homologous ABI5 and EEL transcription factors function antagonistically to fine-tune gene expression during late embryogenesis. Plant Cell. 2002;14:1391–403. doi: 10.1105/tpc.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JY, Choi HI, Im MY, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell. 2002;14:343–57. doi: 10.1105/tpc.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bensmihen S, Giraudat J, Parcy F. Characterization of three homologous basic leucine zipper transcription factors (bZIP) of the ABI5 family during Arabidopsis thaliana embryo maturation. J Exp Bot. 2005;56:597–603. doi: 10.1093/jxb/eri050. [DOI] [PubMed] [Google Scholar]
- 23.Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17:3470–88. doi: 10.1105/tpc.105.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcotte WR, Jr., Russell SH, Quatrano RS. Abscisic acid-responsive sequences from the em gene of wheat. Plant Cell. 1989;1:969–76. doi: 10.2307/3868997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HI, Hong JH, Ha JO, Kang JY, Kim SY. ABFs, a family of ABA-responsive element binding factors. J Biol Chem. 2000;275:1723–30. doi: 10.1074/jbc.275.3.1723. [DOI] [PubMed] [Google Scholar]
- 26.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA. 2000;97:11632–7. doi: 10.1073/pnas.190309197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura S, Lynch TJ, Finkelstein RR. Physical interactions between ABA response loci of Arabidopsis. Plant J. 2001;26:627–35. doi: 10.1046/j.1365-313x.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, et al. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005;44:939–49. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 29.Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA. 2006;103:1988–93. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, et al. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43:1473–83. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- 31.Fujii H, Verslues PE, Zhu JK. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell. 2007;19:485–94. doi: 10.1105/tpc.106.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii H, Zhu JK. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA. 2009;106:8380–5. doi: 10.1073/pnas.0903144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009;50:2123–32. doi: 10.1093/pcp/pcp147. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, et al. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009;50:1345–63. doi: 10.1093/pcp/pcp083. [DOI] [PubMed] [Google Scholar]
- 35.Sirichandra C, Davanture M, Turk BE, Zivy M, Valot B, Leung J, et al. The Arabidopsis ABA-activated kinase OST1 phosphorylates the bZIP transcription factor ABF3 and creates a 14-3-3 binding site involved in its turnover. PLoS ONE. 2010;5:e13935. doi: 10.1371/journal.pone.0013935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Molina L, Chua NH. A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana. Plant Cell Physiol. 2000;41:541–7. doi: 10.1093/pcp/41.5.541. [DOI] [PubMed] [Google Scholar]
- 37.W-K Ng D, Hall TC. PvALF and FUS3 activate expression from the phaseolin promoter by different mechanisms. Plant Mol Biol. 2008;66:233–44. doi: 10.1007/s11103-007-9265-5. [DOI] [PubMed] [Google Scholar]
- 38.Nambara E, Suzuki M, Abrams S, McCarty DR, Kamiya Y, McCourt P. A screen for genes that function in abscisic acid signaling in Arabidopsis thaliana. Genetics. 2002;161:1247–55. doi: 10.1093/genetics/161.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Schumaker KS, Guo Y. Sumoylation of transcription factor MYB30 by the small ubiquitin-like modifier E3 ligase SIZ1 mediates abscisic acid response in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2012;109:12822–7. doi: 10.1073/pnas.1202630109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelstein R, Gampala SSL, Lynch TJ, Thomas TL, Rock CD. Redundant and distinct functions of the ABA response loci ABA-INSENSITIVE(ABI)5 and ABRE-BINDING FACTOR (ABF)3. Plant Mol Biol. 2005;59:253–67. doi: 10.1007/s11103-005-8767-2. [DOI] [PubMed] [Google Scholar]
- 41.Brocard-Gifford IM, Lynch TJ, Finkelstein RR. Regulatory networks in seeds integrating developmental, abscisic acid, sugar, and light signaling. Plant Physiol. 2003;131:78–92. doi: 10.1104/pp.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joung JK, Ramm EI, Pabo CO. A bacterial two-hybrid selection system for studying protein-DNA and protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:7382–7. doi: 10.1073/pnas.110149297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi HI, Park HJ, Park JH, Kim S, Im MY, Seo HH, et al. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol. 2005;139:1750–61. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell. 2007;19:3019–36. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Cho DI, Kang JY, Kim MD, Kim SY. AtNEK6 interacts with ARIA and is involved in ABA response during seed germination. Mol Cells. 2010;29:559–66. doi: 10.1007/s10059-010-0070-7. [DOI] [PubMed] [Google Scholar]
- 46.Mizoguchi M, Umezawa T, Nakashima K, Kidokoro S, Takasaki H, Fujita Y, et al. Two closely related subclass II SnRK2 protein kinases cooperatively regulate drought-inducible gene expression. Plant Cell Physiol. 2010;51:842–7. doi: 10.1093/pcp/pcq041. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Cho DI, Kang JY, Kim SY. An ARIA-interacting AP2 domain protein is a novel component of ABA signaling. Mol Cells. 2009;27:409–16. doi: 10.1007/s10059-009-0058-3. [DOI] [PubMed] [Google Scholar]
- 48.Lee SJ, Kang JY, Park HJ, Kim MD, Bae MS, Choi HI, et al. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol. 2010;153:716–27. doi: 10.1104/pp.110.154617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S, Choi HI, Ryu HJ, Park JH, Kim MD, Kim SY. ARIA, an Arabidopsis arm repeat protein interacting with a transcriptional regulator of abscisic acid-responsive gene expression, is a novel abscisic acid signaling component. Plant Physiol. 2004;136:3639–48. doi: 10.1104/pp.104.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev. 2003;17:410–8. doi: 10.1101/gad.1055803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng DWK, Chandrasekharan MB, Hall TC. Ordered histone modifications are associated with transcriptional poising and activation of the phaseolin promoter. Plant Cell. 2006;18:119–32. doi: 10.1105/tpc.105.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carranco R, Chandrasekharan MB, Townsend JC, Hall TC. Interaction of PvALF and VP1 B3 domains with the beta -phaseolin promoter. Plant Mol Biol. 2004;55:221–37. doi: 10.1007/s11103-004-0512-8. [DOI] [PubMed] [Google Scholar]
- 53.Tang N, Zhang H, Li XH, Xiao JH, Xiong LZ. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012;158:1755–68. doi: 10.1104/pp.111.190389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kakimoto T. Genes involved in cytokinin signal transduction. J Plant Res. 1998;111:261–5. doi: 10.1007/BF02512181. [DOI] [Google Scholar]
- 55.Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–44. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- 56.Weigel D, Glazebrook J. Arabidopsis: A LABORATORY MANUAL. COLD SPRING HARBOR LABORATORY PRESS, Cold Spring Harbor, New York 2002. [Google Scholar]
- 57.James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–36. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimura M, Asai K, Sadaie Y, Yoshikawa H. Interaction of Bacillus subtilis extracytoplasmic function (ECF) sigma factors with the N-terminal regions of their potential anti-sigma factors. Microbiology. 2004;150:591–9. doi: 10.1099/mic.0.26712-0. [DOI] [PubMed] [Google Scholar]
- 59.Gietz RD, Woods RA. Genetic transformation of yeast. Biotechniques. 2001;30:816–20, 822-6, 828 passim. doi: 10.2144/01304rv02. [DOI] [PubMed] [Google Scholar]
- 60.Qin F, Sakuma Y, Tran LS, Maruyama K, Kidokoro S, Fujita Y, et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell. 2008;20:1693–707. doi: 10.1105/tpc.107.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol. 2000;42:819–32. doi: 10.1023/A:1006496308160. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, et al. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010;61:672–85. doi: 10.1111/j.1365-313X.2009.04092.x. [DOI] [PubMed] [Google Scholar]
- 63.Komatsu K, Nishikawa Y, Ohtsuka T, Taji T, Quatrano RS, Tanaka S, et al. Functional analyses of the ABI1-related protein phosphatase type 2C reveal evolutionarily conserved regulation of abscisic acid signaling between Arabidopsis and the moss Physcomitrella patens. Plant Mol Biol. 2009;70:327–40. doi: 10.1007/s11103-009-9476-z. [DOI] [PubMed] [Google Scholar]
- 64.Vicient CM, Delseny M. Isolation of total RNA from Arabidopsis thaliana seeds. Anal Biochem. 1999;268:412–3. doi: 10.1006/abio.1998.3045. [DOI] [PubMed] [Google Scholar]
- 65.Shirzadegan M, Christie P, Seemann JR. An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res. 1991;19:6055. doi: 10.1093/nar/19.21.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.