Abstract
Cellular functions of actin, and associated actin binding proteins (ABPs), have been well characterized with respect to their dynamic cytosolic role as components of the complex cytoskeletal network. In this regard, the collective research in this field has vastly expanded our knowledge of the role of actin to more recently identify a key role within the nucleus as an integral part gene organization and expression. Herein, we describe the requirement of the ABP actin depolymerizing factor-4 (ADF4) as a regulator of resistance to Pseudomonas syringae DC3000 expressing the effector AvrPphB via ADF4’s cytosolic and nuclear functions. In total, our work has identified significant alterations in the expression of the resistance protein RPS5 in an ADF4 phosphorylation dependent manner. In this mini-review, we provide compelling evidence in support of both a nuclear function for ADF4, as well as potential targeting of the actin cytoskeleton bythe bacterial effector AvrPphB.
Keywords: ADF4, nucleus, actin, plant defense
Actin remodeling is required for a multitude of cellular functions in both plants and animals, including growth, development, cell architecture and response to stress.1 As a ubiquitous network linking extracellular perception to intracellular signaling, the actin cytoskeleton is composed of both filamentous-actin (F-actin) and monomeric globular-actin (G-actin), tightly regulated by the precise interplay of a large group of more than 70 actin-binding proteins (ABPs1). In the recent manuscript by Porter et al.,2 the authors demonstrate a cellular function for actin cytoskeletal dynamics, describing a function which links pathogen perception, gene transcription, and the activation of defense signaling. In this mini-review, we will highlight the significance of this work, which provides the first mechanistic description of actin as a cellular platform for defense signaling in plants following perception of a phytopathogenic bacterium.
ADF4 Possesses Both Classic Cellular Functions of Actin-Depolymerizing Factors as well as Confers Resistance to a Bacterial Pathogen
Among the more than 70 ABPs in plants responsible for the regulation and organization of cytoskeletal dynamics and remodeling, the actin depolymerizing factor (ADF) family fulfills a classic biochemical function to both sever and depolymerize F-actin, functioning in large part as a primary regulator of actin turnover.3 In Arabidopsis, there are 11 members of the ADF family, further subdivided into 5 subclasses whose function and expression are hypothesized to both differentiate and specify numerous cellular functions. ADF4 is a member of subclass I which includes ADF1, ADF2 and ADF3, each of which are expressed in a wide variety of tissues, as well as within the cell cytoplasm and nucleus.2-4
Biochemically, ADF4 was initially characterized using a reverse genetics approach, identified in a screen of ABP mutants showing enhanced susceptibility to Pseudomonas syringae.5 Using a complementary series of cell biology and pharmacological experiments, Tian et al. further defined the actin binding specificity of ADF4, demonstrating that the biochemical function of ADF4 is linked to the ability of the host to activate immune signaling following pathogen infection. In total, this work first described a role for actin cytoskeletal dynamics in the activation of plant defense signaling following perception of P. syringae. In a recent publication, the role of ADF4 has been further defined through the application of live cell imaging to monitor actin dynamics.6 Taken together, these two studies provide a platform hypothesizing that the cellular function of ADF4 controls, links, development and defense signaling through modulating the rate of actin turnover. This would suggest that the structural activity of the actin cytoskeleton might serve as a surveillance platform, functioning in large part to both monitor and modulate changes in host cell homeostasis in response to external stimuli.
ADF4 is Required for RPS5 Gene Expression and Supports the Emerging Hypothesis of Nuclear Functions for ABPS
As noted above, in addition to functioning as an ADF, ADF4 has also been demonstrated to play a key role in immunity to P. syringae expressing the bacterial effector AvrPphB (Pst AvrPphB2,5). AvrPphB is a bacterial effector that upon delivery into the host cell via the type three-secretion system utilizes its cysteine protease activities to cleave host targets including PBS1, PBL1 and BIK1.7,8 While the cleavage of BIK1 and PBL1 result in a dampening of PTI, cleavage of PBS1 leads to activation of ETI though PBS1’s association with the cognate resistant (R)-gene RPS5.7,8 In examining the expression of both, RPS5 and PBS1 in wild type Col-0 and the adf4 mutant it was demonstrated that the adf4 mutant has a significant reduction in the mRNA levels of RPS5and no reduction in PBS1.2 This observation is in agreement, and furthermore, supports a growing hypothesis that the fluctuation of nuclear actin levels contribute to the activation of gene transcription,in large part through the association of actin with all three RNA polymerases, including chromatin maintenance machinery.9 Indeed, the recent work by Porter et al., demonstrating abrogation of RPS5 expression in the adf4 mutant coupled with ADF4’s presence within the nucleus,2 suggests a nuclear role for ADF4 in controlling the activation of defense signaling in plants.
The next step in the current work is to understand the “ins and outs” of the temporal and spatial localization of actin, ABPs and the dynamics therein. For example, while plant actin has a nuclear export signal, it does not possess a strong nuclear localization signal.4 Thus, the precise nature by which actin enters the nucleus remains undefined. However, a recent paper has demonstrated that actin, through interactions with both cofilin and importan9, is actively translocated into the nucleus, and furthermore, that cofilin/importin9 dependent differential nuclear actin levels ultimately effect transcription efficiency.10 This would support the hypothesis that ABPs themselves are the chaperones that facilitate nuclear localization of G-actin. If this hypothesis proves correct, it would support a model (Fig. 1) wherebyADF4 association with actin may facilitate active nuclear import of actin, thus regulating expression of RPS5through actin dependent assembly and activation of transcription or chromatin modifying machinery.
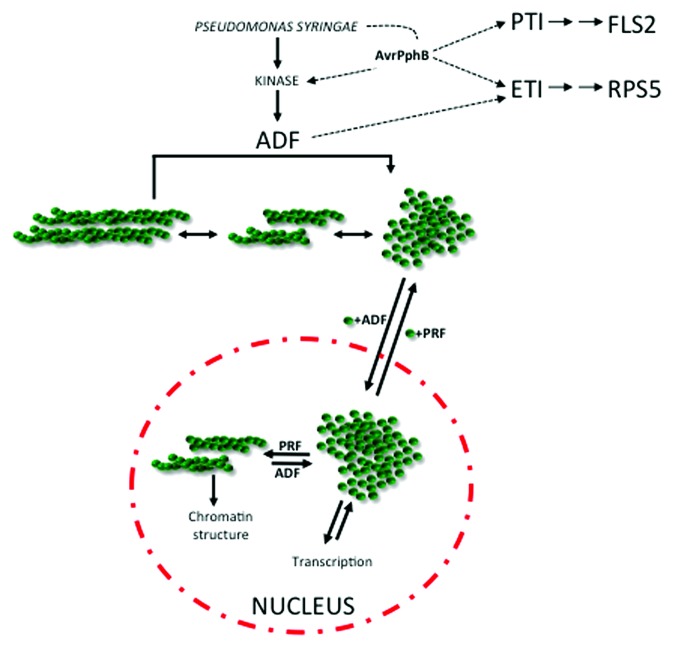
Figure 1. Working hypothesis for the modulation of host resistance and cell signaling through control of actin cytoskeletal dynamics. The virulence activity of the bacterial cysteine protease AvrPphB targets an unidentified kinase that is responsible for the phosphorylation and subsequent regulation of actin depolymerizing factor-4 (ADF4). As a key regulator in controlling not only actin filament organization, but also as a modulator of the balance of globular (G) and filamentous (F) actin, targeting of ADF4 by pathogens represents a key switch in controlling host cell response. At a transcriptional level, the balance of cytoplasmic vs. nuclear actin is required for RNA polymerase function and the general organization and maintenance of chromatin architecture. ETI, effector triggered immunity; PTI, pathogen-associated molecular pattern (PAMP)-triggered immunity; ADF, actin depolymerizing factor; PRF, profilin. This figure was inspired by Vartiainen et al.9
Phosphorylation of ADF4 Regulates its Cellular Function and Reveals a Potential New Virulence Target for Pseudomonas syringae DC3000 AvrPphB
To define the mechanism(s) throughwhich the broader function (e.g., actin binding, filament severing, depolymerization) of ADF4 is regulated, Tian et al.5 investigated the biochemical activity (affinity and depolymerization) of ADF4. To elucidate the link between (in vitro) biochemical function and the regulation of actin cytoskeletal dynamics ultimately leading to immune signaling, Porter et al. investigated phosphorylation as a likely regulatory processes required for activation and attenuation of signaling. Support for this comes from previous work using the vertebrate homolog ADF/cofilin, where numerous factors have been identified as regulatory steps which alter the biochemical function of cofilin, including most importantly, phosphorylation at Serine-3, binding of phosphatidylinositol 4,5-bisphosphate and cellular pH.11 Indeed, our own work not only showed that ADF4 is phosphorylated at the Serine-6 position, but that this phosphorylation event was required for regulating ADF4 affinity for F-actin, as well as for activation of immune signaling through RPS5 mRNA accumulation and MAPK activation.2 Thus, as proposed in Figure 1, our data support the hypothesis that not only is the actin cytoskeleton a virulence target of P. syringae expressing AvrPphB, but that this function regulates a complex network, linking pathogen perception and virulence to nuclear dynamics and the control of transcription.
Final Remarks
Identifying ADF4-dependent expression of RPS5mRNA advances our understanding of the role the actin cytoskeletal network plays within the nucleus, and additionally, provides further evidence in support of the hypothesis that the actin cytoskeleton is a virulence target of not only mammalian pathogens, but of plant pathogens as well.
Acknowledgments
We would like to thank members of the Day lab for critical reading of the manuscript. The work described herein was supported by grants from the Early CAREER award from the National Science Foundation (IOS-0641319) and Arabidopsis 2010 grant from the National Science Foundation (IOS-1021044).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23468
References
- 1.Day B, Henty JL, Porter KJ, Staiger CJ. The pathogen-actin connection: a platform for defense signaling in plants. Annu Rev Phytopathol. 2011;49:483–506. doi: 10.1146/annurev-phyto-072910-095426. [DOI] [PubMed] [Google Scholar]
- 2.Porter K, Shimono M, Tian M, Day B. Arabidopsis Actin-Depolymerizing Factor-4 links pathogen perception, defense activation and transcription to cytoskeletal dynamics. PLoS Pathog. 2012;8:e1003006. doi: 10.1371/journal.ppat.1003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruzicka DR, Kandasamy MK, McKinney EC, Burgos-Rivera B, Meagher RB. The ancient subclasses of Arabidopsis Actin Depolymerizing Factor genes exhibit novel and differential expression. Plant J. 2007;52:460–72. doi: 10.1111/j.1365-313X.2007.03257.x. [DOI] [PubMed] [Google Scholar]
- 4.Kandasamy MK, McKinney EC, Meagher RB. Differential sublocalization of actin variants within the nucleus. Cytoskeleton (Hoboken) 2010;67:729–43. doi: 10.1002/cm.20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian M, Chaudhry F, Ruzicka DR, Meagher RB, Staiger CJ, Day B. Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 2009;150:815–24. doi: 10.1104/pp.109.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henty JL, Bledsoe SW, Khurana P, Meagher RB, Day B, Blanchoin L, et al. Arabidopsis actin depolymerizing factor4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells. Plant Cell. 2011;23:3711–26. doi: 10.1105/tpc.111.090670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science. 2003;301:1230–3. doi: 10.1126/science.1085671. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Zhou J-M. Plant immunity triggered by microbial molecular signatures. Mol Plant. 2010;3:783–93. doi: 10.1093/mp/ssq035. [DOI] [PubMed] [Google Scholar]
- 9.Vartiainen MK, Huet G, Skarp K-P. Nuclear actin levels as an important transcriptional switch. Transcr. 2012;3:226–30. doi: 10.4161/trns.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dopie J, Skarp K-P, Rajakylä EK, Tanhuanpää K, Vartiainen MK. Active maintenance of nuclear actin by importin 9 supports transcription. Proc Natl Acad Sci USA. 2012;109:E544–52. doi: 10.1073/pnas.1118880109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013;25:457–69. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]