Human immunodeficiency virus (HIV)-1 positive drug abusers exhibit greater neurological impairments and have higher rates of motor and cognitive dysfunction in comparison to HIV-1 negative drug abusers or HIV-1 positive individuals who do not abuse drugs”, it reads as “HIV-1 positive individuals who do not abuse drugs (Del Valle et al., 2000; Ferris et al., 2008; Hudson et al., 2010; Nath, 2010). Although the exact neuropathic basis for this HIV-1 drug abuse interaction is unknown, there has been recent recognition of a convergence between the toxicity of HIV-1 trans-activator of transcription (Tat) protein and drugs of abuse within the ventral striatum dopaminergic system (Ferris et al., 2008; Nath, 2010). This putative interaction between the HIV-1 Tat protein and the dopamine (DA) system may produce the neurocognitive/motor impairments associated with chronic HIV-1 infection and drug abuse (Ferris et al., 2008; Harrod et al., 2008). Human imaging studies have found a significant reduction in DA transporter (DAT) density in HIV-1 positive patients, especially in those patients with cognitive/motor deficits and those with co-morbid cocaine abuse (Chang et al., 2008; Wang et al., 2004). We have reported that in vitro exposure to recombinant Tat1-86 decreased the specific [3H]DA uptake and [3H]WIN 35,428 binding sites without a change in total DAT immunoreactivity (Wallace et al., 2006; Zhu et al., 2009). Further, our research using surface plasmon resonance (SPR) analysis has demonstrated that Tat protein interacts directly and specifically with hDAT-GFP in a concentration-dependent manner (Zhu et al., 2009). Thus, the accumulating evidence suggests that the influence of Tat on DAT function and DAT ligand binding sites involves a protein-protein interaction between Tat and DAT, possibly serving as a mechanistic basis for interactions between HIV-1 and cocaine.
In general, drugs interact with transporter proteins in two ways, either as a reuptake inhibitor or as a substrate (Walker et al., 2010). However, there is growing interest in an additional layer of complexity on how compounds can interact with monoamine transporters: allosteric modulation. A recent study reported that the novel allosteric modulator of the DAT, N-(2,2-diphenylethyl)-2-phenyl-4-quinazolinamine (SoRI-20040), decreased the maximal velocity (Vmax) of [3H]DA uptake and the maximal binding sites of [125I]RTI-55 and increased the apparent Km and Kd values in a dose-dependent manner (Pariser et al., 2008). In addition, we have reported that Tat dose-dependently decreased the Vmax of [3H]DA uptake and Bmax of [3H]WIN 35,428 binding, but increased the apparent Km and Kd values (Zhu et al., 2009). These findings suggest that Tat possibly acts as an allosteric modulator of DAT, rather than as either a reuptake inhibitor or a substrate-type releaser, such as cocaine and amphetamine. Thus, the purpose of the current experiment was to further determine the potential mechanisms underlying the inhibitory effects of Tat protein on DAT function.
Adult male Sprague-Dawley rats (200–225 g body weight) were obtained from Harlan (Indianapolis, IN) and housed in standard polyurethane cages with free access to food and water. The colony room was maintained at 21 ± 2°C and 50 ± 10% humidity, with a 12/12-h light/dark cycle (lights on 7:00 AM Eastern Standard Time). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina. Recombinant HIV-1 Tat1-86 protein was used (LA1/Bru strain of HIV-1 clade B, Genbank accession no. K02013, DIATHEVA, Fano, ITALY). SoRI-20040 was provided by Southern Research Institute (Birmingham, AL). Cocaine free base and indatraline hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). HEK 293 cells expressing human DAT (HEK-hDAT) were provided by Dr. Maarten E.A. Reith (New York University School of Medicine). Data analyses were performed using GraphPad Prism 5 (La Jolla, CA).
Results presented in Table 1 demonstrate the effect of Tat on cocaine-induced inhibition of [3H]DA uptake into rat striatal synaptosomes. Synaptosomal preparation and [3H]DA uptake assay were conducted essentially as described previously (Zhu et al., 2009). In brief, aliquots of striatal synaptosomes (30 μl containing 20 μg of protein) were preincubated in assay buffer, containing one of nine concentrations (1 nM – 10 μM, final concentration) of cocaine and the fixed concentrations of indatraline and SoRI-20040 for 5 min at 34°C, and then incubated for 10 min at 34°C with [3H]DA (0.1 μM, final concentration). Indatraline and cocaine are two competitive inhibitors of [3H]DA uptake and SoRI-20040 is a novel allosteric modulator of the DAT (Pariser et al., 2008). Using indatraline and SoRI-20040 as control drugs for competitive inhibition and allosteric modulation, respectively, we examined the effect of Tat protein on cocaine-induced inhibition of [3H]DA uptake. Indatraline (10 nM) decreased specific [3H]DA uptake to 33% of control (cocaine alone) and increased the IC50 value of cocaine from 510 to 2000 nM. SoRI-20040 (12.8 μM) reduced specific [3H]DA uptake to 30% of control and produced a smaller increase in the IC50 value of cocaine (715 nM) than did indatraline. Similarly, Tat (5 μM) decreased specific [3H]DA uptake to 45% of control and induced a similar increase in the IC50 value of cocaine (953 nM) as did SoRI-20040.
Table 1. Effect of Tat on cocaine-induced inhibition of [3H]DA uptake into rat striatal synaptosomes.
Data are presented as mean ± S.E.M. of three independent experiments performed in duplicate.
| Drug | IC50 (nM) | Specific Uptake a (%) |
|---|---|---|
| Cocaine | 510 ± 30 | 100 |
| Cocaine + Indatraline (10 nM) | 2000 ± 210* | 33 ± 2.5 |
| Cocaine + SoRI-20040 (12.8 μM) | 715 ± 48* | 30 ± 2.4 |
| Cocaine + Tat (5 μM) | 953 ± 70* | 45 ± 3.1 |
P < 0.05 compared with the IC50 value of cocaine (Student’s t test).
Each test agent reduced specific [3H]DA uptake and the degree of reduction is reported as a percentage of control.
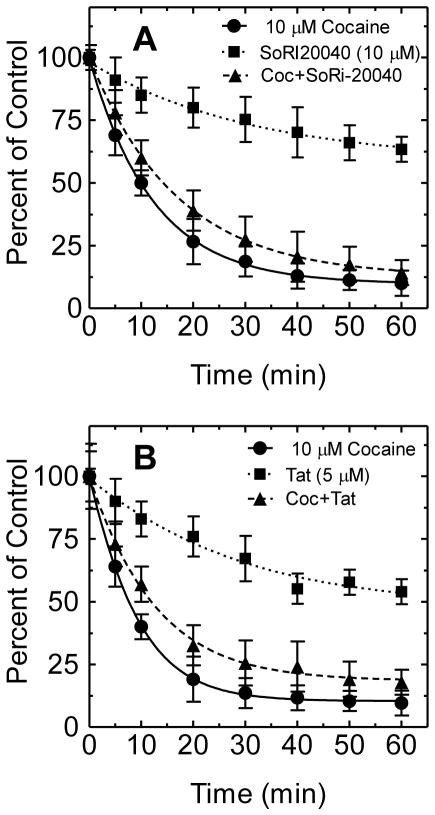
In order to determine if Tat interacts with DAT in an allosteric modulation manner, the effect of Tat on the dissociation of [3H]WIN 35,428 binding to HEK-hDAT was examined. In brief, cells were grown in Dulbecco’s Modified Eagle Medium on Biocoat 24 well tissue culture plates coated with poly-D-lysine. The experiments were carried out in duplicate in a final volume of 500 μl. Once cells were grown to a density of 105 cells per well, the medium was removed and cells were washed twice with 500 μl sodium-phosphate buffer (2.1 mM NaH2PO4, 7.3 mM Na2HPO47H2O, and 320 mM sucrose, pH 7.4). After 2 h incubation with [3H]WIN 35,428, the intact cells were washed twice with assay buffer and the dissociation of [3H]WIN 35,428 binding was initiated by addition of 10 μM cocaine. As reported in Table 2 and Fig. 1A, the dissociation of [3H]WIN 35,428 binding with cocaine proceeded in a monotonic manner and was well described by a single component dissociation model (K−1=0.08 ± 0.005 min−1). The addition of SoRI-20040 following the addition of cocaine significantly slowed the dissociation rate (K−1=0.06 ± 0.001 min−1). The dissociation of [3H]WIN 35,428 binding by the addition of SoRI-20040 alone was described by a single component dissociation model (K−1=0.03 ± 0.008 min−1). In a separate experiment (Fig. 1B), the dissociation of [3H]WIN 35,428 binding with cocaine showed a single component dissociation model (K−1=0.11 ± 0.002 min−1). Tat alone (5 μM) exhibited a similar pattern as did SoRI-20040 alone (K−1=0.03 ± 0.009 min−1). The addition of Tat following the addition of cocaine significantly influenced the dissociation rate (K−1=0.08 ± 0.005 min−1) from hDAT.
Table 2. Effect of SoRI-20040, cocaine and Tat on the dissociation of [3H]WIN 35,428 binding from HEK/hDAT cells.
Data are presented as mean ± S.E.M. of three independent experiments performed in duplicate. A single component dissociation model (K−1, min−1)
| Condition-1 | Condition-2 | Condition-3 |
|---|---|---|
|
| ||
| A. Cocaine | Cocaine + SoRI-20040 | SoRI-20040 |
| 0.08 ± 0.002 | 0.06 ± 0.001* | 0.03 ± 0.007 |
|
| ||
| B. Cocaine | Cocaine + Tat | Tat |
| 0.11 ± 0.003 | 0.08 ± 0.005* | 0.03 ± 0.009 |
P < 0.05 (Student’s t test) compared with the cocaine condition.
Figure 1. Effect of SoRI-20040, cocaine and Tat on the dissociation of [3H]WIN 35,428 binding from HEK/hDAT cells.
HEK293 expressing hDAT cells were incubated with [3H]WIN 35,428 (final concentration, 5 nM) for 2 h on ice. After the 2 h incubation, the incubation reagent was replaced with fresh assay buffer. At the zero time point, cocaine (10 μM) was added to condition-1 and condition-2 (see Table 2). Five minutes later, SoRI-20040 and Tat (5 μM) were added to condition-2 and condition-3, respectively. For the data analysis, the 100% control point (no drug) was time 0 for condition-1 and time 5 min point (no drug) for condition 2 and 3. Each data point is the mean ± S.E.M. (n = 3). * P < 0.05 (Student’s t test) compared with the cocaine condition.
The current study provides novel mechanistic findings, suggesting that Tat protein allosterically modulates the DAT. First, we found that Tat decreased the Vmax of [3H]DA uptake and Bmax of [3H]WIN 35,428 binding and increased the apparent Km and Kd values in a manner unlike that of a competitive inhibitor (Zhu et al., 2009). Second, using indatraline to assess competitive DAT inhibition, Tat reduced specific [3H]DA uptake to 45% of control and produced less of an increase in the IC50 value of cocaine than did indatraline, suggesting that Tat does not interact with DAT in a competitive manner. Third, using SoRI-20040 as a control for allosteric modulation, the addition of Tat after the addition of cocaine significantly slowed the dissociation rate of [3H]WIN 35,428 to 25% of that observed with cocaine alone. In the present study, dissociation experiments were used to detect an allosteric effect of Tat on DAT. This assay has been typically used for comparing the dissociation rate observed when dissociation is initiated by dilution versus the addition of an excess of a competing ligand (Nandi et al., 2004; Pariser et al., 2008). The [3H]WIN 35,428 binding site shares pharmacological identity with the DA uptake carrier and is part of the cocaine binding domain (Pristupa et al., 1994; Reith and Coffey, 1994). We determined the ability of Tat to alter [3H]WIN 35,428 dissociation initiated by the addition of cocaine. Tat was added 5 min after cocaine, ensuring that any effect observed was not due to reassociation of the [3H]WIN 35,428 occurring during the dissociation experiment (Table 2). As reported in Fig. 1B, Tat slowed the dissociation rate, providing clear evidence of an allosteric effect. Regarding the effect of SoRI-20040 on the dissociation rate of [3H]WIN 35,428, our data showed a single component dissociation model.
In summary, the data presented here indicate that Tat allosterically modulates DAT activity. Further development of compounds like SoRI-20040 may be a viable approach to developing therapeutic agents for HIV-1 neurologic impairments. Indeed, a compound that specifically competes with Tat at the DAT, while not altering the transport of dopamine, would be an ideal therapeutic candidate. Alternatively, allosterism signifies a neurobiological mechanism of DAT dysfunction reported in patients with HIV-1 infection and cocaine, which is consonant with susceptibility to increased neurocognitive/motor disorders in the HIV-1 positive, drug abusing, population.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse to Jun Zhu (DA024275 and DA026721) and to Rosemarie M. Booze (DA013137) and by an Award of Research and Productive Scholarship from the University of South Carolina to Jun Zhu.
References
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1-72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008;90:723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LG, Gale JM, Padilla RS, Pickett G, Alexander BE, Wang J, Kusewitt DF. Microarray analysis of cutaneous squamous cell carcinomas reveals enhanced expression of epidermal differentiation complex genes. Mol Carcinog. 2010;49:619–629. doi: 10.1002/mc.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Dersch CM, Kulshrestha M, Ananthan S, Rothman RB. Identification and characterization of a novel allosteric modulator (SoRI-6238) of the serotonin transporter. Synapse. 2004;53:176–183. doi: 10.1002/syn.20048. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- Pariser JJ, Partilla JS, Dersch CM, Ananthan S, Rothman RB. Studies of the biogenic amine transporters. 12. Identification of novel partial inhibitors of amphetamine-induced dopamine release. J Pharmacol Exp Ther. 2008;326:286–295. doi: 10.1124/jpet.108.139675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa ZB, Wilson JM, Hoffman BJ, Kish SJ, Niznik HB. Pharmacological heterogeneity of the cloned and native human dopamine transporter: disassociation of [3H]WIN 35,428 and [3H]GBR 12,935 binding. Mol Pharmacol. 1994;45:125–135. [PubMed] [Google Scholar]
- Reith ME, Coffey LL. [3H]WIN 35,428 binding to the dopamine uptake carrier. II. Effect of membrane fractionation procedure and freezing. J Neurosci Methods. 1994;51:31–38. doi: 10.1016/0165-0270(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Caster JM, Kuhn CM. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J Pharmacol Exp Ther. 2010;335:124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]