Abstract
AIM: To evaluate the impact of body mass index (BMI) on short and long term results after pancreaticoduodenectomies (PD).
METHODS: A consecutive series of PDs performed at the Karolinska University Hospital from 2004 till 2010 were retrieved from our prospective database. The patients were divided by BMI into overweight/obese (O; BMI ≥ 25 kg/m2) and controls (C; BMI < 25 kg/m2). Demographics, peri-operative data, morbidity, mortality, pancreatic fistula (PF) rate, length of stay (LOS), hospital costs, histology, and survival were analyzed. An additional sub analysis of survival was performed in patients with a diagnosis of pancreatic ductal adenocarcinoma (PDAC) and divided in underweight, normal-weight, overweight and obese.
RESULTS: A total of 367 PDs were included (O = 141/C = 226). No differences were found between O and C regarding demographics, peri-operative data, costs, morbidity or mortality. O was associated with higher intra-operative blood loss (1392 ± 115 mL vs 1121 ± 83 mL; P = 0.01), rate of PF (20% vs 9.5%; P = 0.006) and marginally longer LOS (18 ± 0.9 d vs 15 ± 1.1 d; P = 0.05). An increasing risk for PF was observed with increasing BMI. The 1, 3 and 5 years survival rate was similar in O and C in PDAC (68.7%, 26.4% and 8.8% vs 66.1%, 30.9% and 17.9% respectively; P = 0.9). When the survival was analyzed using 4 different categories of BMI (underweight, normal, overweight and obese), a trend was seen toward a difference in survival, with a worse prognosis for the underweight and obese patients compared to normal weight and overweight patients.
CONCLUSION: Overweight increases the risk for intra-operative bleeding and PF, but do not otherwise alter short or long term outcome after PD for pancreatic cancer.
Keywords: Pancreas surgery, Pancreatectomy, Body mass index, Pancreatico- duodenectomy, Oncology, Pancreas cancer
Core tip: In the last decades, the number of overweight individuals has increased dramatically in Western countries. No data are available in the literature that show clearly whether this comorbidity has an impact on short-term or long-term outcomes in these patients or on procedure-related costs. Some studies have shown that pancreatectomies in overweight patients are associated with an increased risk of post-operative complications. The data are even more confusing regarding long-term and oncologic outcomes. In our study, based on a large series of consecutive pancreaticoduodenectomies (PD) performed in a high volume center for pancreatic surgery, we showed that body mass index (BMI) is a risk factor for intra-operative bleeding and post-operative pancreatic fistula, but does not increase the overall morbidity and have no impact on survival of patients with pancreatic ductal adenocarcinoma. Based on these results, BMI should not be considered, per-se, an exclusion criteria for candidates for PD.
INTRODUCTION
In the last decades, the number of overweight individuals has increased dramatically in Western countries. It is estimated that today there are about 1.6 billion individuals in the world who are overweight and 400 million who are obese[1]. At the same time, obesity is a well-recognized risk factor for several chronic degenerative diseases such as cardiovascular diseases[2,3], diabetes[4] and a number of types of cancer[5], including pancreatic cancer[6]. In fact, obese individuals have a twofold to threefold increase in the risk of death from all causes compared to the general population[7]. It is estimated that treatment of obesity-related diseases consumes about 10% of healthcare expenditures in the United States[8].
These data clearly suggest that the number of overweight or obese patients who are candidates for pancreaticoduodenectomy will increase in the future, but no data are available in the literature that show clearly whether this comorbidity has an impact on short-term or long-term outcomes in these patients or on procedure-related costs.
Some studies have shown that pancreatectomies in overweight patients are associated with an increased risk of post-operative complications[9] including a longer hospital stay[9-11], greater blood loss[12], more wound infections[13-15], and an increased rate of pancreatic fistula (PF)[12]. However, some other studies have not shown an overall increase of post-operative morbidity in overweight patients[16,17]. Analysis is even more difficult for long-term and oncologic outcomes. In these areas, few data are available, and the results are not consistent. In a recent paper, Fleming showed shorter long-term survival and an increase in the number of positive node specimens in patients with a body mass index (BMI) > 35 kg/m2 who underwent pancreaticoduodenectomy for pancreatic ductal adenocarcinoma (PDAC)[18]. In contrast, a large single-institution study by Tsai et al[12] found a longer survival time in overweight patients who underwent pancreaticoduodenectomy for PDAC. Due to the worldwide increase in numbers of overweight patients who are candidates for pancreaticoduodenectomy and the lack of data concerning short-term and long-term outcomes for that procedure in these patients, we decided to analyze our own results and investigate the correlation between BMI and survival, post-operative complications, and the cost of pancreaticoduodenectomy in overweight patients.
MATERIALS AND METHODS
Patients who had undergone pancreatoduodenectomy at Karolinska University Hospital 2004–2010 were retrieved from the hospital database containing prospectively collected pre-, intra- and post-operative data. The cohort was divided by BMI into overweight/obese (BMI ≥ 25 kg/m2) and controls (BMI < 25 kg/m2). Demographics, peri-operative data, morbidity, in-hospital mortality, PF rate, length of stay (LOS), histology, and survival were analyzed. Financial files for the patients in the study were obtained from the Economic Department at the Karolinska University Hospital and analyzed. The cost analysis included all the in-hospital diagnostic and therapeutic procedures, such as X rays, blood samples, other tests (e.g., electrocardiogram and spirometry), operations, and drugs, as well as pre- and post-operative stays, and intensive care unit (ICU) or sub-ICU stays. All patients underwent a conventional Whipple resection with vascular resections of large retropancreatic vessels whenever necessary and with radical lymphadenectomy, as described in the Castelfranco Veneto Classification[19]. In all patients, the reconstruction was done by an end-to-side, duct to mucosa, pancreaticojejunostomy, an end-to-side conventional hepatico-jejunostomy and an antecolic gastro-entero anastomosis. The study was approved by the local ethics committee. GraphPad Prism software (R) was used to compare costs, pathological results, intra-operative and post-operative outcomes, and long-term survival between the two groups. The Student’s t test was used for comparison of the means of continuous variables. Fisher’s exact test was used to compare categorical variables. Long-term survival was analyzed using a non-parametric method (Kaplan Meier). Differences in survival were estimated using the Log-rank test.
Statistical analysis
Statistical methods should be described when they are used to verify the results. Choose suitable techniques for the statistical treatments; for example, t-test (group or paired comparisons), χ2 test, Ridit, probit, logit, regression (linear, curvilinear, or stepwise), correlation, analysis of variance (ANOVA), analysis of covariance, etc.
RESULTS
Three hundred sixty-seven consecutive patients were identified of which 141 (38.4%) were overweight/obese. As shown in Table 1, the overweight/obese group had significantly higher mean BMI than the controls (28.6 kg/m2 vs 22.1 kg/m2; P < 0.0001). No significant differences were found in sex distribution, mean age, pre-operative risk assessment according to the American Society of Anesthesiology classification system, or the number of procedures associated with resection of peri-pancreatic vessels.
Table 1.
Pre-operative characteristics of patients
| Patient characteristics | Overweight (n = 141) | Controls (n = 226) | P value |
| BMI (kg/m2) | 28.6 | 22.1 | < 0.0001 |
| Male/Female | 75/66 | 127/99 | 0.5 |
| Mean age (yr) | 63.7 | 65 | 0.3 |
| ASA risk | |||
| I | 10.40% | 5.10% | 0.06 |
| II | 58.30% | 53.40% | 0.40 |
| III | 29.60% | 36.40% | 0.20 |
| IV | 1.70% | 5.10% | 0.30 |
| Vascular resection | 15.60% | 22.10% | 0.10 |
ASA: American Society of Anesthesiology; BMI: Body mass index.
Operative and pathology details
The overweight/obese group had higher intra-operative blood loss (1392 ± 115 mL vs 1121 ± 83 mL; P = 0.01) and a marginally longer LOS (18.0 ± 0.9 d vs 15.0 ± 1.1 d; P = 0.05) compared to the control group. No differences were found between the groups in operation time (446 ± 8 min vs 431 ± 7 min; P = 0.2). Both groups showed a similar panorama of final histological diagnoses even if more patients were treated for chronic pancreatitis in the controls group and for other tumor types in the overweight group (Table 2); about half of the patients were treated for PDAC.
Table 2.
Histological diagnosis of resected specimens and main surgical specific post-operative complications
| Overweight (n = 141) | Controls (n = 226) | P value | |
| Histology | |||
| Ductal adenocarcinoma of the pancreas | 42.5% | 49.5% | 0.2 |
| Periampullary cancers | 20.6% | 17.4% | 0.5 |
| Cholangiocarcinoma | 4.9% | 3.9% | 0.8 |
| Cystic tumors | 12.8% | 10.6% | 0.6 |
| Neuroendocrine tumors | 5.7% | 5.8% | 1.0 |
| Chronic pancreatitis | 3.6% | 9.7% | 0.03 |
| Other tumor types | 9.9% | 3.1% | 0.009 |
| Surgical complications | |||
| Pancreatic fistula | 20.0% | 9.5% | 0.006 |
| Delayed gastric empting | 14.2% | 11.5% | 0.5 |
| Bile leakage | 2.8% | 1.7% | 0.5 |
| Abdominal bleeding | 4.2% | 4.4% | 1.0 |
| Gastro-intestinal bleeding | 2.1% | 2.6% | 1.0 |
| Wound infection | 4.9% | 2.6% | 0.3 |
Peri-operative mortality and morbidity
Overall, 13 of the 367 patients died post-operatively (3.5% in-hospital mortality). No difference in mortality rate was found between the two groups: 5 patients died in the overweight group (3.4%) and 8 in the control group (3.5%). No differences in overall morbidity (47% vs 54%; P = 0.2) or severe post-operative complications (grade ≥ 3b according to the Clavien classification)[20] (15.6% vs 17.2%; P = 0.7) were found between the overweight and control groups. In contrast the overweight patients developed more post-operative medical specific complications (24.1% vs 15%; P = 0.03). When specific surgical complications were compared, only PF occurred more frequently in the overweight group compared to the control group (20% vs 9.5%; P = 0.006). Otherwise, no significant differences were found in the incidence of delayed gastric emptying, bile leakage, wound infection, or intra-abdominal or gastro-intestinal bleeding (Table 2). When the patients were divided into 4 different sub-groups according to BMI, the risk for developing post-operative PF was directly correlated with increasing BMI. Pancreatic fistula developed in none of the underweight patients (BMI < 18.5 kg/m2), 10% of normal weight patients (BMI ≥ 18.5 kg/m2 and ≤ 24.9 kg/m2), in 16% of overweight patients (BMI ≥ 25 kg/m2 and ≤ 29.9 kg/m2), and 32% of obese (BMI ≥ 30 kg/m2).
Cost analysis
The mean overall cost for the overweight group was 30700 ± 1938 euros and 33140 ± 2692 euros in the control group, which was not statistically different (P = 0.4) (Figure 1).
Figure 1.
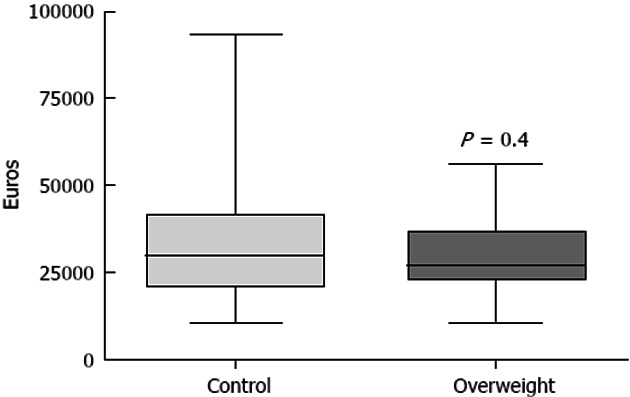
Comparison of in-hospital costs in the overweight and control groups.
Survival analysis
Overall, 60 patients (42.5%) in the overweight group and 112 patients (49.5%) in the control group were resected because of PDAC. One-year, 3-year, and 5-year actuarial survival rates (Figure 2A) were not different between the overweight group and the control group respectively (68.7%, 26.4%, and 8.8% vs 66.1%, 30.9%, and 17.9%; P = 0.9). When the survival of PDAC patients was analyzed using 4 different categories of BMI (underweight, normal, overweight and obese), a trend was seen toward a difference in survival, with a worse prognosis at 1, 3 and 5 years for the underweight and obese patients (57.1%, 0%, 0% and 77.8%, 51.8%, 0%, respectively), compared to normal weight and overweight patients (67%, 32%, 18.4% and 66.5%, 20.6%, 10.3%, respectively) (Figure 2B). However, the survival comparison of the four groups was not significantly different (P = 0.8).
Figure 2.

Actuarial survival curves. A: In overweight and control patients who underwent pancreaticoduodenectomy for pancreatic ductal adenocarcinoma (PDAC); B: For underweight, normal-weight, overweight, and obese patients who underwent pancreaticoduodenectomy for PDAC. BMI: Body mass index.
DISCUSSION
General considerations
Overweight and obesity is becoming one of the most important health problems in Western countries. It is estimated that over $140 million are spent each year on obesity-related diseases in the United States[17,21,22]. Few data are available concerning the impact of overweight on post-operative outcomes after pancreaticoduodenectomy and on the costs associated with the procedure. In addition, the limitations of the published studies make it difficult to analyze the data that are available. The available data were either collected retrospectively, or different criteria were used for dividing patients into sub-groups in the different investigations, or the study populations were not comparable.
Impact of BMI in the short-term outcome of pancreaticoduodenectomies
Some data suggest that pancreaticoduodenectomies (PD) is a more complicated operation in overweight patients. Our study is consistent with others[10,12] that have found comparable operative times in the control and overweight patients but higher intra-operative bleeding in the overweight group. Some studies have reported that overall morbidity after PD was not higher in overweight patients[10]. Similarly, we did not find any differences in overall rates of post-operative complications or in the severity of complications, even though overweight patients seem to be more prone to develop post-operative medical complications. We found an increased risk of PF in the overweight group, which is consistent with many other papers[11,12,23], and found that the risk increased progressively in higher BMI categories. This result may be due to different pancreatic textures in the different BMI groups with an increasing percentage of fat infiltration in overweight patients that affected the quality of the pancreatico-jejunostomy anastomosis[11,24]. In our series, we did not find an increased risk of other BMI-related post-operative complications, such as wound infections, although such an increase has been reported in some other studies[9,15]. Previous papers have not looked at mortality rates, costs, or the LOS in overweight patients after PD. We did not find any differences in those areas, which is not surprising since there were no differences in the overall complication rate.
Impact of BMI in the long-term outcome of PD
Our study didn’t show any statistically significant differences in survival in overweight patients compared to the control group. However, Fleming et al[18] reported decreased survival after PD in patients with a BMI >35 kg/m2. In contrast, a more recent study by Tsai et al[12] found that a higher BMI can be considered a positive long-term prognostic factor for patients undergoing PD for PDAC. In our analysis of patients treated for PDAC, we did not find any difference in survival between the overweight and control subgroups. In our sub-analysis using 4 different BMI categories, the underweight and obese patients had a worse prognosis than the normal weight individuals, although there were no differences between the normal weight and overweight patients. It is possible that the division of patients in two categories did not show a difference because the survival time of controls was negatively affected by the group of underweight patients and the overweight patients had a negative impact on the survival analysis of obese patients. Unfortunately, in our series, the extreme groups of BMI (underweight and obese) were too small to give a definitive answer to this question. It would be interesting to study the impact of BMI on the long-term outcome of PD in a larger number of PDAC patients.
In conclusion, our data shows that PD can be done safely in overweight patients, even though with somewhat higher intra-operative blood loss, postoperative medical complications and PF rate. We also demonstrate that overweight/obesity do not impact survival rates negatively after PD for pancreatic cancer. However, the limited number of patients in the extreme groups of BMI limited the possibility to do a proper sub analysis useful to show the impact of underweight and obese patients in the short and long term outcome[25]. A sub analysis of fat distribution can also offer another area of research to predict the short and long term outcome of these patients[26].
COMMENTS
Background
In the last decades, the number of overweight individuals has increased dramatically in Western countries. No data are available in the literature that show clearly whether this comorbidity has an impact on short-term or long-term outcomes in these patients or on procedure-related costs.
Research frontiers
Some studies have shown that pancreatectomies in overweight patients are associated with an increased risk of post-operative complications. The data are even more confusing regarding long-term and oncologic outcomes.
Innovations and breakthroughs
In their study, based on a large series of consecutive pancreaticoduodenectomies (PD) performed in a high volume center for pancreatic surgery, authors showed that body mass index (BMI) is a risk factor for intra-operative bleeding and post-operative pancreatic fistula, but does not increase the overall morbidity and have no impact on survival of patients with pancreatic ductal adenocarcinoma. Based on these results, BMI should not be considered, per-se, an exclusion criteria for candidates for PD.
Peer review
In the present study, the authors investigated the impact of BMI on short and long term results after PD. This paper clearly demonstrated the relationship between BMI and outcome after PD for pancreatic cancer. It is worth publishing.
Footnotes
Supported by Division of Surgery, Department of Clinical Science, Intervention and Technology, Karolinska Institute at Karolinska University Hospital, Stockholm, Sweden
P- Reviewers Hokama A, Miyazawa R S- Editor Gou SX L- Editor A E- Editor Yan JL
References
- 1.Centers for Disease Control and Prevention (CDC) State-specific prevalence of obesity among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:985–988. [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 5.Bergström A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 7.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: how much, and who’s paying? Health Aff (Millwood) 2003;Suppl Web Exclusives:W3–219-W3-226. doi: 10.1377/hlthaff.w3.219. [DOI] [PubMed] [Google Scholar]
- 9.Benns M, Woodall C, Scoggins C, McMasters K, Martin R. The impact of obesity on outcomes following pancreatectomy for malignancy. Ann Surg Oncol. 2009;16:2565–2569. doi: 10.1245/s10434-009-0573-7. [DOI] [PubMed] [Google Scholar]
- 10.Williams TK, Rosato EL, Kennedy EP, Chojnacki KA, Andrel J, Hyslop T, Doria C, Sauter PK, Bloom J, Yeo CJ, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2009;208:210–217. doi: 10.1016/j.jamcollsurg.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Noun R, Riachy E, Ghorra C, Yazbeck T, Tohme C, Abboud B, Naderi S, Chalhoub V, Ayoub E, Yazbeck P. The impact of obesity on surgical outcome after pancreaticoduodenectomy. JOP. 2008;9:468–476. [PubMed] [Google Scholar]
- 12.Tsai S, Choti MA, Assumpcao L, Cameron JL, Gleisner AL, Herman JM, Eckhauser F, Edil BH, Schulick RD, Wolfgang CL, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg. 2010;14:1143–1150. doi: 10.1007/s11605-010-1201-3. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 14.Su Z, Koga R, Saiura A, Natori T, Yamaguchi T, Yamamoto J. Factors influencing infectious complications after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2010;17:174–179. doi: 10.1007/s00534-009-0128-0. [DOI] [PubMed] [Google Scholar]
- 15.House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, Gonen M, Olson SH, Kurtz RC, Brennan MF, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 16.Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15–23. doi: 10.1016/j.surg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Balentine CJ, Enriquez J, Cruz G, Hodges S, Bansal V, Jo E, Ahern C, Sansgiry S, Petersen N, Silberfein E, et al. Obesity does not increase complications following pancreatic surgery. J Surg Res. 2011;170:220–225. doi: 10.1016/j.jss.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, Lee JE, Crane CH, Pisters PW, Evans DB. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg. 2009;144:216–221. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 19.Jones L, Russell C, Mosca F, Boggi U, Sutton R, Slavin J, Hartley M, Neoptolemos JP. Standard Kausch-Whipple pancreatoduodenectomy. Dig Surg. 1999;16:297–304. doi: 10.1159/000018739. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey AM, Martin RC. Body mass index and outcomes from pancreatic resection: a review and meta-analysis. J Gastrointest Surg. 2011;15:1633–1642. doi: 10.1007/s11605-011-1502-1. [DOI] [PubMed] [Google Scholar]
- 24.Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, Pitt HA. Nonalcoholic fatty pancreas disease. HPB (Oxford) 2007;9:312–318. doi: 10.1080/13651820701504157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaid S, Bell T, Grim R, Ahuja V. Predicting risk of death in general surgery patients on the basis of preoperative variables using american college of surgeons national surgical quality improvement program data. Perm J. 2012;16:10–17. doi: 10.7812/tpp/12-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaujoux S, Torres J, Olson S, Winston C, Gonen M, Brennan MF, Klimstra DS, D’Angelica M, DeMatteo R, Fong Y, et al. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2908–2916. doi: 10.1245/s10434-012-2301-y. [DOI] [PubMed] [Google Scholar]


