Abstract
Objective
Malignant osseous spinal neoplasms are aggressive tumors associated with poor outcomes despite aggressive multidisciplinary measures. While surgical resection has been shown to improve short-term local disease control, it remains debated whether surgical resection is associated with improved overall survival in patients with malignant primary osseous spinal neoplasms. The aim of this manuscript is to review survival data from a US cancer registry spanning 30 years to determine if surgical resection was independently associated with overall survival.
Methods
The SEER registry (1973–2003) was queried to identify cases of histologically confirmed primary spinal chordoma, chondrosarcoma, osteosarcoma, or Ewing’s sarcoma of the mobile spine and pelvis. Patients with systemic metastasis were excluded. Age, gender, race, tumor location, and primary treatments were identified. Extent of local tumor invasion was classified as confined within periosteum versus extension beyond periosteum to surrounding tissues. The association of surgical resection with overall survival was assessed via Cox analysis adjusting for age, radiotherapy, and tumor invasiveness.
Results
827 patients were identified with non-metastatic primary osseous spinal neoplasms (215 chordoma, 282 chondrosarcoma, 158 osteosarcoma, 172 Ewing’s sarcoma). Overall, median survival was histology specific (chordoma, 96 months; Ewing’s sarcoma, 90 months; chondrosarcoma, 88 months; osteosarcoma, 18 months). Adjusting for age, radiation therapy, and extent of local tumor invasion in patients with isolated (non-metastatic) spine tumors, surgical resection was independently associated with significantly improved survival for chordoma [hazard ratio (95 % confidence interval; 0.617 (0.25–0.98)], chondrosarcoma [HR (95 %CI); 0.153 (0.07–0.36)], osteosarcoma [HR (95 %CI); 0.382 (0.21–0.69)], and Ewing’s sarcoma [HR (95 %CI); 0.494 (0.26–0.96)].
Conclusion
In our analysis of a 30-year US population-based cancer registry (SEER), patients undergoing surgical resection of primary spinal chordoma, chondrosarcoma, Ewing’s sarcoma, or osteosarcoma demonstrated prolonged overall survival independent of patient age, extent of local invasion, or location. Surgical resection may play a role in prolonging survival in the multi-modality treatment of patients with these malignant primary osseous spinal neoplasms.
Keywords: Biopsy, Chondrosarcoma, Chordomas, Ewing’s sarcoma, Osteosarcoma, Survival, Surgery
Introduction
The most common malignant primary bone tumors of the spine include chordomas, osteosarcomas, chondrosarcomas, and Ewing’s sarcomas [1]. These tumors can cause significant morbidity and mortality secondary to local invasion and destruction of adjacent structures as well as metastasize to distant organs. Treatment of these tumors usually begins with acquiring tissue for diagnosis [29]. This tissue can be obtained by needle biopsy or surgical resection. The efficacy of surgical resection in prolonging survival, however, is poorly understood. This lack of understanding is primarily due to the rarity of these malignancies, which account for less than 5 % of all osseous neoplasms and less than 0.2 % of all cancers [1]. As a result, previous studies on the efficacy of surgical resection have been limited to small institutional series and controlled trials [29].
Studies, however, using the Surveillance, Epidemiology, and End Results (SEER) registry may provide a better source of understanding for pathologies that are rare. This registry is the most comprehensive source of cancer information because it collects incidence and survival data for patients with cancer from 26 % of the population in the United States. The goal of the present study was to conduct a large population-based study using the SEER registry to understand whether surgical resection as compared to biopsy was associated with improved survival for patients with primary non-metastatic chordomas, chondrosarcoma, osteosarcomas, and Ewing’s sarcoma. This understanding may help clarify the role of surgery in maximizing survival for patients with malignant primary bone tumors.
Methods
The SEER registry, a database maintained by the National Cancer Institute, collects incidence and survival data from 17 population-based cancer registries covering approximately 25 % of the United States population. The database contains information on primary tumor site, histology, stage at diagnosis, treatment regimens including surgery and radiation therapy (XRT), and year of death. We searched the SEER database to identify all registered cases of Ewing’s sarcoma, osteosarcoma, chondrosarcoma, and chordoma in order to assess histology-specific survival during the period from 1973 to 2003.
International classification of disease for oncology, third edition criteria were used to identify cases of histologically confirmed Ewing’s sarcoma (ICD-O code: 9260), osteosarcoma (ICD-O code: 9180–9185, 9190), chondrosarcoma (ICD-O: 9220), and chordoma (ICD-O: 9370). Histological confirmation was obtained in all patients, either from biopsy or surgical pathology. The study population included patients within the SEER database diagnosed between 1973 and 2003. Covariates identified were patient age at diagnosis, year of diagnosis, and site of primary tumor, including vertebral column (ICD-O code: 412) versus (ICD-O code: sacrum/pelvis), metastasis status, whether XRT was administered, extent of tumor invasion, and whether surgical resection was performed. Data regarding chemotherapy was not available in the SEER database. Surgical management was defined at the time of care by the treatment team as: (1) biopsy for tissue diagnosis or (2) surgical resection. Differentiation between intra-lesional and en bloc resection was not made in the SEER registry. Extent of local tumor invasion was defined at the time of care by histological specimen, radiographic analysis, or intra-operative surgeon assessment and classified as: (1) confined (tumor confined to cortex of bone or extension beyond cortex but confined within periosteum) or (2) local invasion (extension beyond periosteum to surrounding tissues, including adjacent skeletal muscle, adjacent bone/cartilage, or skin).
For the purposes of this study, patients presenting with distal site metastasis were excluded. Only patients with isolated primary osseous spine tumors were included. The primary outcome of interest was overall survival. Estimated Kaplan–Meier survival was calculated as the time from diagnosis to death or last follow-up. Observations were censored when a patient was alive at the time of last follow-up. For each tumor histology, the association of surgical resection with overall survival was assessed via Cox proportional-hazards regression analysis adjusting for age, XRT, and extent of local tumor invasion.
Results
Patient population
A total of 827 patients were identified with non-metastatic primary osseous spinal neoplasms (215 chordoma, 282 chondrosarcoma, 158 osteosarcoma, 172 Ewing’s sarcoma). Patients presenting with Ewing’s sarcoma of the spine were on average younger (19 ± 10 years, p < 0.01) and patients presenting with spinal chordoma were on average older (59 ± 16 years, p < 0.01) compared to patients with chondrosarcoma or osteosarcoma (49 ± 20 years) (Table 1). The majority of patients were male for all tumor types, ranging from 55 % for osteosarcoma to 70 % for Ewing’s sarcoma (Table 1). African-Americans comprised a significantly greater proportion of osteosarcoma than all other tumors (13 vs. 4 %, p < 0.01). Otherwise, there was no association between race and histology type.
Table 1.
Characteristics of 827 patients with malignant primary osseous spinal neoplasms without metastasis from the surveillance, epidemiology, and end results (SEER) registry 1973–2003
| Patient characteristic (n, %) | Ewing’s sarcoma (n = 172) | Osteosarcoma (n = 158) | Chondrosarcoma (n = 282) | Chordoma (n = 215) |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD (years) | 19 ± 10 | 46 ± 23 | 49 ± 18 | 59 ± 16 |
| Female sex | 51 (30) | 72 (45) | 93 (33) | 82 (38) |
| Race | ||||
| Caucasian | 158 (90) | 125 (79) | 253 (90) | 192 (89) |
| African-American | 5 (3) | 20 (13) | 15 (5) | 7 (3) |
| Native American | 2 (1) | 1 (1) | 1 (0.4) | 2 (1) |
| Asian | 7 (4) | 12 (7) | 13 (4.6) | 14 (7) |
| Primary site | ||||
| Vertebral column | 58 (33) | 50 (32) | 58 (20) | 95 (44) |
| Sacrum/pelvis | 114 (67) | 108 (68) | 224 (80) | 120 (56) |
| Extent invasion | ||||
| Confined | 53 (31) | 37 (23) | 114 (40) | 80 (37) |
| Local invasion | 119 (69) | 121 (77) | 168 (60) | 135 (63) |
| Radiation therapy | 116 (67) | 45 (29) | 54 (19) | 94 (43) |
| Surgical treatment data available | 80 | 80 | 134 | 95 |
| Diagnostic biopsy | 34 (43) | 26 (32) | 12 (9) | 10 (11) |
| Surgical resection | 46 (57) | 54 (68) | 122 (91) | 85 (89) |
| Median survival | ||||
| All patients | 90 months | 18 months | 88 months | 96 months |
| No surgery | 43 months | 11 months | 16 months | 53 months |
| Surgery | Undetermineda | 37 months | 192 months | 87 months |
| Surgery + XRT | 72 months | 43 months | Undetermineda | 104 months |
| 5-year survival | ||||
| No surgery | 37 | 0 | 33 | 50 |
| Surgery | 74 | 27 | 71 | 71 |
| Surgery + XRT | 60 | 43 | 65 | 80 |
Values in parentheses are expressed in percentage
Confined tumor confined within periosteum; local invasion extension beyond periosteum to adjacent skeletal muscle, bone/cartilage, or skin
aUndetermined: median survival not reached during follow-up period
Presentation and treatment
The sacrum versus mobile spine was more frequently the site of tumor location for all tumor types (Table 1). For all histology types, the majority of tumors had invaded through the periosteum (66 %) rather than being confined within the periosteum (34 %) (Table 1). Surgical resection was performed in the vast majority of patients presenting with chordoma (89 %) and chondrosarcoma (91 %), and in the slight majority for osteosarcoma (68 %) and Ewing’s sarcoma (57 %). XRT was most commonly administered in patients with Ewing’s sarcoma (67 %) and least commonly in patients with chondrosarcoma (19 %) (Table 1).
Survival
For all patients, 401 (48 %) died during their SEER follow-up period. Mean follow-up for surviving patients was 85 ± 50 months. Overall median survival was histology specific (Table 1). Median survival was 90 months for Ewing’s sarcoma, 96 months for chordoma, 88 months for chondrosarcoma, and 18 months for osteosarcoma.
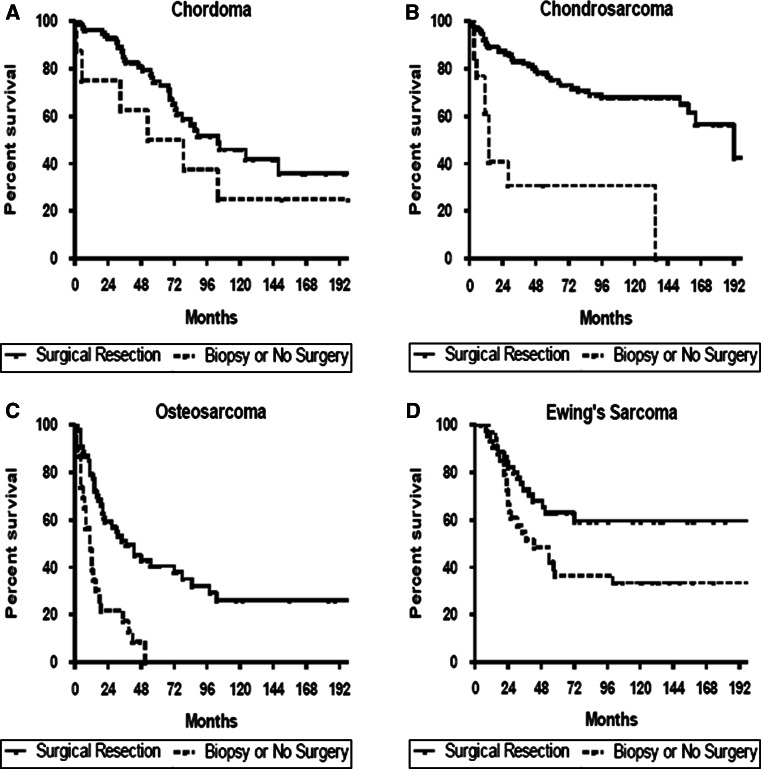
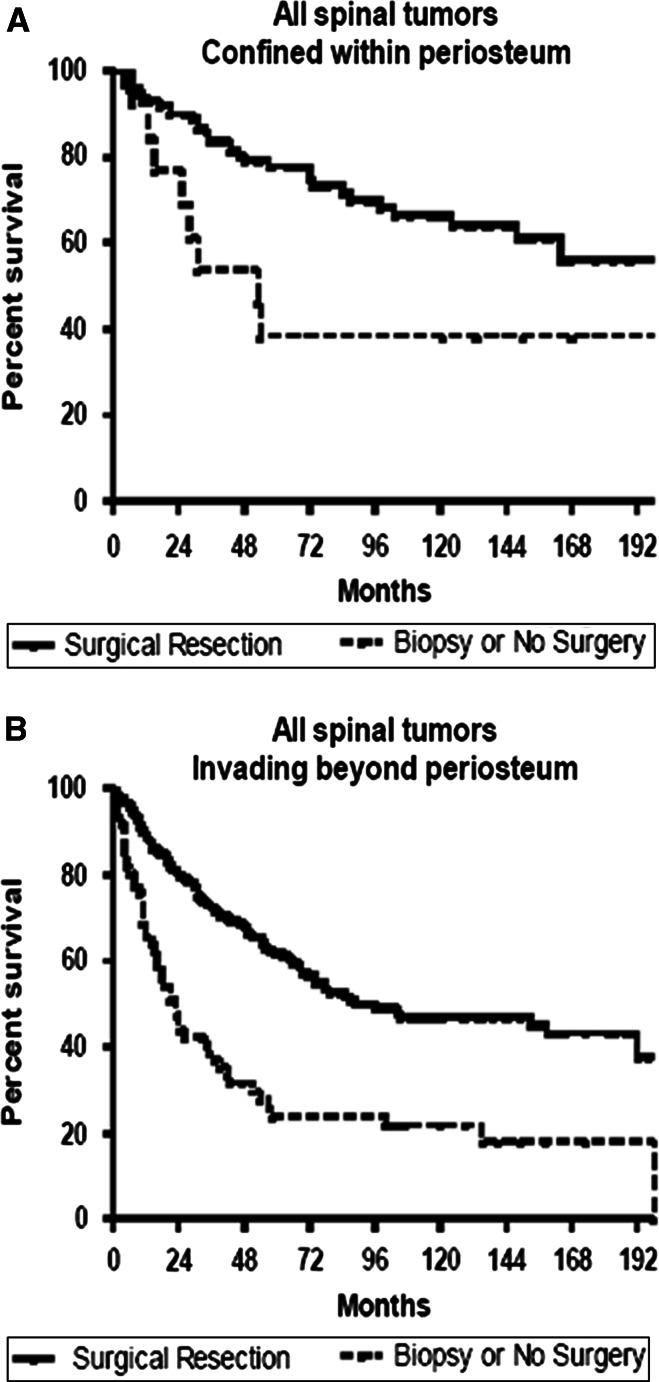
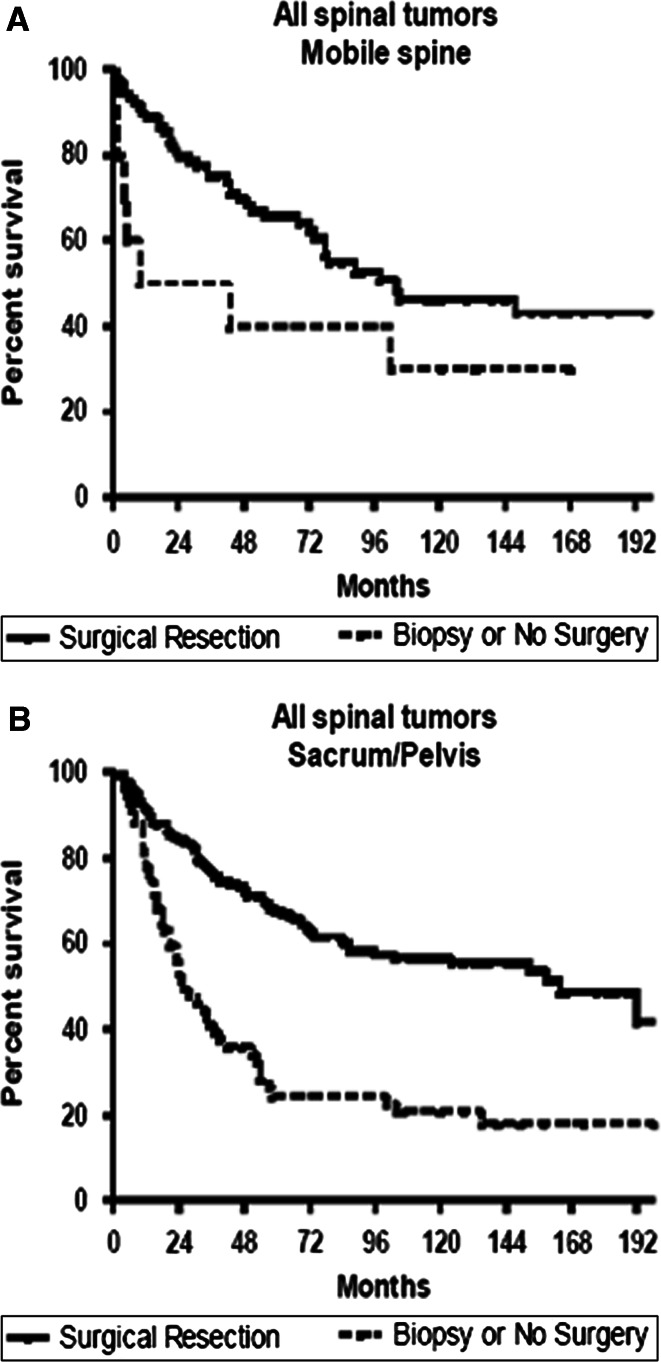
Adjusting for age, XRT, and extent of local tumor invasion in patients with isolated (non-metastatic) spine tumors, surgical resection was associated with significantly improved survival for chordoma [hazard ratio (95 % confidence interval (CI); 0.617 (0.25–0.98)], chondrosarcoma [HR (95 %CI); 0.153 (0.07–0.36)], osteosarcoma [HR (95 %CI); 0.382 (0.21–0.69)], and Ewing’s sarcoma [HR (95 %CI); 0.494 (0.26–0.96)] (Fig. 1). In patients with chondrosarcoma or Ewing’s sarcoma, median and 5-year survival was similar for patients undergoing surgery + XRT versus surgery alone. However, in patients with osteosarcoma and chordoma, median and 5-year survival was greater for patients undergoing surgery + XRT versus surgery alone (Table 1). In analysis of all tumor types, patients undergoing surgery had better survival for both tumors confined to and extending beyond the periosteum relative to patients in the biopsy cohort (Fig. 2). Patients undergoing surgical resection also had better survival for both lesions of the mobile spine and sacrum/pelvis relative to patients in the biopsy cohort (Fig. 3).
Fig. 1.
Kaplan–Meier estimated survival in patients with primary osseous spinal tumors a chordoma, b chondrosarcoma, c osteosarcoma, or d Ewing’s sarcoma stratified by surgical resection versus no surgery. Adjusting for age, radiation therapy, and extent of local tumor invasion in patients with isolated (non-metastatic) spine tumors, surgical resection was associated with significantly improved survival for chordoma [Hazard Ratio (95 % confidence interval; 0.617 (0.25–0.98)], chondrosarcoma [HR (95 %CI); 0.153 (0.07–0.36)], osteosarcoma [HR (95 %CI); 0.382 (0.21–0.69)], and Ewing’s sarcoma [HR (95 %CI); 0.494 (0.26–0.96)]
Fig. 2.
Kaplan–Meier estimated survival in patients with primary osseous spinal tumors that were: a confined (tumor confined to cortex of bone or extension beyond cortex but confined within periosteum) or b locally invasive (extension beyond periosteum to surrounding tissues, including adjacent skeletal muscle, adjacent bone/cartilage, or skin). Surgery was associated with increased survival for both primary spinal tumors that were confined (p < 0.01) or locally invasive (p < 0.001)
Fig. 3.
Kaplan–Meier estimated survival in patients with primary osseous spinal tumors that were located a in the mobile spine or b the sacrum/pelvis. Surgery was associated with increased survival for both primary tumors located in the mobile spine (p < 0.05) as well as those located in the sacrum/pelvis (p < 0.001)
Discussion
In our analysis of the four most common primary spine tumors recorded in the SEER registry over three decades, we evaluated the role of surgical resection and biopsy for patients with isolated malignant primary osseous tumors of the spinal column. Overall median survival was histology specific, where the median survival was 90 months for Ewing’s sarcoma, 96 months for chordoma, 88 months for chondrosarcoma, and 18 months for osteosarcoma. Patients who underwent surgical resection had improved survival as compared to patients who underwent biopsy, even after adjusting for age at the time of surgery, XRT, and extent of local tumor invasion for all four tumor types. Additionally, patients had improved survival with surgical resection regardless of extent of tumor invasion or spinal location. Interestingly, among patients who underwent surgical resection, adjuvant XRT was associated with prolonged survival for patients with osteosarcoma and chordomas.
There are approximately 2,380 new cases of bone cancer diagnosed in the United States each year, with approximately 5 % involving the spine [1]. Among malignant primary osseous neoplasms, the four most common include osteosarcoma (35 %), chondrosarcoma (26 %), Ewing’s sarcoma (16 %), and chordoma (8 %) [8, 11, 18, 24, 27, 33]. The overall 5-year relative survival rates for spine-limited malignant bone tumors range from 10 to 30 % for osteosarcoma [25, 30, 34], 50–75 % for chondrosarcoma [4, 7, 31, 37], 30–65 % for Ewing’s sarcoma [12, 15, 20, 26], and 50–85 % for chordoma [5, 6, 9, 22, 38]. These tumors also are known for having high recurrence rates. The repeated 5-year progression-free survival has ranged from 0 to 25 % for osteosarcoma [25, 30, 34], 50–70 % for chondrosarcoma [4, 7, 16], 30–60 % for Ewing’s sarcoma [2, 3, 15, 20], and 45–65 % for chordomas [13, 17]. The high recurrence rates, limited survival duration, and functional morbidity associated with these tumors have supported the need for aggressive multi-modality strategies for these tumors. Surgical resection decreases tumor burden, may increase chemotherapy and/or XRT efficacy, and allow for neural decompression and spinal stabilization. Additionally, in some cases, surgery may entirely remove the tumor with negative margins [29]. Despite these reported advantages, the overall benefit of surgical resection of various types of primary malignant bone tumors has yet to be demonstrated in a population-based study.
Ewing’s sarcoma is a poorly differentiated, small round cell tumor that typically arises outside of the spine [36]. Classic treatment of these malignancies has typically involved chemotherapy and XRT after obtaining tissue for diagnosis [29]. Surgical resection is typically reserved for cases in which the primary tumor can be completely removed, which is often difficult in the spine due to anatomical limitations [29]. Bacci et al. [3] evaluated 43 patients with spine tumors over an approximate 20-year time span at a single institution and found no difference in survival between patients who were treated locally with radiation and those treated by radiation and surgery. Likewise, Paulino and colleagues [26] evaluated 76 patients with localized Ewing’s sarcoma (only 11 of which had spine involvement) and found there was no difference in survival for patients with radiation, surgery, and radiation with surgery. However, these limited studies were far too underpowered to assess the role of surgery or survival. The present study, however, with 182 patients with isolated spinal Ewing’s sarcoma found a survival advantage for patients treated with surgical resection over biopsy by more than twofold. This was true regardless of extent of local tumor invasion, spine location (mobile vs. sacrum/pelvis), or age.
Osteosarcoma is the most common type of malignant bone cancer. Classic treatment involves a multidisciplinary approach including pre-operative chemotherapy followed by surgical resection [14, 19]. This has resulted in improved survival, but these studies have been primarily limited to patients with limb and not spine involvement. Delaney et al. [10] found a significant 5-year survival advantage between patients who underwent surgical resection (75 %) relative to those who underwent biopsy (25 %) for patients with osteosarcoma. Of the 41 patients in this series, patients with metastatic disease were included and only eight patients had spine involvement [10]. Ozaki et al. [25] evaluated 22 patients with spinal osteosarcoma, with 6 patients having metastatic disease. They found a significant survival difference between 5 patients who underwent wide excision relative to 17 patients (10 biopsy and 7 intra-lesional) who did not undergo wide excision [25]. Similarly, Sundaresan et al. [34] evaluated 24 patients with spinal osteosarcoma treated between 1949 and 1984, and found that patients who underwent more aggressive treatment (surgery, radiation, chemotherapy) had improved survival over biopsy and radiation. The independent effect of surgery, radiation, and/or chemotherapy on survival could not be determined given the low patient numbers [34]. The present study with 158 patients with isolated spinal osteosarcoma without metastasis found a survival advantage for patients treated with surgical resection over biopsy by almost threefold. This was true regardless of extent of local tumor invasion spine location (mobile vs. sacrum/pelvis), or age. Radiation was associated with enhanced survival for those patients who underwent surgery, which has been seen in prior studies [25].
Chondrosarcoma is a cartilage-based bone tumor and is the second most common type of malignant bone tumor [33]. Chordomas are tumors that arise from notochordal elements, and typically occur in the clivus and sacrum [33]. Studies evaluating the role of surgery versus medical management alone for these lesions are few and limited. The majority of studies for have compared intra-lesional versus wide or en bloc resection, and are limited to only resectable lesions [4–7, 13, 16]. The majority of these studies suggest that surgery improved local disease control and prolongs survival when en bloc resection is achieved. The present study with 282 and 215 patients with isolated spinal chondrosarcoma and chordomas found a survival advantage for patients treated with surgical resection over biopsy by more than 6- and 2-fold, respectively. This was true regardless of extent of local tumor invasion, spine location (mobile vs. sacrum/pelvis), or age. Radiation was associated with prolonged survival for patients with chordomas, but not chondrosarcomas.
The present study is the first population-based study on malignant primary osseous tumors of the spine with sufficient power to evaluate the role of surgical resection on survival. We believe this study provides several useful insights. Importantly, this study supports the notion that patients may experience a survival benefit from surgical resection of their lesion. Previous studies on the efficacy of surgical resection as compared to biopsy for patients with primary malignant bone tumors of the spine have been limited to small institutional studies and clinical trials. Additionally, this survival advantage for patients undergoing surgical resection was independent of variables that may affect survival including metastasis status, age, XRT, and degree of invasion which minimizes the bias associated with retrospective analysis. Furthermore, radiation may play a role in enhancing survival following surgical resection, as reported in non-spinal osseous malignancies.
This study, however, has limitations. One limitation is that the variations in specific treatment strategies cannot be accounted for including chemotherapy and spine stabilization techniques. Even though most of these osseous tumors are chemo-resistant, the SEER registry does not contain chemotherapy-specific regimens. Additionally, the SEER registry lacks information on en bloc versus intra-lesional resection. Previous studies have shown that surgical technique may have an impact on survival as may chemotherapy regimens [21, 23, 28, 32, 35, 38]. Moreover, the functional outcome for patients who underwent either surgical resection or biopsy was not available. Although not the focus of this study, neural decompression and stabilization may improve pain and disability in many patients [29]. Despite these inherent limitations, our study focuses on a uniform patient population by utilizing a strict inclusion and exclusion criteria, thus providing more relevant information for patients with primary malignant osseous neoplasms. We included only patients with primary malignant osseous neoplasms and excluded patients with any distant metastatic disease. Furthermore, we performed multivariate analyses and controlled for potential peri-operative confounding variables (age, metastatic disease, extent of invasion, radiation). Hence, the decreased survival obtained with non-surgical patients was not due to increased local invasion and decreased respectability, increased age, or greater distant tumor burden, which decreases but does not eliminate treatment bias. Given these statistical controls and a relatively precise outcome measure, we believe our findings offer useful insights into the management of patients with malignant osseous neoplasms of the spine. Prospective controlled studies are needed to clarify the observations made here.
Conclusion
In our analysis, using the SEER registry over a 30-year period, this study is the largest to evaluate the efficacy of surgery or biopsy for patients with isolated primary malignant osseous tumors of the spine. Patients with osteosarcomas, chondrosarcomas, Ewing’s sarcoma, and chordomas who underwent surgery experienced prolonged survival as compared to patients who underwent medical management alone. This was statistically significant even after controlling for age, XRT, degree of local invasion, and tumor location. XRT was associated with prolonged survival for patients with osteosarcoma and chordomas, but not Ewing’s sarcoma and chondrosarcoma. The findings of this study may help guide treatment strategies aimed at prolonging survival for patients with malignant primary osseous neoplasms of the spine and support the need for a trial to definitively assess the efficacy of surgery at prolonging.
Conflict of interest
None.
Contributor Information
Debraj Mukherjee, Email: debraj.mukherjee@cshs.org.
Matthew J. McGirt, Phone: +1-410-2927026, FAX: +1-615-3439553, Email: mmcgirt1@jhmi.edu
References
- 1.American Cancer Society (2008) Cancer Facts & Figures 2008. American Cancer Society, Atlanta
- 2.Arai Y, Kun LE, Brooks MT, Fairclough DL, Fontanesi J, Meyer WH, et al. Ewing’s sarcoma: local tumor control and patterns of failure following limited-volume radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21:1501–1508. doi: 10.1016/0360-3016(91)90325-X. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Boriani S, Balladelli A, Barbieri E, Longhi A, Alberghini M, et al. Treatment of nonmetastatic Ewing’s sarcoma family tumors of the spine and sacrum: the experience from a single institution. Eur Spine J. 2009;18:1091–1095. doi: 10.1007/s00586-009-0921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergh P, Gunterberg B, Meis-Kindblom JM, Kindblom LG. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201–1212. doi: 10.1002/1097-0142(20010401)91:7<1201::AID-CNCR1120>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–2134. doi: 10.1002/(SICI)1097-0142(20000501)88:9<2122::AID-CNCR19>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M, et al. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976) 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 7.Boriani S, De Iure F, Bandiera S, Campanacci L, Biagini R, Di Fiore M, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000;25:804–812. doi: 10.1097/00007632-200004010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bovill EG, Jr, Kung’u A, Bencivenga A, Jeshrani MK, Mbindyo BS, Heda PM. An epidemiological study of osteogenic sarcoma in Kenya: the variations in incidence between ethnic groups and geographic regions, 1968–1978. Int Orthop. 1985;9:59–63. doi: 10.1007/BF00267039. [DOI] [PubMed] [Google Scholar]
- 9.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC., Jr Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976) 1999;24:1639–1645. doi: 10.1097/00007632-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 10.DeLaney TF, Park L, Goldberg SI, Hug EB, Liebsch NJ, Munzenrider JE, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:492–498. doi: 10.1016/j.ijrobp.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75:203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::AID-CNCR2820751308>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Evans RG, Nesbit ME, Gehan EA, Garnsey LA, Burgert O, Jr, Vietti TJ, et al. Multimodal therapy for the management of localized Ewing’s sarcoma of pelvic and sacral bones: a report from the second intergroup study. J Clin Oncol. 1991;9:1173–1180. doi: 10.1200/JCO.1991.9.7.1173. [DOI] [PubMed] [Google Scholar]
- 13.Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–122. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]
- 14.Gherlinzoni F, Picci P, Bacci G, Campanacci D. Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann Oncol. 1992;3(Suppl 2):S23–S27. doi: 10.1093/annonc/3.suppl_2.S23. [DOI] [PubMed] [Google Scholar]
- 15.Grubb MR, Currier BL, Pritchard DJ, Ebersold MJ. Primary Ewing’s sarcoma of the spine. Spine (Phila Pa 1976) 1994;19:309–313. doi: 10.1097/00007632-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh PC, Xu R, Sciubba DM, McGirt MJ, Nelson C, Witham TF, et al. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine (Phila Pa 1976) 2009;34:2233–2239. doi: 10.1097/BRS.0b013e3181b61b90. [DOI] [PubMed] [Google Scholar]
- 17.Ito E, Saito K, Okada T, Nagatani T, Nagasaka T. Long-term control of clival chordoma with initial aggressive surgical resection and gamma knife radiosurgery for recurrence. Acta Neurochir (Wien) 2010;152:57–67. doi: 10.1007/s00701-009-0535-7. [DOI] [PubMed] [Google Scholar]
- 18.Larsson SE, Lorentzon R. The incidence of malignant primary bone tumours in relation to age, sex and site. A study of osteogenic sarcoma, chondrosarcoma and Ewing’s sarcoma diagnosed in Sweden from 1958 to 1968. J Bone Jt Surg Br. 1974;56B:534–540. [PubMed] [Google Scholar]
- 19.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A et al (1991) Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res 8–14 [PubMed]
- 20.Marco RA, Gentry JB, Rhines LD, Lewis VO, Wolinski JP, Jaffe N, et al. Ewing’s sarcoma of the mobile spine. Spine (Phila Pa 1976) 2005;30:769–773. doi: 10.1097/01.brs.0000157755.17502.d6. [DOI] [PubMed] [Google Scholar]
- 21.Marmor E, Rhines LD, Weinberg JS, Gokaslan ZL. Total en bloc lumbar spondylectomy. Case report. J Neurosurg. 2001;95:264–269. doi: 10.3171/spi.2001.95.2.0264. [DOI] [PubMed] [Google Scholar]
- 22.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/A:1008947301735. [DOI] [PubMed] [Google Scholar]
- 23.Navid F, Willert JR, McCarville MB, Furman W, Watkins A, Roberts W, et al. Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer. 2008;113:419–425. doi: 10.1002/cncr.23586. [DOI] [PubMed] [Google Scholar]
- 24.Oyemade GA, Abioye AA. Primary malignant tumors of bone: incidence in Ibadan, Nigeria. J Natl Med Assoc. 1982;74:65–68. [PMC free article] [PubMed] [Google Scholar]
- 25.Ozaki T, Flege S, Liljenqvist U, Hillmann A, Delling G, Salzer-Kuntschik M, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069–1077. doi: 10.1002/cncr.10258. [DOI] [PubMed] [Google Scholar]
- 26.Paulino AC, Nguyen TX, Mai WY. An analysis of primary site control and late effects according to local control modality in non-metastatic Ewing sarcoma. Pediatr Blood Cancer. 2007;48:423–429. doi: 10.1002/pbc.20754. [DOI] [PubMed] [Google Scholar]
- 27.Price CH, Jeffree GM. Incidence of bone sarcoma in SW England, 1946–74, in relation to age, sex, tumour site and histology. Br J Cancer. 1977;36:511–522. doi: 10.1038/bjc.1977.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao G, Suki D, Chakrabarti I, Feiz-Erfan I, Mody MG, McCutcheon IE, et al. Surgical management of primary and metastatic sarcoma of the mobile spine. J Neurosurg Spine. 2008;9:120–128. doi: 10.3171/SPI/2008/9/8/120. [DOI] [PubMed] [Google Scholar]
- 29.Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine (Phila Pa 1976) 2009;34:S58–S68. doi: 10.1097/BRS.0b013e3181ba6436. [DOI] [PubMed] [Google Scholar]
- 30.Shives TC, Dahlin DC, Sim FH, Pritchard DJ, Earle JD. Osteosarcoma of the spine. J Bone Jt Surg Am. 1986;68:660–668. [PubMed] [Google Scholar]
- 31.Shives TC, McLeod RA, Unni KK, Schray MF. Chondrosarcoma of the spine. J Bone Jt Surg Am. 1989;71:1158–1165. [PubMed] [Google Scholar]
- 32.Stacchiotti S, Marrari A, Tamborini E, Palassini E, Virdis E, Messina A, et al. Response to imatinib plus sirolimus in advanced chordoma. Ann Oncol. 2009;20:1886–1894. doi: 10.1093/annonc/mdp210. [DOI] [PubMed] [Google Scholar]
- 33.Sundaresan N, Rosen G, Boriani S (2009) Primary malignant tumors of the spine. Orthop Clin North Am 40:21–36, v [DOI] [PubMed]
- 34.Sundaresan N, Rosen G, Huvos AG, Krol G. Combined treatment of osteosarcoma of the spine. Neurosurgery. 1988;23:714–719. doi: 10.1227/00006123-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48:132–139. doi: 10.1002/pbc.20697. [DOI] [PubMed] [Google Scholar]
- 36.Weber KL. Current concepts in the treatment of Ewing’s sarcoma. Expert Rev Anticancer Ther. 2002;2:687–694. doi: 10.1586/14737140.2.6.687. [DOI] [PubMed] [Google Scholar]
- 37.York JE, Berk RH, Fuller GN, Rao JS, Abi-Said D, Wildrick DM, et al. Chondrosarcoma of the spine: 1954 to 1997. J Neurosurg. 1999;90:73–78. doi: 10.3171/spi.1999.90.1.0073. [DOI] [PubMed] [Google Scholar]
- 38.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74–79. doi: 10.1097/00006123-199901000-00041. [DOI] [PubMed] [Google Scholar]