Abstract
Background
The increased prevalence of spinal fusion surgery has created an industry focus on bone graft alternatives. While autologous bone graft remains the gold standard, the complications and morbidity from harvesting autologous bone drives the search for reliable and safe bone graft substitutes. With the recent information about the adverse events related to bone morhogenetic protein use, it is appropriate to review the literature about the numerous products that are not solely bone morphogenetic protein.
Purpose
The purpose of this literature review is to determine the recommendations for use of non-bone morphogenetic protein bone graft alternatives in the most common spine procedures based on a quantifiable grading system.
Study design
Systematic literature review.
Methods
A literature search of MEDLINE (1946–2012), CINAHL (1937–2012), and the Cochrane Central Register of Controlled Trials (1940–April 2012) was performed, and this was supplemented by a hand search. The studies were then evaluated based on the Guyatt criteria for quality of the research to determine the strength of the recommendation.
Results
In this review, more than one hundred various studies on the ability of bone graft substitutes to create solid fusions and good patient outcomes are detailed.
Conclusion
The recommendations for use of bone graft substitutes and bone graft extenders are based on the strength of the studies and given a grade.
Keywords: Spine fusion, Bone graft substitute, Bone graft extender, Allograft, Spine ceramics, Platelet-derived gel bone graft enhancers
Introduction
Spinal fusion surgery has significantly increased over the past few decades as new technology emerges to facilitate patient comfort and mobility [1]. Accordingly, the percentage of total spending on spine surgery including spinal fusion and new spinal fusion devices has also grown [1]. Arthrodesis is important in a wide range of spinal disorders including, but not limited to, scoliosis, degenerative disk disease, spondylolisthesis, spinal stenosis, and vertebral fractures [2–7]. Classically, autologous bone graft has been used to create fusion masses and in some cases to provide immediate structural support [8, 9]. Harvesting of autologous bone graft has limitations and has significant morbidity, as shown in Table 1 [10–14]. Previously, with the development of bone morphogenetic protein, many surgeons felt that the search for a suitable bone graft alternative was complete. Now that the adverse event profile has recently changed for bone morphogenetic protein, there is currently a re-evaluation of its application. The search for alternative biomaterials that will extend and supplement local bone graft continues.
Table 1.
Reported complication rates associated with iliac crest bone harvest
| Complication | Reported rate of occurrence |
|---|---|
| Minor complications | 10–39 % |
| Superficial infections | |
| Superficial seromas | |
| Superficial hematomas | |
| Major complications | 5.8–10 % |
| Herniation | |
| Vascular injuries | |
| Deep infections at the donor site | |
| Neurologic injuries | |
| Deep hematoma formation requiring OR | |
| Iliac wing fractures | |
| Chronic donor site pain (>24 months) | 25–60 % |
| Reoperation due to wound complications | 2–5 % |
| Poor appearance of graft site | 5–16 % |
| Harvest site numbness | 24 % |
| Pain, paresthesias, hematoma, infection | Up to 50 % |
| Difficulty in daily activities at 36 mo post-op | 18–19 % |
Significant progress has been made in the field of bone graft alternatives with multiple different types such as cadaver-derived bone, animal-derived bone, synthetics, and combinations of the above. However, iliac crest bone graft (ICBG) remains the “gold standard” of graft options as it is both osteoconductive, providing a scaffold for osteoblast adherence and neovascularization, and osteoinductive, stimulating precursor cells to migrate into the graft site and differentiate into osteoblasts [8, 9]. Autologous laminectomy bone (ALB) is often used as a bone graft instead of ICBG and also has excellence fusion rates as it contains three critical elements for enhanced osteogenesis: the trabeculae of the bone provide an osteoconductive scaffold, it contains bone morphogenetic proteins (BMPs) with osteoinductive potential, and it contains osteoblasts as a source of osteogenic cells [6, 15]. Despite the associated donor site morbidities, as shown in Table 1, increased blood loss, operating time and hospitalization time, iliac crest autograft is still the graft of choice for spine surgery because of the excellent fusion rates [10–14].
There are an overwhelming amount of alternatives to enhance or substitute for autologous bone. We will evaluate the use of various forms of allograft, platelet gels, and ceramics as alternatives to bone graft. Allograft bone is neither osteogenic or osteoinductive, and thus does not induce new bone formation in the same manner as autogenous bone graft. The porosity of allograft is comparable to live bone, and promotes new bone formation by providing an osteoconductive scaffold. Fresh-frozen allograft contains some growth factors for osteoinduction. Demineralized bone matrix (DBM) is not used as a structural graft because of its consistency, but is able to act as graft extender due to its osteoinductive and osteoconductive characteristics. Platelet gels are osteoinductive, and are used as bone graft enhancers in conjunction with ICBG, ALB, or allograft bone. Ceramics are osteoconductive and biodegradable bone graft scaffolds [16–19]. ALB and ceramics may be combined with bone marrow aspirate (BMA) to enhance the osteoinductive and osteogenic potential [15]. Each graft alternative has advantages and disadvantages, and the options must be considered on an individual, risk–benefit basis to select the best option for each patient undergoing spinal fusion. The purpose of this manuscript is to review the efficacy of different non-bone morphogenetic protein bone graft alternatives in the most common spine procedures, and to summarize the research that has been done on their safety and efficacy in the literature, and provide recommendations based on the level of evidence for each bone graft substitute.
Methods
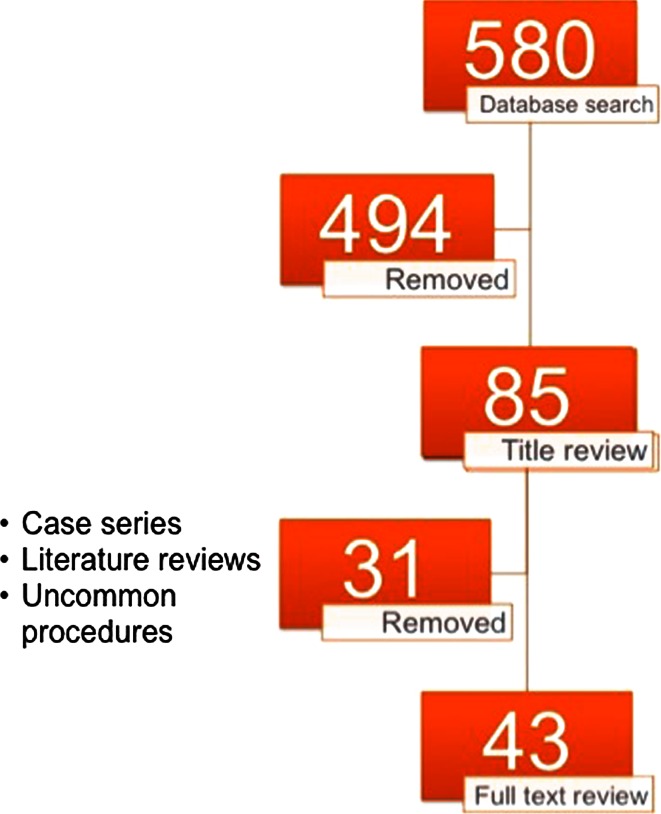
A literature search of MEDLINE (1946–2012), CINAHL (1937–2012), and the cochrane central register of controlled trials (1940–April 2012), was performed, and this was supplemented by a hand search. The search strategy was designed to with the concept of maximum sensitivity. The only limitations were the studies were in the English language and on human subjects. The studies included in this review are only studies of a comparative nature, such as randomized controlled trials (RCTs), prospective comparative studies between iliac crest bone graft and a bone graft substitute, retrospective comparative studies between different bone graft substitutes. Due to the importance of their contribution, well done cohort studies with mid to long-term outcomes of either iliac crest bone graft or a bone graft substitute were included if they were felt to clearly help understand the outcomes of the bone graft substitute. The spinal procedures reviewed in this review are the most common procedures with enough comparative studies to evaluate the strength of evidence. These procedures include anterior cervical discectomy and fusion, anterior lumbar interbody fusion, posterolateral lumbar fusion, transformational lumbar interbody fusion, and posterior spinal fusion for adolescent idiopathic scoliosis. Figure 1 demonstrates the number of studies found at each stage of the literature search process.
Fig. 1.
Diagram of literature search with articles removed at each stage
The articles were then reviewed based on the quality of the research. The recommendations are based on a standardized reference for the quality of research supporting use of different products. In a literature review conducted by Miyazaki et al., the authors created a standardized recommendation grading scale based on the Guyatt criteria shown in Table 2 [17, 20]. The Guyatt criteria grading scale is currently the most compelling and innovative approach, in which a recommendation is given a number (1 or 2) and letter (A, B, or C) designation. The number designation is based on the trade-off between the benefits of a medication/surgery/device and the risk, harms, or cost. If, based on current literature, the benefits clearly do or do not outweigh the risks, a Grade 1 (strong) recommendation is made. For example, use of short-term aspirin after myocardial infarction has been shown through randomized controlled trials to reduce the relative risk of death with minimal side effects and low cost, and has been given a strong Grade 1 recommendation from the American College of Chest Physicians Task Force [21]. With less certainty of the balance between benefits and risks, in which patients’ or societal values or circumstances may be more influential because the evidence is weak, a Grade 2 (weak) recommendation is made. The letter designation represents the quality of the underlying evidence and is based on study methodology, and corresponds to the strength of the evidence of the literature for the grade of recommendation [22]. Randomized trials with consistent results provide Grade A (high) support, randomized trials with inconsistent results or methodological flaws provide Grade B (moderate) support, while observational studies provide Grade C (low) support. The second highest grade of all studies in a category became the grade for the recommendation of use of the bone graft substitute for that category. We feel this most appropriately captures the range of quality of the studies for a particular indication.
Table 2.
Grading of recommendations according to Gyatt et al.
| Grade | Clarity of risk/benefit | Methodologic strength of supporting evidence | Implications |
|---|---|---|---|
| 1A | Clear | Randomized trials without important limitations | Strong recommendation; can apply to most patients in most circumstances without reservation |
| 1B | Clear | Randomized trials with important limitations (inconsistent results, methodologic flaws) | Strong recommendations, likely to apply to most patients |
| 1C+ | Clear | No RCTs, but RCT results can be unequivocally extrapolated, or overwhelming evidence from observation studies | Strong recommendation: can apply to most patients in most circumstances |
| 1C | Clear | Observation studies | Intermediate-strength recommendation; may change when stronger evidence available |
| 2A | Unclear | Randomized trials without important limitations | Intermediate-strength recommendation; best action may differ depending on circumstances or patients’ or societal values |
| 2B | Unclear | Randomized trials with important limitations (inconsistent results, methodologic flaws) | Weak recommendation; alternative approaches likely to be better for some patients under some circumstances |
| 2C | Unclear | Observation studies | Very weak recommendations; other alternatives may be equally reasonable |
Various techniques have been described in the literature regarding methods to determine whether an arthrodesis had been achieved postoperatively, including static AP and lateral radiographs alone, inclusion of dynamic flexion/extension radiographs, computed tomography (CT) scan, and surgical exploration during revision surgery. Radiographically, adequate fusion has been assessed for the presence of bony bridge formation between endplates on static films, progression of deformity, or uninterrupted bone bridging between transverse processes on at least one side of a fusion mass without breaks, clefts, or regions of resorption, with interobserver correlation of 86 % [23–25]. Flexion/extension radiographs allow evaluation of instrumentation motion or screw radiolucencies that may suggest loosening [23]. Outcomes of fusion analysis vary between radiographic and CT-reconstruction analysis. In a randomized animal model, CT scan was found to have a much higher accuracy for detecting fusion or pseudarthrosis than static or dynamic radiographs [26]. However, the correlation between the radiographic evidence of solid arthodesis and clinical outcomes has not been fully established. The specific manner of fusion analysis used in the literature will be discussed in subsequent sections.
Allograft
Fresh-frozen or freeze-dried allograft
Clinical studies
There are two important studies that pertain to all applications of the use of allograft in spine surgery. The first is a study evaluating the rate of post-operative bacterial infections after allograft use because there is the small chance that allograft could be infected with bacteria at the time of implantation. Mikhael et al. [27] performed a retrospective chart review on 1,435 patients who underwent spine fusion procedures with irradiated allograft (144), non-irradiated allograft (441), and autograft (850), and who had a minimum of 1-year follow-up. They found surgical site infections rates of 1.7, 3.2, and 4.3 % respectively, with no significant difference (p = 0.51). Thus, there is no increased risk of post-operative infections associated with the use of allograft bone. The second pivotal study is the one conducted by Buck et al. [28] evaluating the risk of viral disease transmission with allograft bone use. Buck et al. determined that the chance of receiving an allograft implantation from an HIV positive donor is 1 in 1.67 million. This is less than the risk of death from flying, 1 in 500,000, or the risk of death from driving, 1 in 7,000. Nasca et al. [29] reviewed the use of cryopreserved bone grafts in all of spine surgery, and deemed the use of allograft as safe and similar fusion rates to autograft. They did not evaluate based on procedure, so the fusion rates cited in the study are not as helpful as those cited below.
Anterior cervical
The most commonly studied use of isolated allograft bone in the literature is for anterior cervical discectomy and fusion, and according to a literature review conducted by Glassman et al., the reported fusion rates range between 94 and 98 %, in which the studies used dynamic radiographs and CT scan [30]. A review of bone graft substitutes by Chau et al. [31] found a range of fusion rates between 54 and 100 %. Suchomel et al. conducted a prospective semi-randomized study comparing allograft to ICBG in 79 patients (113 disc levels) undergoing one or two level ACDF, with anteroposterior (AP) and lateral radiographs used to assess fusion over time [32]. They found no difference in graft migration or collapse. They found a significantly slower fusion rate at 3 and 6 months, which was no longer present at 1 and 2 years. Thus, the allograft group had similar fusion rates, just slightly longer time to fusion. Ryu et al. [33] prospectively randomized 40 patients to compare allograft to ICBG in a carbon fiber cage in ACDF. They found equivalent 100 % fusion rates at 12 and 24 months follow-up, utilizing dynamic radiographs to assess fusion and instability. Using similar analysis, Samartzis et al. [23] retrospectively reviewed 66 patients who underwent ACDF with either allograft or ICBG. They found fusion rates of 100 % in the allograft group and 90.3 % in the autograft group and concluded that allograft is a sufficient alternative to autograft. In long-term follow-up, Yue et al. [34], report fusion rates of 92.6 % of disc spaces at an average of 7.2 years after surgery. They found a 47.9 % rate of graft subsidence, but only an 8.5 % rate of kyphosis (Recommendation: 1A).
Posterolateral lumbar
The reported fusion rates with the use fresh-frozen or freeze-dried allograft bone in posterolateral lumbar fusions have wide variability in the literature. Gibson et al. [35] conducted a prospective, randomized trial comparing fresh-frozen femoral head allograft to ICBG in 69 patients undergoing instrumented posterolateral lumbar fusions. At 1-year follow-up, 5 of 30 of the ICBG group complained of donor site pain. When they excluded the patients with donor site pain, the Roland-Morris scores were equivalent in the allograft and autograft groups. At more than 6 years follow-up, the clinical outcome scores were significantly better in the allograft groups. In another study, a prospective comparative study of 20 patients by An et al. [36], they reported on fusion rates of different graft substitutes. The order of the rates of fusion as assessed via static and dynamic radiographs from highest to lowest were as follows: autograft bone, followed by a mixture of autograft and allograft, then fresh-frozen allograft, and finally freeze-dried allograft. Autograft bone had the highest bone density. This study supported autograft, but the numbers are too small to be conclusive (Recommendation: 1B).
Anterior lumbar interbody
The use of allograft in anterior lumbar interbody fusions has similar success to posterolateral fusions. In a prospective, blind, single-site study, Thalgott et al. evaluated the outcomes and fusion rates of anterior lumbar interbody fusions with fresh-frozen or freeze-dried femoral ring allograft as part of a circumferential fusion in 50 patients. Upon radiographic assessment, fusion was found in 71.4 % of the 56 fusion levels [37]. The fusion rates in patients with freeze-dried allograft and fresh-frozen allograft were 65.38 and 76.67 %, respectively. In this study, there were 24 fusion levels in smoking patients, and 28 fusion levels in non-smoking patients. Smoking was strongly associated with pseudarthrosis with 58.3 % of freeze-dried patients and 16.7 % of the fresh-frozen patients requiring revision for pseudarthrosis. The highest fusion rate was seen in the non-smoking patients who underwent fusion with freeze-dried allograft, but there was no significant difference in clinical outcome scores. McKenna et al. conducted a prospective randomized controlled trial comparing femoral ring allograft to a titanium cage in circumferential lumbar fusions and found superior clinical results for the femoral ring allograft group on ODI, VAS for leg pain, and SF-36. This shows that allograft can have excellent outcome scores when used as structural interbody bone graft substitutes. Putzier et al. [38] conducted a prospective, randomized study on 40 patients undergoing anterior interbody fusion and transpedicular fixation for spondylolisthesis. All patients underwent placement of a PEEK cage, but group 1 had ICBG placed in the cage and group 2 had allograft placed in the PEEK cage. They found that at 6 months follow-up, group 2 had lower fusion rates, but by 1-year follow-up, both groups had equal fusion rates assessed via either CT or radiographs. This study shows that allograft is also a sufficient bone graft substitute to place inside a cage (Recommendation: 1B).
Adolescent idiopathic scoliosis
The use of allograft bone in adolescent idiopathic scoliosis surgery has limited results. Dodd et al. conducted a randomized clinical trial in which allograft was utilized for scoliosis repair in 20 patients averaging 15 years old. The radiographic assessment showed no significant difference in bone graft mass or maintenance of curvature correction when compared to autograft patients [5]. Aurori et al. [39] conducted a retrospective comparative study on 208 patients and found that there was no significant difference in the incidence of pseudarthrosis between allograft and autograft patients between 10 and 17 years old on radiographs or CT scan. Another study by Betz et al. [40] on 91 patients found that when adolescents underwent spinal fusion surgery without the addition of any material had a 100 % fusion rate at a minimum of 2-year follow-up. This is likely due to the large volume of local autograft bone harvested and also the ability of children and adolescents to heal and create a robust fusion mass. Knapp et al., in a multi-center, long-term retrospective review found a fusion rate of 97.8 % in patients treated with allograft, utilizing radiographs, CT scan, or bone scan [41] (Recommendation: 1A).
Recommendations
The grade of recommendations for the use of allograft bone in various spine fusions is summarized in Table 3, which is based upon the studies cited above. In summary, because it has a low rate of viral disease transmission and is not associated with increase in surgical site infections, allogenic bone use for spinal surgery does not appear to pose a significant risk to the allograft recipient and we recommend its use in cervical and lumbar fusions. The use of allograft bone in adolescent idiopathic scoliosis cases is likely necessary in cases of a low volume of local bone available for fusion.
Table 3.
Recommendations for fresh-frozen or freeze-dried allograft use
| Recommendation | Usage | Grade |
|---|---|---|
| Anterior cervical discectomy and fusion | Structural allograft | 1A |
| Posterolateral lumbar fusion | Allograft alone | 1B |
| Anterior lumbar interbody fusion | Structural and non-structural allograft | 1B |
| Posterior spinal fusion for AIS | Allograft alone | 1A |
Demineralized bone matrix
Anterior Cervical The use of demineralized bone matrix has poor results in the cervical spine. An et al. in a prospective study at two centers found a significantly higher rate of graft collapse and pseudarthrosis in patients who underwent multilevel ACDF and were fused with allograft plus demineralized bone compared to autograft. The authors attributed this difference due to the high prevalence of smokers in the study group and also to the radiographic evaluation techniques (AP, lateral, flexion/extension radiographs) to assess fusion, as no advanced imaging was obtained for borderline cases ultimately considered to have pseudarthrosis [42] (Recommendation: 2A).
Anterior Lumbar Interbody Anterior lumbar interbody fusions require structural support in addition to osteoconductive and osteoinductive materials. Because DBM lacks structural strength, DBM composites used for anterior spinal fusions should include a material imparting strength such as a metal cage or structural allograft. The use of DBM with structural allograft theoretically increases the risk of disease transmission due to the source of allograft bone from two donors. Thalgott et al. conducted a long-term prospective cohort study on a series of 50 patients with average 4 years follow-up using DBM composites with coralline hydroxyapatite and titanium mesh cages for anterior spinal fusions. They found this particular composite effective when it was used as part of a rigidly held in place circumferential fusion [43] (Recommendation: 1C).
Posterolateral Lumbar Clinical data on DBM is limited, yet most studies support the efficacy of DBM as a bone graft extender for posterolateral lumbar fusion. Kang et al. [44] performed a multicenter prospective randomized study to compare fusion rates of single-level posterolateral lumbar fusions with ICBG to Grafton plus local bone. The fusion rates were 85 and 83 %, respectively, with no statistically significant difference. Similar results of equivalent fusion rates using radiographic analysis were found in prospective study by Sassard et al., and a prospective pilot study by Schizas et al. [24, 45]. At a 1-year follow-up, the Grafton group showed a trend toward better patient-reported outcomes; however, results are limited by the small number of patients per study group [44]. Cammisa et al. [46] conducted a prospective, multicenter equivalency trial on 81 patients. They compared Grafton DBM gel to an iliac crest autograft, and found that Grafton DBM gel could not extend the size of autograft required for a solid spinal fusion (Recommendation: 1C).
Adolescent Idiopathic Scoliosis The use of DBM as an alternative to autograft for scoliosis spinal fusion surgery still needs further investigation to confirm its efficacy. Due to the long constructs and multiple fusion levels in many scoliosis corrections, utilizing a readily available graft substitute such as DBM is desirable. Price et al. retrospectively analyzed the efficacy of DBM in combination with bone marrow for a posterior spinal fusion in 77 scoliosis cases, and found that the fusion rates were comparable to those of iliac bone autograft combined with DBM composites [47]. Weinzapfl et al. [48] compared the use of allograft to DBM as a bone graft substitute in patients undergoing video assisted anterior thoracoscopic release and fusion for adolescent idiopathic scoliosis. At more than 1-year follow-up, fusion was observed on lateral radiographs in 82 % of the disc spaces in the allograft group and in 92 % in the DBM group, although this difference was not significant. Again, it should be noted, that allograft alone, not demineralized allograft in conjunction with iliac crest, is sufficient as a bone graft substitute in the fusion of adolescent idiopathic scoliosis patients [41] (Recommendation: 2C).
Recommendations
As outlined in Table 4, the recommendations for use of DBM depend on whether it is being used as a bone graft substitute or bone graft extender. It also depends on the location of use. The results of DBM use in ACDF from the two studies have conflicting results, but the study with the better design has results in favor of DBM use in ACDF. The use of DBM as a bone graft extender or in conjunction with bone marrow aspirate in lumbar posterolateral fusions has good to excellent outcomes therefore we recommend its use. Anterior lumbar procedures require structural support and thus we recommend the use of DBM with structural non-allograft cages and/or in conjunction with posterolateral fusions. As discussed below, the use of structural allograft can be augmented with different bone graft extenders that do not increase the risk of disease transmission. DBM use in adolescent idiopathic scoliosis fusions is recommended as bone graft extender.
Table 4.
Recommendations for use of demineralized bone matrix for spinal fusion
| Recommendation | Usage | Grade |
|---|---|---|
| Anterior cervical discectomy and fusion | Bone graft substitute | 2A |
| Anterior lumbar interbody fusion | Bone graft substitute with circumferential structural support | 1C |
| Posterolateral lumbar fusion | Bone graft extender | 1C |
| Posterior spinal fusion for AIS | Bone graft extender | 2C |
Platelet gels
Anterior Cervical In a double blind, randomized study by Feiz-Erfan et al., platelet concentrate was evaluated for evidence of superior fusion rates in ACDF. They followed 50 patients after ACDF in which half of the patients also had platelet concentrate added to the interbody fusion. They followed the patients radiographically at 6, 12, 52 weeks, and 2 years after surgery. The overall fusion rate was 84 % and the patients with degenerative disc disease treated with allograft plus platelet rich concentrate had higher rates of fusion at 12 weeks. The patients with soft disc herniations treated with allograft plus platelet-rich concentrate had lower rates of fusion at 12 weeks. At 1-year follow-up, all patients had equivalent fusion rates [49] (Recommendation: 2C).
Anterior Lumbar Interbody The clinical results of using platelet-enriched allograft are good, but with just one study the recommendation cannot be strong. Jenis et al. [50] conducted a prospective clinical study comparing platelet-rich plasma enhanced allograft to ICBG in patients undergoing anterior–posterior lumbar fusion. They followed a total of 37 patients (52 levels) with static radiographs and CT scan, and at 6 months post-op, the fusion rates were equivalent at 56 %. At 12 and 24 months follow-up, the fusion rates were 85 % in the ICBG and 89 % in the PRP-enriched allograft group, although the difference was not significant. While the authors concluded that platelet-enriched allograft was a successful alternative to ICBG, they did not comment on the similar success rate of non-platelet enriched allograft. They also did not comment on the use of a unit of blood from the patient to get the volume of PRP to supplement the allograft, and whether this led to an increased need for post-operative transfusions (Recommendation: 2C).
Posterolateral Lumbar The results of clinical studies do not support the use of platelet gels as a bone graft extender. Weiner and Walker retrospectively compared the rates of posterolateral lumbar fusion using iliac crest autograft alone and iliac crest autograft combined with platelet gel in 59 patients. The iliac crest fusion rate was significantly higher than the fusion rate of iliac crest in combination with platelet gel, at 91 versus 62 %, respectively, as assessed on static and dynamic radiographs. The authors concluded that platelet gel is not an effective enhancer of fusion and in fact lowered the efficacy of ICBG alone [51]. Carreon et al. [52] also retrospectively looked at the discrepancy between iliac crest autograft alone and iliac crest autograft in combination with platelet gel for one, two, or three level posterolateral lumbar fusion. At a minimum of 2 years follow-up in 76 patients utilizing CT scan and exploration during revision surgery, there was a 25 % nonunion rate for patients in the platelet gel group, while the iliac crest alone group experienced a 17 % nonunion rate. Tsai et al. [53] prospectively studied 34 patients who underwent posterolateral lumbar fusion with the addition of platelet gel to ALB. At 2 years follow-up with dynamic radiographs and CT scan, the nonunion rate was 15 % in the platelet gel group, compared to 10 % in the control group. Thus, the authors concluded that platelet gel does not enhance fusion. Thus, Tsai et al. agreed with the prior authors’ conclusions, that platelet gel is not a beneficial bone graft enhancer for posterolateral lumbar spinal fusion [52] (Recommendation: 2C).
Transforaminal Lumbar Interbody Castro et al. studied a different application of platelet gel, a transforaminal lumbar interbody fusion (TLIF) in 86 patients. They observed a 19 % decrease in fusion rate in patients where platelet gel was used in comparison to ICBG alone. The decrease in fusion rate seen radiographically did not achieve significance due to the relatively small number of patients in the study, but Castro et al. concluded that there is no benefit to using platelet gel for TLIF surgery [54]. Hee et al. prospectively studied 23 patients and compared them to historical controls to evaluate the impact of platelet gels on instrumented TLIF surgery. They found that although the use of platelet gels do not increase the fusion rates, it may promote a faster rate of fusion [55] (Recommendation: 2C).
Recommendations
It would make logical sense that the addition of platelet gel to autologous iliac crest would enhance fusion rates due to the presence of TGF-β and PGDF. However, clinical study findings refute this hypothesis. Potential reasons for these results include rapid dissolving of the platelet gel and diffusion of growth factors, the concentration of growth factors may be insufficient to enhance fusion status, or possible presence of bone growth-inhibiting factors [52]. The required concentrations of growth factors within platelet gel to promote osteogenesis is currently unknown [19]. Thus, our level of recommendation is to not use platelet gels as a bone graft substitute or extender for spinal fusion.
Ceramics
Anterior cervical The overall fusion rates of anterior cervical fusion with ceramic bone grafts substitutes ranges between 74 and 100 % [31]. Cho et al. [56] conducted a prospective, randomized study comparing a biphasic calcium phosphate ceramic to ICBG in a PEEK cage for patients undergoing ACDF. They found that the fusion rate on static and dynamic radiographs of the calcium phosphate group was significantly lower than the ICBG group in the first 5 months, but by 6 months post-op, the fusion rates were equal. When evaluated by number of fusion levels, they found that with increasing number of fusion levels, the time to fusion was delayed. They also found decreased EBL and shorter hospital stays for the calcium phosphate group. Thus, the authors concluded that calcium phosphate is a good substitute for ICBG (Recommendation: 1B).
The use of hydroxyapatite also has good clinical results for anterior cervical fusions. McConnell et al. prospectively randomized 29 patients to undergo ACDF with either coralline hydroxyapatite or ICBG [57]. While both groups had 100 % fusion rates as seen on radiographs and CT scan, the hydroxyapatite group had a significantly higher rate of graft fragmentation (89 vs. 11 %) and settling (50 vs. 11 %). Iseda et al. [58] conducted a scintigraphic study in 12 patients comparing hydroxyapatite grafts to ICBG and found the both had the same rate of uptake until fusion. They concluded that the radiolucent line is not always a sign of pseudarthrosis in patients with hydroxyapatite (Recommendation: 1C).
Anterior Lumbar Interbody The studies evaluating the use of ceramics in interbody fusions have good results. Thalgott et al. [43] conducted a retrospective review of the long-term efficacy of hydroxyapatite as a bone graft replacement for anterior interbody fusion in lumbar spine in 50 patients. They concluded that hydroxyapatite, when combined with DBM and a titanium mesh cage, can be a bone replacement for anterior fusion when part of a circumferential fusion, with a fusion rate of 96 % as evaluated with dynamic radiographs. These results are difficult to interpret the success of the fusion as due to the hydroxyapatite or to the DBM (Recommendation 1C).
The use of Healos in anterior lumbar interbody fusions has also been evaluated. Neen et al. [59] prospectively studied 50 patients’ age and gender matched to historical controls to evaluate the difference in fusion between Healos with BMA versus ICBG. Healos plus BMA was less effective at achieving radiographic anterior lumbar interbody fusions than historical controls, but achieved clinical interbody fusion (Recommendation: 2A).
Posterolateral Lumbar Similar to the results of anterior lumbar studies, ceramics have been shown in clinical studies to have good outcomes when used as a bone graft extender for posterolateral lumbar fusions. Chen et al. [60] conducted a prospective clinical trial in 74 patients to study the efficacy of calcium sulfate pellets with local autograft bone versus iliac crest bone graft for instrumented short segment posterolateral lumbar fusion. Patients underwent single or multiple level fusions where one side was fused with ICBG and the test side was fused with autologous laminectomy bone (ALB) and calcium sulfate. For single-level fusions, the fusion rate as seen on radiographs of ALB and calcium sulfate was 87.2 versus 89.7 % on the ALB and ICBG side. For multiple level fusions, the fusion rates were 82.9 and 85.7 % respectively. A similar study by Chang et al. [61] demonstrated a fusion rate of 92.3 % by CT, but another study by Niu et al. [15] showed a fusion rate of only 45.5 % by radiographs at 1-year follow-up. In the prospective study by Niu et al., the 43 patients each underwent posterolateral fusion with ICBG in one gutter as a control, and in the other gutter either ALB plus BMA or calcium sulfate plus BMA. The 1-year fusion rate, assessed radiographically and on CT scan, for the ICBG gutters was 90.5 %, the ALB plus BMA was 85.7 %, and calcium sulfate plus BMA was 45.5 %. The difference between ICBG and calcium plus BMA was statistically significant. In a prospective, randomized study by Kasai et al. on 35 patients undergoing uninstrumented posterolateral lumbar fusion after multilevel laminectomy, they found fusion rates of 80 % using ALB mixed with various ratios of apatite and wolatsonite glass–ceramic granules. This demonstrates the ability of ceramics to function as an adequate bone graft extender even when instrumentation is lacking (Recommendation: 1C).
The use of β-TCP as a bone graft extender has had better results in the literature. Dai and Jiang conducted a prospective, randomized clinical trial in 62 patients comparing β-TCP and ALB to iliac crest autograft plus ALB for single-level posterolateral fusion, and found that there were no differences in the clinical outcome, nor the fusion rates on static and dynamic radiographs [62]. This study shows that ceramics can serve as a bone graft extender. With only one comparative study in the literature that does not state specific fusion rates or statistical significance values, the flaws of this study prevent a higher recommendation (Recommendation: 1C).
The clinical results for hydroxyapatite have mixed results. Acharya et al. [63] found that hydroxyapatite is not suitable as a bone graft substitute. They conducted a prospective, matched, controlled study comparing hydroxyapatite bioactive glass ceramic composite on one side of the posterolateral fusion to autologous bone on the contralateral side in 24 patients. At 1-year follow-up, 95 % of the patients had poor consolidation of the hydroxyapatite side of the fusion evaluated with AP radiographs, and the study was prematurely terminated. The authors concluded that this study clearly shows that hydroxyapatite is inferior to ICBG in posterolateral lumbar fusions (Recommendation: 2C).
One study shows that hydroxyapatite is not effective as a bone graft extender. Korovessis et al. conducted a prospective longitudinal randomized clinical and radiological study on 57 patients comparing iliac crest bone autograft to coralline hydroxyapatite with BMA and local autograft in instrumented posterolateral lumbar fusion [64]. They concluded through dynamic radiographs and CT scan evaluation that iliac bone autograft achieved a solid fusion while the coralline hydroxyapatite mixture needed a large bony surface. They do not recommend the use of hydroxyapatite for posterolateral fusion, but recommended its use over decorticated laminae (Recommendation: 2C).
Hydroxyapatite may still have a role as a bone graft extender in the formulation of Healos (20 % hydroxyapatite and 80 % type I bovine collagen). Findings from the study cited above by Neen et al. [59] supports the use of Healos with BMA as a bone graft substitute for posterolateral and anterior lumbar interbody fusion. They found that the fusion rates were equivalent for posterolateral and anterior interbody. Ploumis et al. [65] also found that hydroxyapatite may be a good bone graft extender. They conducted a prospective clinical trial in 28 patients who underwent posterolateral spinal fusion for degenerative scoliosis. They compared Healos combined with local autograft and BMA to local autograft with cancellous allograft. They found that both groups had similar results, with one case of pseudarthrosis in each group at 2-year follow-up. However, the Healos group on average took more time to achieve radiographic fusion (Recommendation: 1C).
Adolescent Idiopathic Scoliosis Successful results have been reported for the use of ceramics in scoliosis cases. Ransford et al. [18] conducted a prospective randomized clinical study on the efficacy of a synthetic porous ceramic as a bone graft substitute for posterior spinal fusion in idiopathic scoliosis utilizing static radiographs for fusion analysis, and concluded that it is a safe and effective substitute for autologous bone graft. LeHeuc et al. [66] conducted a comparative study evaluating allograft and autologous bone to β-TCP and autologous bone in 54 scoliosis patients with a minimum of 4-year follow-up. Both groups developed a radiographic fusion by 6 months and neither group had evidence of pseudarthrosis. The allograft group had a loss of correction of 33 % and the β-TCP group had a loss of correction of 8 %, but the authors did not determine significance. Muschik et al. [67] conducted a prospective clinical study and also reported that β-TCP is a viable alternative to allograft as a bone extender, even when large amounts are needed for scoliosis cases. Delecrin et al. [68] followed 58 patients who underwent posterior spinal fusion with local bone graft combined with either iliac crest bone graft or hydroxyapatite and tricalcium phosphate. They found radiographic evidence of successful incorporation and bone mass formation in both groups. Lerner et al. [69] conducted a prospective randomized trial 40 patients with adolescent idiopathic scoliosis in which patients underwent posterior spinal fusion with autologous local bone and either ICBG or β-TCP. One patient in the β-TCP group developed a pseudarthrosis at the distal aspect of the fusion seen on static radiographs and confirmed at surgical exploration. Ilharreborde et al. [70] conducted a retrospective review of 88 patients and found the bioactive glass group had a significantly better maintenance of correction on standing full-length static radiographs at 38 months follow-up than the ICBG group (Recommendation: 1C).
Recommendations
Overall, we recommend the use of ceramics as osteoconductors, but not without the use of additional osteoinductive material. Confirmation of fusion is based on X-ray or CT, so it is impossible to differentiate between ceramics and bone ingrowth [17]. Thus, it may be unclear whether the fusion masses represent new bone formation or the presence of an unfused ceramic mass. Since patients have good outcomes with the use of ceramics, we hypothesis that these radiographic findings represent new bone formation. There are multiple studies showing good patient outcomes when ceramics are used as bone graft extenders in posterolateral lumbar fusions. β-TCP is a strongly recommended bone graft substitute in posterolateral lumbar fusions, and hydroxyapatite is an intermediate-level recommendation as a bone substitute is for anterior cervical interbody fusion, when combined with rigid internal fixation for necessary support. The published studies on using ceramics in adolescent idiopathic scoliosis show that this application can yield successful results, and they show fusion when no local allograft bone is used. The summary of our recommendations for use of ceramic products is seen in Table 5.
Table 5.
Recommendations for ceramic product use in spinal fusion surgery
| Recommendation | Ceramic | Application | Grade |
|---|---|---|---|
| Anterior cervical fusion | β-TCP | Bone graft substitute with synthetic cage | 1B |
| Anterior cervical fusion | Hydroxyapatite | Bone graft substitute | 1C |
| Anterior lumbar interbody fusion | Hydroxyapatite | Bone substitute | 1C |
| Anterior lumbar interbody fusion | Healos | Bone extender | 2A |
| Posterolateral lumbar fusion | Calcium sulfate | Bone graft extender | 1C |
| Posterolateral lumbar fusion | β-TCP | With BMA as bone graft extender | 1C |
| Posterolateral lumbar fusion | Hydroxyapatite | With BMA as bone graft extender | 2C |
| Posterolateral lumbar fusion | Hydroxyapatite | With BMA as bone graft substitute | 2C |
| Posterolateral lumbar fusion | Healos | With BMA as bone graft extender | 1C |
| Posterior spinal fusion for AIS | β-TCP | Bone graft substitute | 1C |
Conclusion
Though much progress has been made in the field of bone graft alternatives for spinal fusion, we believe that further research must be conducted. Approximately 1,400 products are available on the international market for use as bone void fillers including not only the substitutes discussed in this article, and must be evaluated to confirm the safety, efficacy, and associated risk factors. An ideal bone graft substitute with little or no associated complications and risks does not exist at this time. Currently, autograft is considered to be the gold standard. Allograft works well as an osteoconductive scaffold. Demineralized bone matrix leads to variable outcomes depending on the batch, due to the variability in growth factor content. Platelet gels have little clinical evidence of their efficacy. Ceramics are promising as osteoconductors and thus bone graft extenders, and bone graft substitute when used with bone marrow aspirate. A summary of the discussed bone graft substitutes’ advantages and disadvantages can be seen in Table 6 and a summary of our recommendations is shown in Table 7. We believe that patients and their surgeons should carefully select a treatment option that is the best match for the patient’s particular circumstance.
Table 6.
Summary of bone graft options
| Graft type | Iliac crest | Local bone | Allograft | DBM | Ceramics |
|---|---|---|---|---|---|
| Osteoconductive |  |
 |
 |
 |
 |
| Osteoinductive |  |
 |
|||
| Bioresorbable |  |
 |
 |
 |
 |
| Biocompatible |  |
 |
 |
||
| Cost effective |  |
 |
 |
 |
 |
| Growth factors |  |
 |
 |
 |
|
| Live cells |  |
 |
|||
| Structural |  |
 |
|||
| Large availability |  |
 |
 |
||
| Donor site morbidity | |||||
| Finite amount | |||||
| Increased blood loss | |||||
| Increased operative time | |||||
| Disease transmission risk | |||||
| Requires carrier | |||||
| Synthetic |
Table 7.
Summary of recommendations
| Allograft | DBM | PRP | CaSO4 | β-TCP | HA | Healos | |
|---|---|---|---|---|---|---|---|
| ACDF | 1A | 2A | 2C | 1B | 1C | ||
| ALIF | 1B | 1C | 2C | 1C | 2A(e) | ||
| PLSF | 1B | 1C(e) | 2C | 1C(e) | 1C(e) | 2C 2C(e) |
1C (e) |
| TLIF | 2C | ||||||
| PSF AIS | 1A | 2C(e) | 1C |
(e)—use as a bone graft extender instead of substitute
ACDF anterior cervical discectomy and fusion, ALIF anterior lumbar interbody fusion, TLIF tranforaminal interbody fusion, PLSF posterolateral spinal fusion, PSF AIS posterior spinal fusion for adolescent idiopathic scoliosis
Conflict of interest
None.
References
- 1.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine (Phila Pa 1976) 2006;31(23):2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bono CM, Lee CK (2004) Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976) 29(4):455–463; discussion Z455. pii:00007632-200402150-00019 [DOI] [PubMed]
- 3.Burkus JK, Schuler TC, Gornet MF, Zdeblick TA. Anterior lumbar interbody fusion for the management of chronic lower back pain: current strategies and concepts. Orthop Clin North Am. 2004;35(1):25–32. doi: 10.1016/S0030-5898(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 4.Dawson EG, Lotysch M, 3rd, Urist MR. Intertransverse process lumbar arthrodesis with autogenous bone graft. Clin Orthop Relat Res. 1981;154:90–96. [PubMed] [Google Scholar]
- 5.Dodd CA, Fergusson CM, Freedman L, Houghton GR, Thomas D. Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg Br. 1988;70(3):431–434. doi: 10.1302/0301-620X.70B3.3286656. [DOI] [PubMed] [Google Scholar]
- 6.Kho VK, Chen WC. The results of posterolateral lumbar fusion with bone chips from laminectomy in patients with lumbar spondylolisthesis. J Chin Med Assoc. 2004;67(11):575–578. [PubMed] [Google Scholar]
- 7.Dai LY, Jiang LS, Jiang SD. Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine (Phila Pa 1976) 2008;33(23):2536–2544. doi: 10.1097/BRS.0b013e3181851bc2. [DOI] [PubMed] [Google Scholar]
- 8.Dimar JR, Glassman SD. The art of bone grafting. Curr Opin Orthop. 2007;18(3):8. doi: 10.1097/BCO.0b013e328112f35d. [DOI] [Google Scholar]
- 9.Dimar JR, 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Two-year fusion and clinical outcomes in 224 patients treated with a single-level instrumented posterolateral fusion with iliac crest bone graft. Spine J. 2009;9(11):880–885. doi: 10.1016/j.spinee.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A(5):716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 1995;20(9):1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Delawi D, Dhert WJ, Castelein RM, Verbout AJ, Oner FC. The incidence of donor site pain after bone graft harvesting from the posterior iliac crest may be overestimated: a study on spine fracture patients. Spine (Phila Pa 1976) 2007;32(17):1865–1868. doi: 10.1097/BRS.0b013e318107674e. [DOI] [PubMed] [Google Scholar]
- 14.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Niu CC, Tsai TT, Fu TS, Lai PL, Chen LH, Chen WJ. A comparison of posterolateral lumbar fusion comparing autograft, autogenous laminectomy bone with bone marrow aspirate, and calcium sulphate with bone marrow aspirate: a prospective randomized study. Spine (Phila Pa 1976) 2009;34(25):2715–2719. doi: 10.1097/BRS.0b013e3181b47232. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CJ, Chou WY, Teng HP, Chang WN, Chou YJ. Coralline hydroxyapatite and laminectomy-derived bone as adjuvant graft material for lumbar posterolateral fusion. J Neurosurg Spine. 2005;3(4):271–275. doi: 10.3171/spi.2005.3.4.0271. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki M, Tsumura H, Wang JC, Alanay A. An update on bone substitutes for spinal fusion. Eur Spine J. 2009;18(6):783–799. doi: 10.1007/s00586-009-0924-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ransford AO, Morley T, Edgar MA, Webb P, Passuti N, Chopin D, Morin C, Michel F, Garin C, Pries D. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80(1):13–18. doi: 10.1302/0301-620X.80B1.7276. [DOI] [PubMed] [Google Scholar]
- 19.Rihn JA, Kirkpatrick K, Albert TJ. Graft options in posterolateral and posterior interbody lumbar fusion. Spine (Phila Pa 1976) 2010;35(17):1629–1639. doi: 10.1097/BRS.0b013e3181d25803. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G, Schunemann H, Cook D, Jaeschke R, Pauker S, Bucher H. Grades of recommendation for antithrombotic agents. Chest. 2001;119(1 Suppl):3S–7S. doi: 10.1378/chest.119.1_suppl.3S. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schunemann H. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129(1):174–181. doi: 10.1378/chest.129.1.174. [DOI] [PubMed] [Google Scholar]
- 22.Fisher CG, Wood KB. Introduction to and techniques of evidence-based medicine. Spine (Phila Pa 1976) 2007;32(19 Suppl):S66–S72. doi: 10.1097/BRS.0b013e318145308d. [DOI] [PubMed] [Google Scholar]
- 23.Samartzis D, Shen FH, Goldberg EJ, An HS. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976) 2005;30(15):1756–1761. doi: 10.1097/01.brs.0000172148.86756.ce. [DOI] [PubMed] [Google Scholar]
- 24.Sassard WR, Eidman DK, Gray PM, Block JE, Russo R, Russell JL, Taboada EM. Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: avoiding second site autologous bone harvest. Orthopedics. 2000;23(10):1059–1064. doi: 10.3928/0147-7447-20001001-17. [DOI] [PubMed] [Google Scholar]
- 25.Christensen FB, Laursen M, Gelineck J, Eiskjaer SP, Thomsen K, Bunger CE. Interobserver and intraobserver agreement of radiograph interpretation with and without pedicle screw implants: the need for a detailed classification system in posterolateral spinal fusion. Spine. 2001;26(5):538–543. doi: 10.1097/00007632-200103010-00018. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama S, Wullschleger M, Wilson K, Williams R, Goss B. Reliability of clinical measurement for assessing spinal fusion: an experimental sheep study. Spine. 2012;37(9):763–768. doi: 10.1097/BRS.0b013e31822ffa05. [DOI] [PubMed] [Google Scholar]
- 27.Mikhael MM, Huddleston PM, Nassr A. Postoperative culture positive surgical site infections after the use of irradiated allograft, nonirradiated allograft, or autograft for spinal fusion. Spine (Phila Pa 1976) 2009;34(22):2466–2468. doi: 10.1097/BRS.0b013e3181b1fef5. [DOI] [PubMed] [Google Scholar]
- 28.Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS) Clin Orthop Relat Res. 1989;240:129–136. [PubMed] [Google Scholar]
- 29.Nasca RJ, Whelchel JD. Use of cryopreserved bone in spinal surgery. Spine (Phila Pa 1976) 1987;12(3):222–227. doi: 10.1097/00007632-198704000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Glassman SD, Howard JM, Sweet A, Carreon LY. Complications and concerns with osteobiologics for spine fusion in clinical practice. Spine (Phila Pa 1976) 2010;35(17):1621–1628. doi: 10.1097/BRS.0b013e3181ce11cc. [DOI] [PubMed] [Google Scholar]
- 31.Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18(4):449–464. doi: 10.1007/s00586-008-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchomel P, Barsa P, Buchvald P, Svobodnik A, Vanickova E. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. Eur Spine J. 2004;13(6):510–515. doi: 10.1007/s00586-003-0667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu SI, Mitchell M, Kim DH. A prospective randomized study comparing a cervical carbon fiber cage to the Smith-Robinson technique with allograft and plating: up to 24 months follow-up. Eur Spine J. 2006;15(2):157–164. doi: 10.1007/s00586-005-0951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976) 2005;30(19):2138–2144. doi: 10.1097/01.brs.0000180479.63092.17. [DOI] [PubMed] [Google Scholar]
- 35.Gibson S, McLeod I, Wardlaw D, Urbaniak S. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine (Phila Pa 1976) 2002;27(15):1599–1603. doi: 10.1097/00007632-200208010-00002. [DOI] [PubMed] [Google Scholar]
- 36.An HS, Lynch K, Toth J. Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord. 1995;8(2):131–135. doi: 10.1097/00002517-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Thalgott JS, Fogarty ME, Giuffre JM, Christenson SD, Epstein AK, Aprill C. A prospective, randomized, blinded, single-site study to evaluate the clinical and radiographic differences between frozen and freeze-dried allograft when used as part of a circumferential anterior lumbar interbody fusion procedure. Spine (Phila Pa 1976) 2009;34(12):1251–1256. doi: 10.1097/BRS.0b013e3181a005d7. [DOI] [PubMed] [Google Scholar]
- 38.Winters HA, van Engeland AE, Jiya TU, van Royen BJ. The use of free vascularised bone grafts in spinal reconstruction. J plast reconst aesthet surg JPRAS. 2010;63(3):516–523. doi: 10.1016/j.bjps.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Aurori BF, Weierman RJ, Lowell HA, Nadel CI, Parsons JR. Pseudarthrosis after spinal fusion for scoliosis. A comparison of autogeneic and allogeneic bone grafts. Clin Orthop Relat Res. 1985;199:153–158. [PubMed] [Google Scholar]
- 40.Emovon OE, King JA, Holt CO, Singleton B, Howell D, Browne BJ. Effect of cyclosporin pharmacokinetics on renal allograft outcome in African-Americans. Clin Transplant. 2003;17(3):206–211. doi: 10.1034/j.1399-0012.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 41.Knapp DR, Jr, Jones ET, Blanco JS, Flynn JC, Price CT. Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech. 2005;18(Suppl):S73–S76. doi: 10.1097/01.bsd.0000128694.21405.80. [DOI] [PubMed] [Google Scholar]
- 42.An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine (Phila Pa 1976) 1995;20(20):2211–2216. doi: 10.1097/00007632-199510001-00006. [DOI] [PubMed] [Google Scholar]
- 43.Thalgott JS, Giuffre JM, Klezl Z, Timlin M. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002;2(1):63–69. doi: 10.1016/S1529-9430(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 44.Kang JD, An H, Hilibrand AS (2008) Grafton and local bone has comparable outcomes to iliac crest bone in single level lumbar fusions. Paper presented at the American Academy of Orthopaedic Surgeons 75th Annual Meeting, San Francisco, CA, March 5–9, 2008
- 45.Schizas C, Triantafyllopoulos D, Kosmopoulos V, Tzinieris N, Stafylas K. Posterolateral lumbar spine fusion using a novel demineralized bone matrix: a controlled case pilot study. Arch Orthop Trauma Surg. 2008;128(6):621–625. doi: 10.1007/s00402-007-0495-4. [DOI] [PubMed] [Google Scholar]
- 46.Cammisa FP, Jr, Lowery G, Garfin SR, Geisler FH, Klara PM, McGuire RA, Sassard WR, Stubbs H, Block JE. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine (Phila Pa 1976) 2004;29(6):660–666. doi: 10.1097/01.BRS.0000116588.17129.B9. [DOI] [PubMed] [Google Scholar]
- 47.Price CT, Connolly JF, Carantzas AC, Ilyas I. Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2003;28(8):793–798. [PubMed] [Google Scholar]
- 48.Weinzapfel B, Son-Hing JP, Armstrong DG, Blakemore LC, Poe-Kochert C, Thompson GH. Fusion rates after thoracoscopic release and bone graft substitutes in idiopathic scoliosis. Spine (Phila Pa 1976) 2008;33(10):1079–1083. doi: 10.1097/BRS.0b013e31816f69b3. [DOI] [PubMed] [Google Scholar]
- 49.Feiz-Erfan I, Harrigan M, Sonntag VK, Harrington TR. Effect of autologous platelet gel on early and late graft fusion in anterior cervical spine surgery. J Neurosurg Spine. 2007;7(5):496–502. doi: 10.3171/SPI-07/11/496. [DOI] [PubMed] [Google Scholar]
- 50.Jenis LG, Banco RJ, Kwon B. A prospective study of Autologous Growth Factors (AGF) in lumbar interbody fusion. Spine J. 2006;6(1):14–20. doi: 10.1016/j.spinee.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Weiner BK, Walker M (2003) Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine (Phila Pa 1976) 28 (17):1968–1970; discussion 1971. doi:10.1097/01.BRS.0000083141.02027.48 [DOI] [PubMed]
- 52.Carreon LY, Glassman SD, Anekstein Y, Puno RM (2005) Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine (Phila Pa 1976) 30(9):E243–246; discussion E247. pii:00007632-200505010-00026 [DOI] [PubMed]
- 53.Tsai CH, Hsu HC, Chen YJ, Lin MJ, Chen HT. Using the growth factors-enriched platelet glue in spinal fusion and its efficiency. J Spinal Disord Tech. 2009;22(4):246–250. doi: 10.1097/BSD.0b013e3181753ae2. [DOI] [PubMed] [Google Scholar]
- 54.Castro FP., Jr Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17(5):380–384. doi: 10.1097/01.bsd.0000110342.54707.19. [DOI] [PubMed] [Google Scholar]
- 55.Hee HT, Majd ME, Holt RT, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12(4):400–407. doi: 10.1007/s00586-003-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho DY, Lee WY, Sheu PC, Chen CC (2005) Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg Neurol 63(6):497–503; discussion 503–494. doi:10.1016/j.surneu.2004.10.016 [DOI] [PubMed]
- 57.McConnell JR, Freeman BJ, Debnath UK, Grevitt MP, Prince HG, Webb JK. A prospective randomized comparison of coralline hydroxyapatite with autograft in cervical interbody fusion. Spine (Phila Pa 1976) 2003;28(4):317–323. doi: 10.1097/01.BRS.0000048503.51956.E1. [DOI] [PubMed] [Google Scholar]
- 58.Iseda T, Nakano S, Suzuki Y, Miyahara D, Uchinokura S, Moriyama T, Sameshima T, Goya T, Wakisaka S. Radiographic and scintigraphic courses of union in cervical interbody fusion: hydroxyapatite grafts versus iliac bone autografts. J Nucl Med. 2000;41(10):1642–1645. [PubMed] [Google Scholar]
- 59.Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine (Phila Pa 1976) 2006;31(18):E636–E640. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 60.Chen WJ, Tsai TT, Chen LH, Niu CC, Lai PL, Fu TS, McCarthy K. The fusion rate of calcium sulfate with local autograft bone compared with autologous iliac bone graft for instrumented short-segment spinal fusion. Spine (Phila Pa 1976) 2005;30(20):2293–2297. doi: 10.1097/01.brs.0000182087.35335.05. [DOI] [PubMed] [Google Scholar]
- 61.Chang CH, Lin MZ, Chen YJ, Hsu HC, Chen HT (2008) Local autogenous bone mixed with bone expander: an optimal option of bone graft in single-segment posterolateral lumbar fusion. Surg Neurol 70 Suppl 1:S1:47–49; discussion S41:49. doi:10.1016/j.surneu.2008.05.022 [DOI] [PubMed]
- 62.Dai LY, Jiang LS. Single-level instrumented posterolateral fusion of lumbar spine with beta-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine (Phila Pa 1976) 2008;33(12):1299–1304. doi: 10.1097/BRS.0b013e3181732a8e. [DOI] [PubMed] [Google Scholar]
- 63.Acharya NK, Kumar RJ, Varma HK, Menon VK. Hydroxyapatite-bioactive glass ceramic composite as stand-alone graft substitute for posterolateral fusion of lumbar spine: a prospective, matched, and controlled study. J Spinal Disord Tech. 2008;21(2):106–111. doi: 10.1097/BSD.0b013e31805fea1f. [DOI] [PubMed] [Google Scholar]
- 64.Korovessis P, Koureas G, Zacharatos S, Papazisis Z, Lambiris E. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur Spine J. 2005;14(7):630–638. doi: 10.1007/s00586-004-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ploumis A, Albert TJ, Brown Z, Mehbod AA, Transfeldt EE. Healos graft carrier with bone marrow aspirate instead of allograft as adjunct to local autograft for posterolateral fusion in degenerative lumbar scoliosis: a minimum 2-year follow-up study. J Neurosurg Spine. 2010;13(2):211–215. doi: 10.3171/2010.3.SPINE09603. [DOI] [PubMed] [Google Scholar]
- 66.Le Huec JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A. Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg. 1997;63(3):202–211. [PubMed] [Google Scholar]
- 67.Muschik M, Ludwig R, Halbhubner S, Bursche K, Stoll T. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(Suppl 2):S178–S184. doi: 10.1007/s005860100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delecrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine (Phila Pa 1976) 2000;25(5):563–569. doi: 10.1097/00007632-200003010-00006. [DOI] [PubMed] [Google Scholar]
- 69.Lerner T, Bullmann V, Schulte TL, Schneider M, Liljenqvist U. A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J. 2009;18(2):170–179. doi: 10.1007/s00586-008-0844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ilharreborde B, Morel E, Fitoussi F, Presedo A, Souchet P, Pennecot GF, Mazda K. Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis: a comparative study with iliac crest autograft. J Pediatr Orthop. 2008;28(3):347–351. doi: 10.1097/BPO.0b013e318168d1d4. [DOI] [PubMed] [Google Scholar]