Abstract
Purpose
Metastatic spinal cord compression (MSCC) requires expeditious treatment. While there is no ambiguity in the literature about the urgency of care for patients with MSCC, the effect of timing of surgical intervention has not been investigated in detail. The objective of our study was to investigate whether or not the ‘timing of surgery’ is an important factor in survival and neurological outcome in patients with MSCC.
Methods
All patients with MSCC presenting to our unit from October 2005 to March 2010 were included in this study. Patients were divided into three groups—those who underwent surgery within 24 h (Group 1, n = 45), between 24 and 48 h (Group 2, n = 23) and after 48 h (Group 3, n = 53) from acute presentation of neurological symptoms. The outcome measures studied were neurological outcome (change in Frankel grade post-operatively), survival (survival rate and median survival in days), incidence of infection, length of stay and complications.
Results
Patients’ age, gender, revised Tokuhashi score, level of spinal metastasis and primary tumour type were not significantly different between the three groups. Greatest improvement in neurology was observed in Group 1, although not significantly when compared against Group 2 (24–48 h; (p = 0.09). When comparisons of neurological outcome were performed for all patients having surgery within 48 h (Groups 1 and 2) versus after 48 h (Group 3), the Frankel grade improvement was significant (p = 0.048) favouring surgery within 48 h of presentation. There was a negative correlation (−0.17) between the delay in surgery and the immediate neurological improvement, suggesting less improvement in those who had delayed surgery. There was no difference in length of hospital stay, incidence of infection, post-operative complications or survival between the groups.
Conclusions
Our results show that surgery should be performed sooner rather than later. Furthermore, earlier surgical treatment within 48 h in patients with MSCC resulted in significantly better neurological outcome. However, the timing of surgery did not influence length of hospital stay, complication rate or patient survival.
Keywords: Timing, Surgery, Metastatic spinal cord compression
Introduction
Metastatic spinal cord compression (MSCC) is a challenging clinical problem and represents an oncological emergency, which requires expeditious treatment. MSCC occurs in 5–14 % of patients with cancer during the course of their disease [1, 2]. This results in considerable morbidity for cancer patients and imposes an increased pressure on palliative care. However, the survival of patients with cancer has steadily improved over the years due to advances in diagnosis and treatment, multi-disciplinary management and improved palliative care. It has been well established that MSCC requires prompt recognition and immediate treatment to relieve neurological compression, restore function, prevent disability and improve the quality of life [3, 4]. Without this expeditious treatment, the spinal cord can be irreversibly damaged resulting in deteriorating or permanent sensorimotor deficits, bowel and bladder dysfunction and possible requirement for 24-h nursing care.
Earlier studies perhaps until the mid-1980s collectively showed that decompressive laminectomy alone did not offer any additional benefit compared to conventional radiotherapy [5–8]. Then evolved a trend towards posterior decompression combined with stabilisation surgery resulting in improved outcome and survival [9, 10]. More recent literature in the last decade including a randomised controlled trial has shown that direct decompression and stabilisation surgery improved the functional outcome and ambulation in patients with MSCC [11, 12].
While there is no ambiguity in the literature about the urgency of care for patients with MSCC, the effect of timing of surgical intervention has not been investigated in detail. A small retrospective series found improved neurological outcome if surgery was performed within 48 h [12]. Another study also reported an improved ambulatory function if the symptoms were of less than 48-h duration prior to the intervention [13]. Without urgent treatment, the spinal cord is irreversibly damaged with the consequences of permanent or increasing sensorimotor deficit and bladder/bowel dysfunction [4]. The NICE guidelines further outline the importance of timing of surgery as an important factor contributing to the likely neurological outcome—if it is gradual, careful monitoring is required and unless further deterioration occurs, surgery can be planned for the next scheduled list after staging to permit optimal decision making. If rapid deterioration is obvious, surgical intervention is an emergency and needs to be done as soon as possible [14]. The objective of our study was to investigate whether or not the ‘timing of surgery’ was an important factor in neurological outcome, complications and survival in patients with MSCC.
Patients and methods
We performed a retrospective analysis of all patients undergoing emergent surgery for MSCC at a tertiary referral spinal unit between October 2005 and March 2010. All patients who were surgically treated for MSCC with a known or unknown primary and neurological deficit were included in the study. Exclusion criteria included patients with spinal metastasis without cord compression, treatment solely by radiotherapy, patients having vertebroplasty/kyphoplasty procedure without decompression, patients who had undergone previous surgery for spinal metastasis and those patients who had other causes of neurological dysfunction such as stroke.
The information was recorded for all patients in their medical notes and included age, gender, clinical presentation, type of primary tumour, revised Tokuhashi score [15], level of spinal involvement as determined by pre-operative Magnetic Resonance Imaging (MRI) findings and type of surgical procedure. Data were analysed as a retrospective audit. All patients who presented with MSCC were given dexamethasone 16 mg daily and then reduced gradually to stop 5 to 7 days after surgery. The surgical approach was dictated by the location of the epidural cord compression and surgical preference of the operating surgeon. Patients received adjuvant radiotherapy post-operatively as required.
There were five outcome variables—the neurological outcome was assessed using the Frankel grade [16] in the immediate post-operative period, and at regular intervals until death; the presence or absence of any surgical or post-operative complications and infection, length of hospital stay as well as the survival period.
Statistical analysis was performed using SPSS 17.0 version software (Statistical Package for Social Sciences, SPSS Inc.). The differences in demographics between the groups were analysed using the Fisher’s exact test or Pearson Chi square test. Change in Frankel grade was analysed using ANOVA (3 way repeated) and Kendall tau rank correlation coefficient. Morbidity was analysed by comparing the patients’ length of stay in hospital using P–P plot and comparing the incidence of complications using Pearson Chi square test. Survival data were computed using Kaplan–Meier product limit method, and the survival curves were drawn for each group. Log rank Mantel–Cox test was used to show difference in survival between groups. The Pearson correlation test was used to calculate the correlation between histology of primary tumour, level of spinal metastases, revised Tokuhashi score and number of spinal metastases versus the outcome variables; p values less than 0.05 were considered significant.
Results
Patient characteristics
A total of 153 patients underwent surgery for MSCC during this period. Thirty-two patients did not have any motor or sensory loss (Frankel E) and were excluded. The timing of surgical intervention from the patients’ acute presentation with neurological deterioration was determined in the remaining 121 patients (75 males, 46 females; mean age of 61 years; 95 % Confidence Interval for Mean (CI) 59–63; range 17–86; see Table 1). These patients were divided into three groups based on the timing of intervention—patients who were operated on within 24 h (Group 1, n = 45), those who were operated on between 24 and 48 h (Group 2, n = 23) and patients who underwent surgery after 48 h (Group 3, n = 53).
Table 1.
Comparison of patients’ characteristics in all groups
| Group 1 (n = 45) | Group 2 (n = 23) | Group 3 (n = 53) | Total | p value | |
|---|---|---|---|---|---|
| Mean age (range) | 60 (17–86) | 66 (47–79) | 61 (31–81) | 61 (17–86) | 0.16 |
| Male | 25 | 19 | 31 | 75 | 0.07 |
| Female | 20 | 4 | 22 | 46 | 0.07 |
| Mean Tokuhashi score (range) | 8.4 (1–14) | 9.1 (3–14) | 8.9 (2–14) | 8.7 (1–14) | 0.59 |
| Primary tumour | 0.84 | ||||
| Breast | 8 | 2 | 10 | 20 | |
| Lung | 5 | 1 | 6 | 12 | |
| Prostate | 10 | 5 | 3 | 18 | |
| Renal | 4 | 3 | 11 | 18 | |
| Myeloma | 5 | 3 | 10 | 18 | |
| GI | 1 | 5 | 3 | 9 | |
| Other | 12 | 3 | 6 | 21 | |
| Unknown | 0 | 1 | 4 | 5 | |
| Level of spinal involvement | 0.86 | ||||
| Cervical | 5 | 3 | 7 | 15 | |
| Upper thoracic | 15 | 8 | 14 | 37 | |
| Lower thoracic | 15 | 8 | 16 | 39 | |
| Lumbar | 10 | 3 | 14 | 27 | |
| Sacrum | 0 | 1 | 2 | 3 | |
There was no statistically significant difference in any of these characteristics: age (p = 0.16), revised Tokuhashi score (p = 0.59), gender (p = 0.07), type of primary tumour (p = 0.84) or the level of spinal involvement (p = 0.86). Most tumours metastasised to the thoraco-lumbar region as expected. Majority of the patients underwent a posterior procedure (n = 105; 87 %), while three patients (2.5 %) had isolated anterior procedure and 13 patients (11 %) had a combined anterior and posterior procedure.
Neurological outcome
Neurological grade was studied in detail. Table 2 shows the pre and post-operative Frankel grade in the three groups. There were more patients in Group 1 (surgery within 24 h) who had a greater neurological deficit pre-operatively, i.e. Frankel A or B compared to the other two groups (p = 0.001). There was no statistical significance (p = 0.09) in Frankel grade improvement when comparing Group 1 (less than 24 h) with Group 2 (24–48 h). We did, however, find a negative correlation (Kendall tau rank correlation coefficient, r = −0.176) between the timing of surgery and change in Frankel grade, suggesting that the greater the delay, the lesser the neurological improvement. Table 3 further shows that four patients who improved by more than 1 grade were all in Group 1.
Table 2.
Results of all outcome variables in groups
| Group 1 | Group 2 | Group 3 | p value | |
|---|---|---|---|---|
| Frankel grade pre-op (and post-op) | ||||
| A | 6 (4) | 0 (1) | 0 (1) | Group 1 versus 2 p = 0.09 Group 1 and 2 versus 3 p = 0.048 |
| B | 3 (4) | 0 (0) | 1 (0) | |
| C | 15 (8) | 4 (3) | 7 (11) | |
| D | 21 (20) | 9 (10) | 45 (27) | |
| E | 0 (9) | 0 (9) | 0 (14) | |
| Mean length of stay in days (range) | 20 (2–75) | 22 (3–40) | 20 (2–66) | 0.67 |
| Complications | 18/45 (40 %) | 10/23 (43 %) | 22/53 (42 %) | 0.97 |
| Infection | 7/45 (16 %) | 2/23 (9 %) | 9/53 (17 %) | 0.64 |
| Mean survival (days) | 573 | 820 | 643 | 0.99 |
Table 3.
Changes in Frankel grade in each group
| Group | Changes in Frankel grade | |||||
|---|---|---|---|---|---|---|
| Deterioration by 1 grade | No change | Improvement by 1 grade | Improvement by 2 grades | Improvement by 3 grades | Total | |
| 1 | 4 | 18 | 19 | 3 : B to D (n = 2); A to C (n = 1) | 1 (A to D) | 45 |
| 2 | 1 | 12 | 10 | 0 | 0 | 23 |
| 3 | 7 | 27 | 19 | 0 | 0 | 53 |
Complications, infection, length of stay and survival (Table 2)
Overall, there were 50 patients who had one or more complications (41 %). The incidence of complications was similar in the three groups, with 40 % in group 1 (18/45), 43 % in Group 2 (10/23) and 42 % in Group 3 (22/53) and was not statistically different (p = 0.97). The overall incidence of post-operative surgical site infection was 15 % (18/121). There were seven patients in Group 1 (7/45, 16 %), two patients in Group 2 (2/23, 9 %) and nine patients in Group 3 (9/53, 17 %); there was no statistically significant difference between the three groups (p = 0.64). The mean length of stay was 20 days in Group 1 (range 2–75), 22 days in Group 2 (3–40) and 20 days in Group 3 (2–66). There was no difference in the length of post-operative stay in hospital between the groups (ANOVA p = 0.67).
Post-operatively, 12/121 (9.9 %; 4 patients in Group 1, 1 patient in Group 2, 7 patients in Group 3) deteriorated by at least one Frankel grade [7 patients by 1 grade, 2 patients by 2 grades (Frankel D to B) and three patients by 3 grades (Frankel D to A)] (Table 3). Of these, four patients were re-admitted for revision surgery.
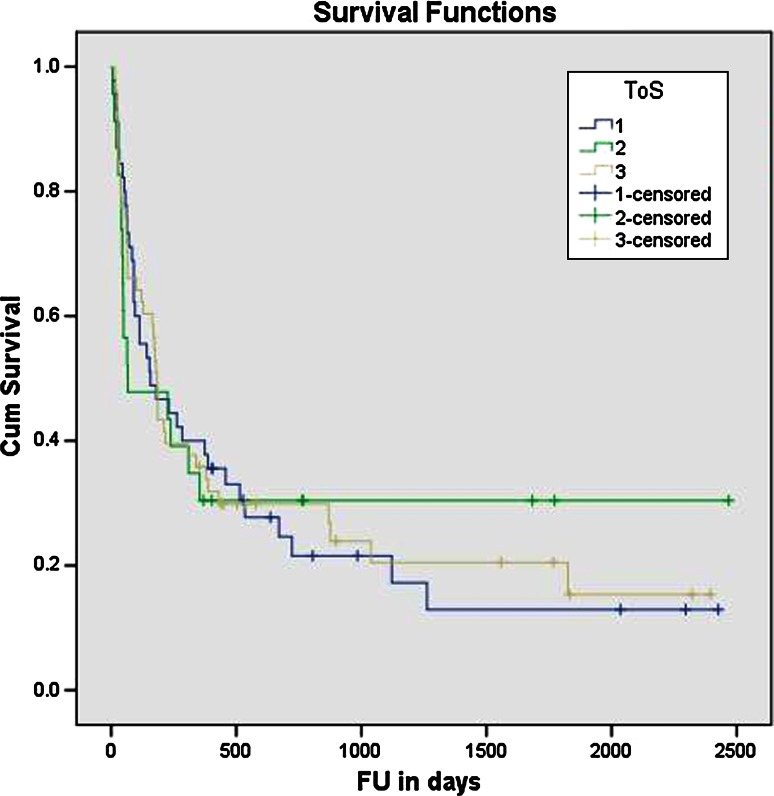
Overall, 93 patients (77 %) had died and 28 (23 %) were alive at the end of the study. The mean survival in our patients was 21 months (95 % CI 15–26 months; median survival 6 months (95 % CI 4–8 months). Figure 1 shows the Kaplan–Meier survival analysis for all the three groups. There was no statistically significant difference (Log rank Mantel–Cox test) between the three groups in terms of survival (p = 0.99).
Fig. 1.
Kaplan–Meier survival analysis for all three groups (showing no significant difference in survival between groups)
Surgery ‘within 48 h’ versus ‘after 48 h’ (Table 4)
Table 4.
Comparisons of patients undergoing surgery within 48 h versus after 48 h
| Surgery within 48 h (Groups 1 and 2) | Surgery after 48 h (Group 3) | p value | |
|---|---|---|---|
| No. | 68 | 53 | |
| Mean age | 62 | 61 | 0.68 |
| Gender (M:F) | 44:24 | 31:22 | 0.31 |
| Mean Tokuhashi score | 8.7 | 8.9 | 0.71 |
| Frankel grade pre-op (and post-op) | 0.048 | ||
| A | 6 (3) | 0 (1) | |
| B | 3 (4) | 1 (0) | |
| C | 19 (11) | 7 (11) | |
| D | 30 (30) | 45 (27) | |
| E | 0 (18) | 0 (14) | |
| Mean length of stay in days (range) | 21 (2–75) | 20 (2–66) | 0.4 |
| Complications % (no./total) | 41 % (28/68) | 42 % (22/53) | 0.97 |
| Infection % (no./total) | 13 % (9/68) | 17 % (9/53) | 0.37 |
| Mean survival (days) | 657 | 643 | 0.79 |
A sub-analysis was performed by comparing all patients who had surgery ‘within 48 h’ from presentation (Groups 1 and 2, n = 68) with those that had surgery ‘after 48 h’ from presentation (Group 3, n = 53). The groups were again equally distributed in terms of age, gender, revised Tokuhashi score, primary tumour type and level of spinal involvement (Table 4). There were a larger number of patients with greater neurological deficit, i.e. Frankel A and B in the ‘within 48 h’ group compared to the ‘after 48 h’ group. There was a statistically greater improvement in neurological function post-operatively in the ‘within 48 h’ Group 1 (p = 0.048) when compared with the ‘after 48 h’ group. But, there was no difference in survival (Log rank Mantel–Cox test p = 0.79), length of stay (p = 0.4), incidence of infection (p = 0.37) or complications (p = 0.97).
Primary tumour type, levels of spinal involvement, revised Tokuhashi score and number of metastases versus outcome variables (Table 5)
Table 5.
Primary tumour type, levels of spinal involvement, revised Tokuhashi score and number of metastases versus outcome variables
| Length of stay | Change in Frankel grade | Survival | Complications | |
|---|---|---|---|---|
| Histology of primary tumour | ||||
| Pearson correlation | −0.05 | 0.11 | −0.09 | 0.14 |
| p value | 0.54 | 0.14 | 0.22 | 0.07 |
| Levels of spinal metastases | ||||
| Pearson correlation | −0.06 | −0.02 | 0.06 | −0.03 |
| p value | 0.40 | 0.73 | 0.40 | 0.68 |
| Revised Tokuhashi score | ||||
| Pearson correlation | −0.07 | −0.06 | 0.33 | 0.09 |
| p value | 0.37 | 0.39 | 0.01** | 0.26 |
| Number of metastases in the spine | ||||
| Pearson correlation | −0.05 | 0.01 | 0.004 | −0.16 |
| p value | 0.53 | 0.84 | 0.96 | 0.05** |
A sub-analysis was performed comparing the histology of the primary tumour, revised Tokuhashi score, levels of spinal involvement, number of metastases in the spinal column with the outcome variables including length of stay, change in Frankel grade, survival and complications. The revised Tokuhashi score correlated significantly with survival (p = 0.01) and the complication rate increased in patients who had a greater number of metastases in the spinal column (p = 0.05).
Discussion
We found that in patients with MSCC and neurological deterioration, earlier surgical treatment resulted in better neurological outcome. In particular, surgical decompression within 48 h of acute presentation resulted in significantly better neurological outcome post-operatively compared to surgery after 48 h. There have been previous reports in the literature relating to the urgency of treatment for MESCC [12, 13]. Furstenberg et al. [12] had shown similar results with improved neurological recovery in patients who underwent surgery within 48 h of the development of symptoms. They found that a poor prognosis was associated with surgery delayed by more than 48 h, a low functional score pre-operatively, sensorimotor deficits, and bladder and sphincter dysfunction. If surgery was delayed, then the outcome was equally poor in those patients whose neurological symptoms developed acutely or chronically. This study, however, was limited by the small patient numbers (n = 35) and short follow-up of 6 weeks.
Chaichana et al. [13] analysed the predictors of ambulatory function after surgery for MESCC and found that patients who presented with symptoms for less than 48 h had a significantly increased likelihood of recovering ambulation. Our study was also able to demonstrate no difference in the survival of patients despite early surgical intervention in MSCC. We have reported a median survival of 6 months (95 % CI 4–8 months) and a mean survival of approximately 21 months. This is comparable, if not a little better, than other reports with a mean survival time of 11–16 months [9, 17]. Tancioni et al. [18] concluded in their study that the prognosis was dependent upon the histology of the primary tumour and visceral metastases. Our results were similar in that the Tokuhashi score correlated statistically significant to the survival of the patients. However, the histology of the primary tumour did not give a statistically significant correlation in our study. We opine that survival is influenced by several factors—the type and nature of the primary tumour, extent of secondary disease, the duration of the metastatic disease and by the sensitivity to the adjuvant treatment, rather than timing of acute decompressive surgical intervention per se.
Due to the large difference in the number of patients in our series who underwent the various surgical approaches [posterior (n = 105) vs. anterior (n = 3) vs. combined (n = 13)], we were not able to perform any meaningful statistics to make comparisons with our outcome variables. However, Li et al. [19] showed that en bloc surgery (n = 32) could achieve a lower local recurrence rate than the debulking surgery (n = 99), but with similar survival, neurological salvage, and incidence of complications.
The main strength of our work lies in the large sample size; all patients were followed up in spinal oncological clinics (until deceased). We have comprehensively reported on differences in neurological outcome, complication and survival in all groups. Limitations include a larger number of patients in Group 1 with greater neurological dysfunction, and reliance on patient history for duration of symptoms. Nevertheless, this study does succeed in providing retrospective evidence on the importance of timely intervention in these patients.
Conclusion
Our results show that surgery should be performed sooner rather than later. Furthermore, early decompressive surgery within 48 h of acute presentation with neurological deficit in patients with metastatic spinal cord compression results in a better neurological improvement compared to surgery after 48 h. However, the incidence of post-operative length of hospital stay, complications or patient survival was not influenced by earlier surgical intervention.
Acknowledgments
The authors would like to thank Dr Graham Warren, Research Fellow in Medical Statistics, Nottingham Integrated Clinical Research Centre, Queen’s Medical Centre, Nottingham for statistical support.
Conflict of interest
None.
References
- 1.Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614–619. doi: 10.1056/NEJM199208273270907. [DOI] [PubMed] [Google Scholar]
- 2.Nelson KA, Walsh D, Abdullah O, McDonnell F, Homsi J, Komurcu S, et al. Common complications of advanced cancer. Semin Oncol. 2000;27(1):34–44. [PubMed] [Google Scholar]
- 3.Bucholtz JD. Metastatic epidural spinal cord compression. Semin Oncol Nurs. 1999;15(3):150–159. doi: 10.1016/S0749-2081(99)80002-3. [DOI] [PubMed] [Google Scholar]
- 4.McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486–491. doi: 10.1038/sj.bjc.6602957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111–118. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 7.Findlay GF. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry. 1984;47(8):761–768. doi: 10.1136/jnnp.47.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen S, Borgesen SE, Rohde K, Rasmusson B, Bach F, Boge-Rasmussen T, et al. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer. 1990;65(7):1502–1508. doi: 10.1002/1097-0142(19900401)65:7<1502::AID-CNCR2820650709>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Sundaresan N, Sachdev VP, Holland JF, Moore F, Sung M, Paciucci PA, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330–2335. doi: 10.1200/JCO.1995.13.9.2330. [DOI] [PubMed] [Google Scholar]
- 10.Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614–1627. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1614::AID-CNCR12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 12.Furstenberg CH, Wiedenhofer B, Gerner HJ, Putz C. The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Jt Surg Br. 2009;91(2):240–244. doi: 10.1302/0301-620X.91B2.20894. [DOI] [PubMed] [Google Scholar]
- 13.Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62(3):683–692. doi: 10.1227/01.neu.0000317317.33365.15. [DOI] [PubMed] [Google Scholar]
- 14.Metastatic Spinal Cord Compression. Diagnosis and management of patients at risk of or with metastatic spinal cord compression (November 2008). Developed for NICE by the National Collaborating Centre for Cancer (CG75). http://www.nice.org.uk/nicemedia/pdf/CG75NICEguideline.pdf [PubMed]
- 15.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 16.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 17.Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1999;24(18):1943–1951. doi: 10.1097/00007632-199909150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Tancioni F, Navarria P, Pessina F, Attuati L, Mancosu P, Alloisio M, Scorsetti M, Santoro A, Baena RR. Assessment of prognostic factors in patients with metastatic epidural spinal cord compression (MESCC) from solid tumor after surgery plus radiotherapy: a single institution experience. Eur Spine J. 2012;21(Suppl 1):S146–S148. doi: 10.1007/s00586-012-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Gasbarrini A, Cappuccio M, Terzi S, Paderni S, Mirabile L, Boriani S. Outcome of excisional surgeries for the patients with spinal metastases. Eur Spine J. 2009;18(10):1423–1430. doi: 10.1007/s00586-009-1111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]