Abstract
Purpose
To evaluate safety of coblation of simulated lytic metastases in human cadaveric vertebral bodies by measuring heat distribution during thermal tissue ablation and comparing it to radiofrequency ablation (RFA).
Materials and methods
Three devices were compared: a 10 mm single-needle RFA electrode, a 20 mm array RFA electrode and the coblation device. To simulate bone metastases, a spinal tumor model was used stuffing a created lytic cavity with muscle tissue. Measuring of heat distribution was performed during thermal therapy within the vertebral body, in the epidural space and at the ipsilateral neural foramen. Eight vertebral bodies were used for each device.
Results
Temperatures at heat-sensitive neural structures during coblation were significantly lower than using RFA. Maximum temperatures measured at the end of the procedure at the neural foramen: 46.4 °C (±2.51; RFA 10 mm), 52.2 °C (±5.62; RFA 20 mm) and 42.5 °C (±2.88; coblation). Maximum temperatures in the epidural space: 46.8 °C (±4.7; RFA 10 mm), 49.5 °C (±6.48; RFA 20 mm) and 42.1 °C (±2.5; coblation). Maximum temperatures measured within the vertebral body: 50.6 °C (±10.48; RFA 10 mm), 61.9 °C (±15.39; RFA 20 mm) and 54.4 °C (±15.77; coblation).
Conclusion
In addition to RFA, the application of coblation is a safe method to ablate vertebral lesions with regards to heat distribution at heat-sensitive neural spots. The measured temperatures did not harbor danger of thermal damage to the spinal cord or the spinal nerves.
Keywords: Spine, Metastases, RFA, Coblation
Introduction
Metastases of the spine represent the most common type of bone metastases in tumor patients. At the point of their death, about 36 % of all tumor patients possess osseous metastases of the spine [1]. The most frequent primary tumors creating lytic metastases are breast cancer and bronchial carcinoma [2].
The treatment is usually palliative and comprises different methods including chemotherapy, radiotherapy and interventional techniques like thermal ablation therapy. Concerning pain reduction, the combination of thermal ablation and vertebroplasty shows high success rates, which can be up to 100 % [3]. In general, there are two different thermal ablation methods: radiofrequency ablation (RFA) and radiofrequency ionization (coblation). RFA is a well-known technique, which is also used in the treatment of primary tumors like renal cell carcinoma [4], hepatocellular carcinoma [5] or osteoid osteoma [6]. There are two different types of RFA applicators: monopolar and bipolar systems. The most commonly used RFA systems operate in a monopolar way, as did the system that was used in our cadaver study. In addition to the active electrode, which is placed in the target tissue, grounding pads are placed on the patient’s body. The high electrical resistance of the tissue, compared to the resistance of the electrodes, leads to an ionic agitation of the adjacent tissue. Thus, the agitated tissue represents technically the source of heating, not the electrode itself that is placed within it. Therefore, the heating is not limited to the device-tip, but concerns a certain field including the target tissue in the vicinity of the electrode [7].
The radiofrequency ionization (plasma-mediated ablation, coblation) represents a new thermal ablation method in the treatment of bone metastases, which tumor-patients could benefit from. With this technique, at the tip of the device, a plasma field of highly energetic ionized particles is created which is capable of breaking molecular bonds and dissolving the target tissue [8]. To maintain this plasma field, a continuous flow of saline solution through the device is necessary. In contrast to RFA, a cavity is created in the target tissue, caused by the slightly curved design of the device and its circumferential moving in different clock positions while performing coblation. The created cavity allows a more controlled injection of cement during the following vertebroplasty [9]. Recently, plasma-mediated ablation has found its way into treatment of both bone tumors and bone metastases. Jakanani et al. [10] described the successful coblation of a acetabular metastasis followed by cementoplasty; Gerszten and Monaco [11] performed the ablation of vertebral metastases in patients with malignant vertebral compression fractures prior to vertebroplasty.
Nevertheless, coblation represents—like the RFA—a thermal ablation procedure, which may harbor danger of thermal injury when the metastases are adjacent to the spinal canal or nerve root. Because vertebral metastases typically affect the posterior part of the vertebral body first, nerve structures are particularly endangered.
Because of the risk of thermal damage in heat-sensitive [12–16] nerve tissue, it is important to measure the generated temperatures during this procedure. Concerning RFA of vertebral lesions, this issue was evaluated by Adachi et al. [17]. Wegener et al. [18] recorded temperatures in the spinal canal to investigate safety of vertebroplasty. We performed this study to evaluate the risk of therapy-associated neural damage during coblation therapy in human cadaveric vertebral bodies and to compare it with the temperatures occurring during conventional RFA.
Materials and methods
Human cadaveric lumbar vertebral bodies were used in this study to compare the heat distribution during the application of RFA and coblation under realistic anatomic circumstances. A tumor-mimic model, as developed by Kawai et al. [19] to simulate RFA of tumor tissue, was used to create a lytic lesion in the posterior part of the vertebral body. For this purpose, a transpedicular access was established with a 9 G biopsy needle. Via this access, a 1.5 cc cavity was created within the posterior part of the vertebral body, with a Soteira Shield Kyphoplasty System (Soteira Inc., Natic, USA). The created cavity was filled up with 1.5 cc tumor paste, which was made of fresh muscle tissue from swine. To enhance visibility under fluoroscopic view, barium sulfate was added to the muscle paste, at a ratio of 1:10. This model was suggested for performing the RFA using the standard ablation algorithms.
Eight human vertebral bodies were used for each of the three tested devices: a 20 mm RFA array electrode (LeVeen™ Electrode System, Boston Scientific, Natick, USA), a 10 mm single-needle RFA electrode (Soloist™ Electrode System, Boston Scientific, Natick, USA) and the coblation device (Cavity SpineWand, ArthroCare, Sunnyvale, USA). Under fluoroscopic guidance, the devices were placed in position through the same access used to create the tumor mimic model. Thermocouples (k-type) were placed on three positions at the level of the thermosensitive regions of interest: via contralateral transpedicular access within the central vertebral body, at the ipsilateral neural foramen and in the epidural space (Fig. 1). All placements were accomplished under fluoroscopic control. The thermocouples were connected to a four-channel thermometer (Voltcraft 304/K204, Conrad Electronic SE, Hirschau, Germany). The vertebral body was placed in a plastic case filled with saline solution, continuously tempered at 37 °C to simulate standard body temperature. At the bottom of the case, a copper plate was mounted to place the RFA grounding pads at (Fig. 2).
Fig. 1.
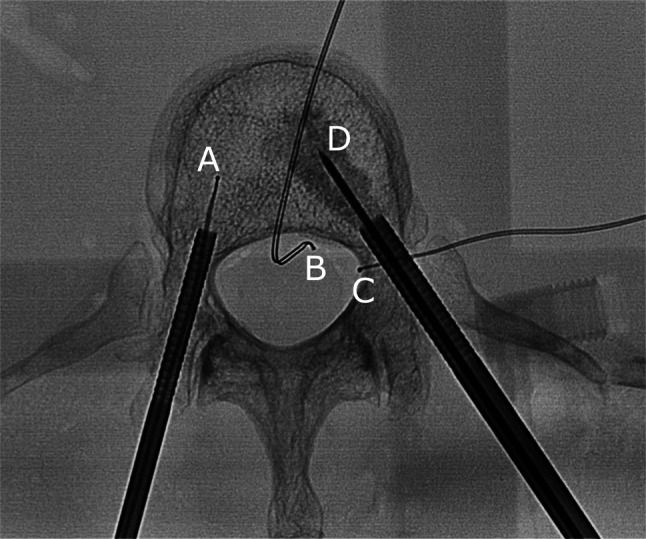
Fluoroscopic view of the vertebral body with thermocouples within the vertebral body (A), in the epidural space (B) and at the neural foramen (C). The metastasis simulating lytic lesion was filled with muscle tissue via the same transpedicular access the 10 mm Soloist electrode (D) was inserted
Fig. 2.
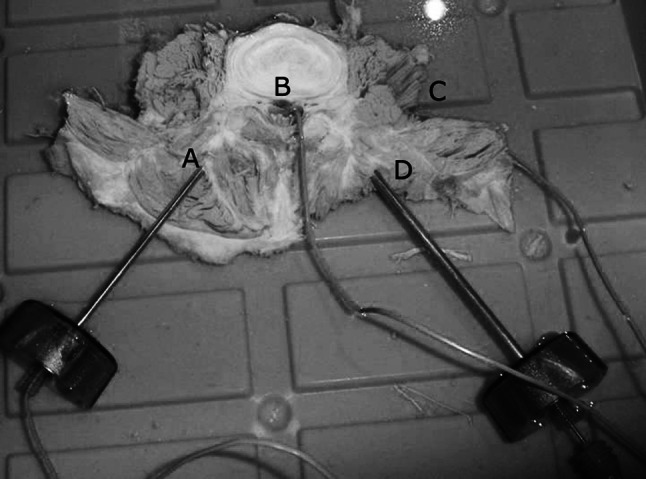
Experimental setup with positions of the thermocouples within the contralateral vertebral body (A) and at the level of heat-sensitive neural structures: in the epidural space (B) and at the neural foramen (C). The ablation device (D) was inserted via transpedicular access
Temperatures were measured during thermal ablation as previously described by Adachi et al. [17]. The RFA electrode was connected with a RF 3000 generator (Boston Scientific, Natick, USA), whereas the coblation electrode received power from an ArthroCare System 2000 Controller (ArthroCare, Sunnyvale, USA). RFA started at 2 W using the single-needle electrode and at 20 W using the array-electrode. During the application, the power was raised according to the algorithms given by the manufacturer. The coblation system was set to level 6 during the whole operation. In contrast to RFA, during coblation, the device was moved back and forth repetitively within the vertebral body, using different clock positions to create the cavity.
Results
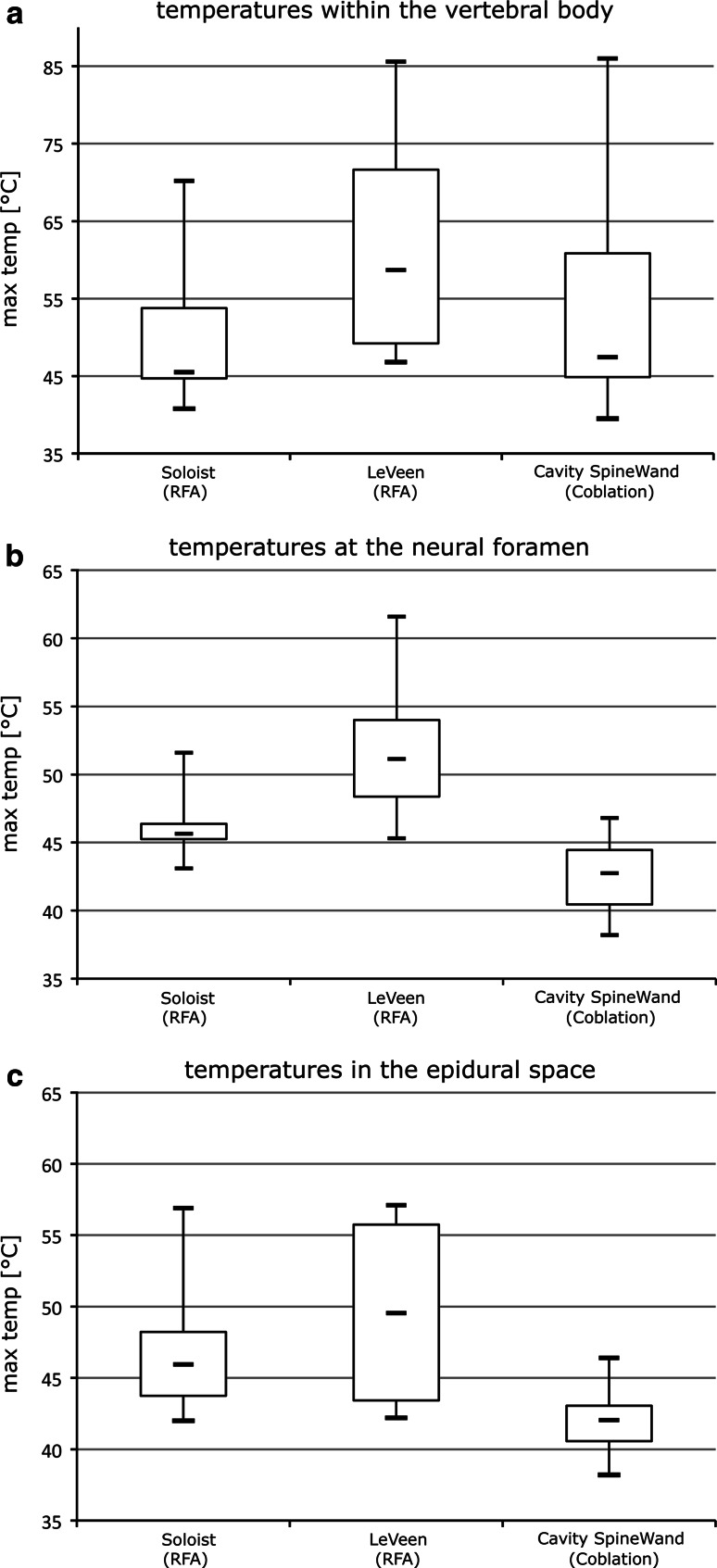
The maximum temperature at the neural foramen was 46.4 °C (±2.51) using the RFA 10 mm device, 52.2 °C (±5.62) using the RFA 20 mm electrode and 42.5 °C (±2.88) with the coblation device. In the epidural space, the temperature reached 46.8 °C (±4.7) with the RFA 10 mm device, 49.5 °C (±6.48) using the RFA 20 mm electrode and 42.1 °C (±2.5) while the appliance of coblation. The highest temperature within the vertebral body was 50.6 °C (±10.48) using the RFA 10 mm electrode, 61.9 °C (±15.39) with the RFA 20 mm device and 54.4 °C (±15.77) using coblation (Fig. 3).
Fig. 3.
Temperatures within the vertebral body (a), at the neural foramen (b) and in the epidural space (c). Data displayed as a boxplot (box-and-whisker diagram): the bottom and top of the box represent the lower and upper quartiles. The band within the box represents the median. The whiskers display maximum and minimum values
At the monitoring point within the vertebral body, the differences between the RFA Soloist, the RFA LeVeen electrode and the coblation electrode were not significant (RFA 10 mm/coblation: P = 0.579; RFA 20 mm/coblation: P = 0.353; unpaired t test). The highest temperature measured at the neural foramen when applying coblation was significantly lower than the temperatures reached by both RFA devices (Soloist/coblation: P = 0.012; LeVeen/coblation: P = 0.0007; unpaired t test). Also, the maximum temperature in the epidural space recorded using coblation was significantly lower than the highest temperatures measured during RFA (Soloist/coblation: P = 0.023; LeVeen/coblation: P = 0.008; unpaired t test). The mean duration of appliance using the three devices was 9.61 min (RFA Soloist electrode), 7.73 min (RFA LeVeen electrode) and 1.43 min (Cavity SpineWand), (Soloist/coblation: P < 0.0001; LeVeen/coblation: P < 0.0001; unpaired t test).
Discussion
Damage of nerve tissue has been identified as one of the most serious complications associated with thermal ablation therapy. Neural damage occurs within the spinal canal when temperature reaches 45 °C [15, 16, 20, 21]. Nour et al. [22] performed RFA animal studies in porcine models. Depending on the proximity to the adjacent neural structures, neural injury occurred varying from radiculopathy to paraplegia. Nakatsuka et al. [23] described transient damage as well as permanent injury of neural structures in patients. However, the monitoring of the temperature within the spinal canal helps to render the procedure safer. In another study of Nakatuska et al. [24], neural damage occured only in one case and, furthermore, was only transient.
The results of our measurements within the vertebral body show no significant difference between RFA and coblation regarding the distribution of heat. Temperatures generated at this point were relatively high compared to the other measuring points. However, the assumption that higher temperatures within the vertebral body could cause positive effects regarding pain relief was excluded by Anselmetti et al. [25]; in their study, thermal damage to the intravertebral nerve tissue was ruled out as the principal reason for pain relief. Thus, at the sensible spots like the neural foramen and in the epidural space, coblation generates significantly lower temperatures than RFA does. Both the 10 mm Soloist electrode and the 20 mm LeVeen electrode caused temperatures higher than 45 °C in the majority of cases, whereas coblation exceeded the 45 °C mark only once at the neural foramen and the epidural space, respectively. In both cases, the temperature did not overrun 47 °C, so that we did not classify this exceedance as dangerous because duration of coblation was very short: Froese et al. [16] determined the effective dose (ED50) for heating nerve tissue, or rather the spinal cord, at 45 °C as 10.8 min. An increase of temperature by 1 °C decreases the exposure time necessary to cause the same effect, by the factor of 2,25. According to this, the ED50 for heating neural tissue at 47 °C is 2.13 min. However, in our experimental series, coblation did not exceed 1.58 min, the mean application time was 1.42 min (84.9 s).
In contrast, temperatures during RFA exceeded 45 °C at the neural foramen in 87.5 % of all treatments (Soloist 75.0 %; LeVeen 100.0 %). In the epidural space, the temperature exceeded 45 °C in 62.5 % of all runs (Soloist 62.5 %; LeVeen 62.5 %).
Adachi et al. [17] recorded temperatures in the spinal canal while performing RFA ex vivo in vertebral bodies with and without a cortical bone defect of the posterior wall. Temperatures above 45 °C were only reached in the vertebral bodies with a defect of the posterior wall. However, in opposition to our study, the test arrangement was not tempered at standard body temperature but at a value between 23 and 25 °C. This significant lower ambient temperature causes a considerable cooling effect that may be the reason for lower values recorded in this study. Unlike this study, we monitored temperature also at the neural foramen, because the nerve roots represent, beside the spinal cord, another vulnerable structure adjacent to the vertebral column. Although this measuring point is more distant from the device-tip than the epidural measuring point, it exceeded the 45 °C mark more often whilst using RFA. Thus, the spinal nerves seem even more endangered to sustain thermal injury than the spinal cord. We assumed that the access-needle used in coaxial technique, which the RFA-electrode was inserted through, acts as a conductor of heat. Its transpedicular location implies proximity to the ipsilateral foramen intervertebrate that presents the passage point of the spinal nerves.
The values measured during the RFA in our cadaver study could lead to the conclusion that neural injury might be a rather frequent complication in patients. Nevertheless, our experiments were performed ex vivo, prohibiting simulation of a factor affecting the heat distribution. In vivo, cerebrospinal fluid and the blood flow through the spinal and vertebral vessels would cause a certain amount of cooling to the structures we focused our measurements at. In our study, we did not simulate the convective effect of blood-flow and liquor circulation: temperatures generated in vivo would be lower [25].
In conclusion, our study shows that in addition to RFA, the application of coblation is a safe method to ablate vertebral lesions concerning heat distribution at heat-sensitive neural spots, because the temperatures reached by coblation are lower than those reached by RFA (neural foramen: RFA 10 mm/coblation: P < 0.05; RFA 20 mm/coblation: P < 0.001. epidural space: RFA 10 mm/coblation: P < 0.05, RFA 20 mm/coblation: P < 0.01). During coblation, our measured data did not show risk of thermal injury to the spinal cord or the nerve roots, while RFA constantly reached higher temperatures at the adjacent neural structures. According to this, coblation may be attended at an even lower rate of complication than RFA. It is convenient to choose coblation as the preferred therapy when the location of the vertebral lesion is adverse and the application of RFA appears to harbor the danger of injuring the spinal cord or the nerve roots. This can be the case when the metastasis invades the posterior wall of the vertebral body or infiltrates the pedicles. So minimal-invasive interventional therapy of metastatic disease of the spine may come into consideration for patients where RFA cannot be performed for reasons of safety.
To confirm these results, further studies, performed in vivo, will be needed. Furthermore, it will be necessary to analyze the reaction of the tissue treated by coblation. This should include a comparison between the volumes of ablated metastatic tissue using both methods to evaluate the capability of coblation concerning this aspect.
Conflict of interest
None.
References
- 1.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15:1–4. doi: 10.1097/00007632-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327:614–619. doi: 10.1056/NEJM199208273270907. [DOI] [PubMed] [Google Scholar]
- 3.Toyota N, Naito A, Kakizawa H, et al. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. 2005;28:578–583. doi: 10.1007/s00270-004-0208-0. [DOI] [PubMed] [Google Scholar]
- 4.Salagierski M, Salagierski MS, Salagierska-Barwińska A. Radiofrequency ablation in kidney tumour management: a method of real-time monitoring. Scand J Urol Nephrol. 2010;44:84–90. doi: 10.3109/00365590903555385. [DOI] [PubMed] [Google Scholar]
- 5.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: current status. World J Radiol. 2010;2:417–424. doi: 10.4329/wjr.v2.i11.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hempfing A, Hoffend J, Bitsch RG et al (2007) The indication for gamma probe-guided surgery of spinal osteoid osteomas. Eur Spine J 16(10):1668–1672 [DOI] [PMC free article] [PubMed]
- 7.Clasen S, Pereira PL (2009) Radiofrequency ablation—technical basics. In: Mahnken AH, Ricke J (eds) CT- and MR-guided interventions in radiology. Springer, Berlin, pp 159–166
- 8.Chen YC, Lee SH, Saenz Y, et al. Histologic findings of disc, end plate and neural elements after coblation of nucleus pulposus: an experimental nucleoplasty study. Spine J. 2003;3:466–470. doi: 10.1016/S1529-9430(03)00143-8. [DOI] [PubMed] [Google Scholar]
- 9.Georgy BA. Bone cement deposition patterns with plasma-mediated radio-frequency ablation and cement augmentation for advanced metastatic spine lesions. AJNR Am J Neuroradiol. 2009;30:1197–1202. doi: 10.3174/ajnr.A1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakanani GC, Jaiveer S, Ashford R, et al. Computed tomography-guided coblation and cementoplasty of a painful acetabular metastasis: an effective palliative treatment. J Palliat Med. 2010;13:83–85. doi: 10.1089/jpm.2009.0176. [DOI] [PubMed] [Google Scholar]
- 11.Gerszten PC, Monaco EA. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurg Focus. 2009;27:E9. doi: 10.3171/2009.9.FOCUS09184. [DOI] [PubMed] [Google Scholar]
- 12.Haveman J, Sminia P, Wondergem J, et al. Effects of hyperthermia on the central nervous system: what was learnt from animal studies? Int J Hyperthermia. 2005;21:473–487. doi: 10.1080/02656730500159079. [DOI] [PubMed] [Google Scholar]
- 13.Wondergem J, Haveman J, Rusman V, et al. Effects of local hyperthermia on the motor function of the rat sciatic nerve. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:429–438. doi: 10.1080/09553008814552561. [DOI] [PubMed] [Google Scholar]
- 14.Letcher FS, Goldring S. The effect of radiofrequency current and heat on peripheral nerve action potential in the cat. J Neurosurg. 1968;29:42–47. doi: 10.3171/jns.1968.29.1.0042. [DOI] [PubMed] [Google Scholar]
- 15.Yamane T, Tateishi A, Cho S, et al. The effects of hyperthermia on the spinal cord. Spine. 1992;17:1386–1391. doi: 10.1097/00007632-199211000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Froese G, Das RM, Dunscombe PB. The sensitivity of the thoracolumbar spinal cord of the mouse to hyperthermia. Radiat Res. 1991;125:173–180. doi: 10.2307/3577885. [DOI] [PubMed] [Google Scholar]
- 17.Adachi A, Kaminou T, Ogawa T, et al. Heat distribution in the spinal canal during radiofrequency ablation for vertebral lesions: study in swine. Radiology. 2008;247:374–380. doi: 10.1148/radiol.2472070808. [DOI] [PubMed] [Google Scholar]
- 18.Wegener B, Zolyniak N, Gülecyüz MF, et al. Heat distribution of polymerisation temperature of bone cement on the spinal canal during vertebroplasty. Int Orthop. 2012;36:1025–1030. doi: 10.1007/s00264-011-1382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Kaminou T, Sugiura K, et al. Creation of a tumor-mimic model using a muscle paste for radiofrequency ablation of the lung. Cardiovasc Intervent Radiol. 2009;32:296–302. doi: 10.1007/s00270-008-9463-9. [DOI] [PubMed] [Google Scholar]
- 20.Dupuy DE, Hong R, Oliver B, et al. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 21.Diehn FE, Neeman Z, Hvizda JL, et al. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol. 2003;14:1569–1576. doi: 10.1097/01.RVI.0000096769.74047.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nour SG, Aschoff AJ, Mitchell ICS, et al. MR imaging-guided radio-frequency thermal ablation of the lumbar vertebrae in porcine models. Radiology. 2002;224:452–462. doi: 10.1148/radiol.2242011269. [DOI] [PubMed] [Google Scholar]
- 23.Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15:707–712. doi: 10.1097/01.RVI.0000133507.40193.E4. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuka A, Yamakado K, Takaki H, et al. Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol. 2009;32:70–75. doi: 10.1007/s00270-008-9390-9. [DOI] [PubMed] [Google Scholar]
- 25.Anselmetti GC, Manca A, Kanika K, et al. Temperature measurement during polymerization of bone cement in percutaneous vertebroplasty: an in vivo study in humans. Cardiovasc Intervent Radiol. 2009;32:491–498. doi: 10.1007/s00270-009-9509-7. [DOI] [PubMed] [Google Scholar]