Abstract
Background
The postoperative analgesic potential of periarticular anesthetic infiltration (PAI) after TKA is unclear as are the complications of continuous femoral nerve block on quadriceps function.
Questions/purposes
We asked (1) whether PAI provides equal or improved postoperative pain control in comparison to a femoral nerve block in patients who have undergone TKA; and (2) if so, whether PAI improves early postoperative quadriceps control and facilitates rehabilitation.
Methods
We randomized 60 patients to receive either PAI or femoral nerve block. During the first 5 days after TKA, we compared narcotic consumption, pain control, quadriceps function, walking distance, knee ROM, capacity to perform a straight leg raise, and active knee extension. Medication-related side effects, complications, operating room time, and hospitalization duration were compared.
Results
Opioid consumption was lower in the PAI group during the first 8 postoperative hours (12.5 mg versus 18.7 mg morphine), as was reported pain at rest (1.7 versus 3.5 on a 10-point VAS). Thereafter, narcotic consumption and reported pain were similar up to 120 hours. More subjects in the femoral nerve block group experienced quadriceps motor block (37% versus 0% in the PAI group). On Days 1 to 3, subjects in the PAI group experienced better capacity to perform the straight leg raise, active knee extension, and had longer walking distances.
Conclusions
PAI provided pain control equivalent to that of a femoral nerve block while avoiding a motor block and its negative functional impacts. The data suggest it should be considered an alternative to a femoral nerve block.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Perioperative pain control in TKA may be insufficient and may interfere with patients’ ability to sleep, walk, and participate in other activities [13, 25]. Continuous femoral nerve block and/or sciatic nerve blocks improve postoperative pain control and reduce narcotic consumption [23]. However, 0.1% to 2.5% of patients experience complications associated with nerve blocks including muscle weakness, nerve damage, local infection, or “double crush” (in which a distal nerve branch becomes more sensitive to injury if the proximal root is injured, by spinal stenosis or lumbar disc herniation, for examples) with peripheral nerve blocks and tourniquet [7, 14, 15, 23].
Parenteral narcotics continue to play a major role in postoperative pain control strategies despite major side effects [1, 11, 23]. With the aim of decreasing the occurrence of side effects, a good analgesia protocol preferably should be multimodal and should block pain influx at its origin. Furthermore, it should maintain maximum muscle control to optimize postoperative rehabilitation and inhibit venous stasis. Periarticular anesthetic infiltration (PAI) reportedly achieves these goals [27].
Evidence from the literature supports the use of PAI to manage early postoperative pain. In a previous randomized controlled trial (RCT) evaluating PAI, we showed that mean morphine consumption was lower in the PAI group than the control group up to 40 hours postoperatively (46.7 mg compared with 68.6 mg, respectively) [27]. Other authors compared different PAI protocols versus femoral nerve block and have reported lower pain scores and consumption of opioids in their PAI group [21, 26]. Carli et al. [8] compared the analgesic effect of PAI versus femoral nerve block plus PAI into the posterior knee capsule and found that the femoral nerve block plus posterior PAI was associated with lower opioid consumption and better recovery at 6 weeks as measured by functional walking capacity (2- and 6-minute walk tests) than PAI alone. Although a femoral nerve block may affect quadriceps function in the early postoperative period, is time-consuming, and is associated with complications, it remains the most commonly used pain-control method. However, the analgesic potential and functional benefits of PAI versus femoral nerve block remain controversial.
We therefore asked whether: (1) a PAI protocol would reduce morphine consumption with patient-controlled analgesia (PCA) and pain levels in comparison to a femoral nerve block; (2) a PAI protocol would provide better postoperative lower limb function versus a femoral nerve block measured with patient capacity to perform active knee extension, straight leg raise, knee ROM, and daily walking distance; (3) PAI or femoral nerve block is associated with a higher postoperative complication rate; and (4) a PAI protocol would decrease operating room time and hospitalization duration.
Patients and Methods
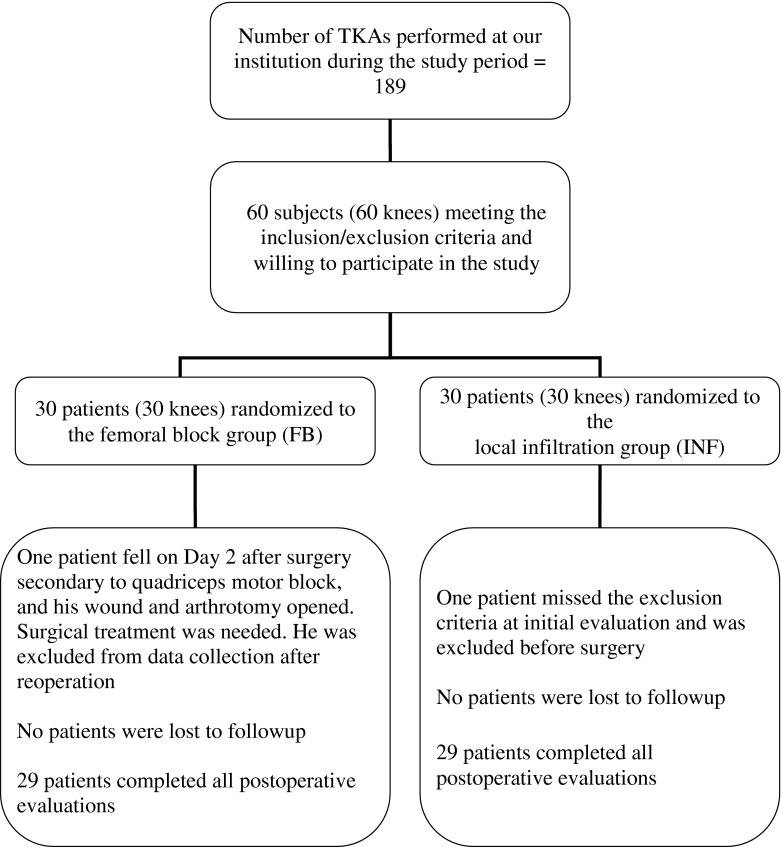
Between August 2006 and February 2009, 189 TKAs were performed by one of four orthopaedic surgeons (PAV, ML, MF, AR) at the Maisonneuve-Rosemont Hospital in Montreal, Quebec, Canada. Patients were included in the study if they were older than 18 years and were undergoing primary, unilateral TKA under spinal anesthesia. We excluded 129 patients with one of the following exclusion criteria: simultaneous bilateral TKAs, previous patellectomy, acute or chronic knee infection, regular narcotic use, neuromuscular deficit affecting the lower limbs, major systemic illness (heart failure, renal insufficiency, coagulopathy), and known allergy or intolerance to one of the study drugs, or who did not agree to participate in the study. A randomization table was created with SPSS 10.04 software (SPSS Inc, Chicago, IL, USA), and the results were kept in opaque, sealed envelopes. Randomization of each patient was revealed by the research nurse to the operative team (anesthetist, nurse, and surgeon) on the morning of surgery. Patients and postoperative teams (pain control unit, nurses, and physiotherapists) were blinded to the treatment group, making this trial double-blind. A total of 60 patients were randomized to one of two treatment groups: PAI (N = 30) or continuous femoral nerve block (N = 30). One subject randomized to the PAI group could not receive spinal anesthesia at initial evaluation and was removed from the study just before surgery, leaving 29 patients in the PAI group. The protocol was approved by the ethics and scientific committees of the hospital. All patients gave written, informed consent.
An estimated sample size of 27 in each group was needed to provide 80% power to detect a clinically meaningful difference of 15 mg morphine consumption from PCA during the first 48 hours [27] between the two groups with a SD of 19.4 mg and alpha error of 0.05.
The demographics and characteristics on both groups were comparable (Table 1).
Table 1.
Demographic and perioperative patient data
| Variable | Femoral block† | Infiltration† | p Value |
|---|---|---|---|
| (n = 30) | (n = 29) | ||
| Age (years) | 66.6 (36–78, 9.5) | 67.3 (54–78, 6.8) | 0.719 |
| Weight (kg) | 83.7 (52.2–121.6) | 89.0 (64.7–121.0) | 0.253 |
| Height (cm) | 164.8 (147.3–177.8, 8.9) | 165.6 (151.1–185.0, 9.3) | 0.757 |
| Sex: female/male | 23/7 | 16/13 | 0.081 |
| Side: right/left | 16/14 | 18/11 | 0.497 |
| Preoperative flexion (degrees) | 115.0 (90.0–135.0, 14.0) | 115.3 (80.0–150.0, 18.5) | 0.959 |
| Diagnosis | 0.383 | ||
| Osteoarthritis | 28 | 28 | |
| Avascular necrosis | 2 | 0 | |
| Rhumatoid arthritis | 0 | 1 | |
| Surgery duration (minutes) | 102.8 (73.0–148.0, 17.4) | 108.9 (70.0–160.0, 19.1) | 0.205 |
| Total operating room time (minutes)* | 170.8 (125.0–215.0, 22.3) | 161.4 (106.0–224.0, 27.1) | 0.152 |
| Postoperative days when patient met discharge criteria | 5.0 (3–15, 2.3) | 4.9 (3–8, 1.3) | 0.550 |
| Length of hospital stay (days) | 6.8 (5–16, 2.6) | 6.6 (5–15, 2.1) | 0.919 |
†Mean (minimum–maximum, SD); * minus time to perform placebo femoral block in the infiltration group (mean, 9.9 minutes).
All patients received spinal anesthesia with 2 to 3 mL bupivacaine 0.5% without narcotics. Intravenous narcotics and ketamine were not allowed during surgery. No bladder catheter was inserted routinely. One gram of vancomycin was given intravenously preoperatively and every 12 hours for 24 hours after surgery. TKAs were performed with tourniquet inflation up to 300 mm Hg during draping and released before or after skin closure according to the surgeon’s preference. A standard medial parapatellar arthrotomy was performed, and cemented, posterior-stabilized components were implanted. All components were fixed with Simplex® (Stryker Allendale, NJ, USA) cement. According to the surgeon’s preference, an evacuation drain was inserted before joint closure in 16 of 29 and 16 of 30 knees from the PAI and femoral nerve block groups, respectively. The drain was removed on postoperative Day 1. A Jones-type dressing was applied and discarded on postoperative Day 1. All patients received the cyclooxygenase-2 antiinflammatory drug Celebrex® (Pfizer, New York, NY, USA) (200 mg twice a day) and acetaminophen (500 mg four times a day) preoperatively (morning of the surgery) and regularly postoperatively until discharge. Low-molecular-weight heparin (Fragmin®; Eisai, NJ, USA) at 5000 units subcutaneously daily for 10 days was administered from postoperative Day 1 as thromboprophylaxis. PCA was available for additional analgesia during the first 48 hours. The decision to prolong PCA over 48 hours was determined by the anesthetist and not based on any standardized criteria. Afterward, oral narcotics (hydromorphone, codeine, and oxycodone) were given as needed.
In the PAI group, deep local anesthetic infiltration was prepared by adding 7.5 mL of 10.0 mg/mL Naropin® sterile pack (10 mL; AstraZeneca, Mississauga, Ontario, Canada), 30 mg ketorolac, and 0.5 mL adrenaline (1/1000) into a 100-mL sterile pack of 0.2 mg/mL Naropin®. This yielded 108 mL of a mixture containing 275 mg ropivacaine. Before component implantation, the entire mixture was infiltrated in deep tissues (collateral ligaments, posterior capsule, quadriceps tendon, patellar tendon, fat pad, periosteum, and synovial lining) with two 60-mL syringes fitted with 22-gauge needles. Before wound closure, subcutaneous tissues were infiltrated with 125 mg ropivacaine (2.5 mL of 10.0 mg/mL Naropin® 10 mL sterile pack plus 50 mL of 0.2 mg/mL Naropin® 100 mL sterile pack [total 52.5 mL through a 60-mL syringe]). A 16-gauge infiltration catheter passing through the vastus lateralis muscle was inserted in the joint for intraarticular injection on Day 1 after surgery. To keep patients and evaluators blinded to the treatment group, the anesthetist performed a sham femoral nerve block before spinal anesthesia by inserting a femoral catheter like in the femoral nerve block group. The postoperative continuous infiltration pump was filled with saline serum prepared by the pharmacy department. On postoperative Day 1 (between 16 and 24 hours after surgery), the evacuation drain was clamped, if present, and 150 mg ropivacaine (15 mL of Naropin® 10 mg/mL sterile pack [20 mL; AstraZeneca, London, UK]) was injected into the knee through the 16-gauge infiltration catheter, which then was removed. To avoid ropivacaine drainage through the skin hole, the clamped evacuation drain was discarded 3 to 6 hours after injection.
We used a paravascular approach to achieve anesthesia in the femoral nerve block (control) group before spinal anesthesia was administered in the operating room. Under local skin anesthesia with 0.5 to 1 cc of xylocaine 1%, an insulated Tuohy 18-gauge needle attached to a peripheral nerve stimulator was inserted in proximity to the femoral nerve using a 20-gauge femoral catheter. The needle was redirected until a persistent muscular response was obtained at 0.5 mA and 0.1 ms. Once this was achieved, 20 mL ropivacaine 0.25% was infiltrated. Ropivacaine 0.2% was perfused continuously by a pump (Hospira Abbott APM, Lake Forest, IL, USA) through the femoral catheter at 8 to 10 mL/hour for 48 to 72 hours. The anesthetist team on the floor adjusted the pump debit once 12 hours after surgery (starting with 8 mL/hour and modified by adding a 5-mL bolus with flow increased to 10 mL/hour) to maximize pain control and avoid quadriceps motor block. To maintain blinding, an intraarticular infiltration catheter was inserted in the knee during surgery (like in the PAI group); the catheter was removed on Day 1 after injection simulation (patients were asked not to look at the procedure). On Day 1, four of 29 intraarticular infiltration catheters were not infused in the PAI group because they were blocked (n = 3) or no longer intraarticular (n = 1). Regarding the femoral nerve block group, four femoral catheters were removed before 48 hours: one after a patient’s fall secondary to a quadriceps motor block, one after catheter disengagement on Day 1, one on postoperative Day 2 because of skin discharge and displacement, and one as a result of persistent quadriceps motor block limiting mobilization. The patient who fell secondary to quadriceps motor block overflexed his knee resulting in opening of his skin wound and arthrotomy incision. He was brought back to the operating room emergently for joint lavage and wound closure; thereafter he was excluded from data collection (Fig. 1).
Fig. 1.
The study screening and randomization flowchart shows the number of patients who completed the study.
Our standard postoperative regimen was used for all subjects. Postoperative knee radiographs were taken before the patient left the operating room. On the ward, all postoperative staff members (nurses, residents, physiotherapists, and ward anesthetists) were kept blind to the treatment group. Patients received physiotherapy evaluation and treatment twice a day, starting the evening of the surgery. They were allowed to mobilize as tolerated, and full weightbearing was permitted from the day of surgery. After surgery, passive and active exercises were supervised by a senior physiotherapist. A continuous passive motion machine was used 1 hour twice a day with increases in ROM as tolerated. At each visit, physiotherapists were asked to identify patients who had femoral nerve motor block (whatever the group); evaluate patients’ free and assisted knee flexion, measured by a goniometer; evaluate capacity to perform the straight leg raise test (yes or no); and evaluate the ability to extend the knee when sitting (yes or no). Patients were encouraged to stand and walk, under supervision, as soon as they could, and walking distance was recorded. Wounds were assessed daily by a nurse, noting zones of skin ischemia and cellulitis and recording number of days before wound discharge ceased.
A research nurse visited the ward staff and patients twice a day to collect all data and record medication side effects, especially those associated with ropivacaine such as blurred vision, hearing problems, mouth paresthesia, dizziness, uncontrolled muscle contraction, convulsion, hypotension, bradycardia, headache, and pruritus. Each day, starting on the evening of the surgery, patients were evaluated by an orthopaedic resident and an internal medicine doctor. Ultrasound to assess the presence of a deep vein thrombosis (DVT) was requested if there was clinical suspicion. At each visit, postoperative complications were assessed and, when present, classified according to Dindo et al. [12]. The patients’ pain control was assessed daily by an anesthetist. The primary outcome measure was morphine consumption with PCA the first 48 hours after surgery. It was recorded every 8 hours for the first 48 hours. Secondary outcome measures were narcotic consumption between 48 and 120 hours postoperatively noted every 8 hours and transformed into morphine equivalents according to a conversion table (Table 2); pain level was estimated daily by patients on a VAS (0–10) at rest and during physiotherapy exercises during the first 5 postoperative days. Although all patients stayed in the hospital at least 5 days postoperatively, recorded length of hospitalization was based on the following discharge criteria: active knee flexion greater than 80°, extension lag less than 10°, walking without help for more than 30 m, and a dry surgical wound. Ambulatory postoperative visits were at 6 weeks, 3 months, and 1 year.
Table 2.
Conversion of narcotics use into morphine equivalents
| Narcotics | Dose in morphine equivalents (mg) |
|---|---|
| Morphine, subcutaneous or intramuscular | 10 |
| Hydromorphone, subcutaneous or intramuscular | 1.5 |
| Hydromorphone, oral | 7.5 |
| Codeine, subcutaneous or intramuscular | 120 |
| Codeine, oral | 200 |
| Oxycodone, oral | 20 |
Continuous variables are reported as mean (SD, minimum, and maximum). Categorical variables are presented as frequency and percentage. Comparisons between groups (Table 1) were conducted with Student’s t-tests for normally distributed, continuous variables, Mann–Whitney tests for nonnormally distributed, continuous variables, and chi-square tests for categorical variables. Total narcotic consumption during the first 48 hours and 120 hours was compared by Student’s t-tests, whereas differences in narcotic consumption between the groups with time was analyzed by a two-way repeated-measures ANOVA. Because the narcotic consumption pattern differed with time between groups, contrasts were used to assess differences between groups at each time. Daily evaluations of pain, knee flexion, and walking distances also were analyzed by two-way repeated-measures ANOVA. For the variable straight leg raise, chi-square tests were performed each day to assess groups. Complications were compared by chi-square tests. Statistical analysis was performed with SPSS Version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
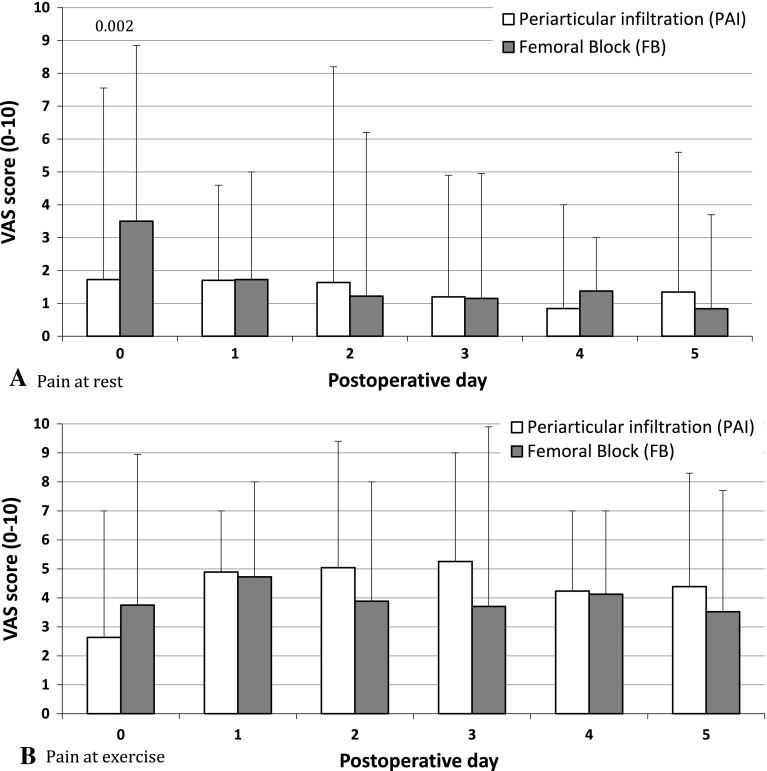
Both groups had similar (p = 0.884 and 0.740, respectively) mean morphine consumption during the first 48 and 120 hours after surgery (Table 3). However, the pattern of narcotic consumption differed (p = 0.015) with time between groups (Fig. 2). Higher (p = 0.037) morphine consumption was observed in the 0- to 8-hour period in the femoral nerve block group (Table 3). A difference (p = 0.016) was observed between groups in the pattern of postoperative pain at rest as reported on the VAS (Fig. 3A). The difference (p = 0.002) at rest was mainly the result of higher pain levels reported on the day of surgery by the femoral nerve block group (Table 3; Fig. 3A). There was no difference (p = 0.088) in the pattern between the groups during exercise (Fig. 3B).
Table 3.
Study population postoperative outcome data
| Variable | Femoral block* | Infiltration* | p Value |
|---|---|---|---|
| (N = 30) | (N = 29) | ||
| Morphine consumption in morphine equivalent (mg) | |||
| 0–8 hours postoperative | 18.7 (2–49, 11.3) | 12.5 mg (1–48, 10.7) | 0.037 |
| 0–48 hours postoperative | 57.2 (4.9–153.0, 37.6) | 57.9 (5.0–167.0, 39.9) | 0.884 |
| 0–120 hours postoperative | 115.9 (14–361, 81.5) | 123.7 (25–423, 94.6) | 0.740 |
| Pain on VAS scale | |||
| First postoperative day | 3.5 (0–9, 3.1) | 1.7 (0–8, 2.2) | 0.088 |
| Motor femoral nerve block during postoperative period | 37% (11/30) | 0 | < 0.001 |
| Number of patients | |||
| Unable to stand on Day 1 | 22% (6/27) | 0 | 0.018 |
| Number of patients unable to perform SLR | |||
| Day 1 | 74% (20/27) | 13% (3/24) | < 0.001 |
| Day 2 | 50% (13/26) | 4% (1/24) | 0.001 |
| Day 3 | 55% (11/20) | 5% (1/20) | 0.002 |
| Number of patients able to perform active knee extension | |||
| Day 1 | 41% (12/29) | 96% (23/24) | < 0.001 |
| Day 2 | 56% (15/27) | 100% (25/25) | 0.001 |
| Day 3 | 60% (12/20) | 100% (20/20) | 0.002 |
| Complications | |||
| Total | 15 | 10 | 0.228 |
| Confusion episode | 1 | 1 | |
| Urinary catheterization | 5 | 7 | 0.517 |
| Infection | 2 (1 deep, 1 superficial) | 0 | |
| Fall and wound disruption | 1 | 0 | |
| DVT | 6 | 2 | 0.142 |
* Mean (minimum–maximum, SD); SLR = straight leg raise; DVT = deep vein thrombosis.
Fig. 2.
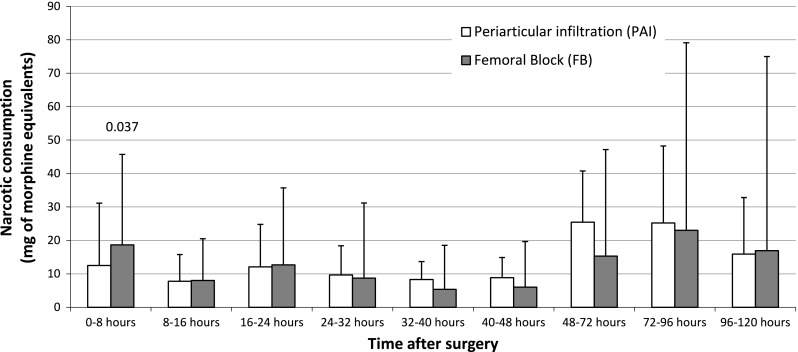
The postoperative narcotics consumption per period in milligrams of morphine equivalents is shown. The bars represent means and the lines represent 95% CI.
Fig. 3A–B.
The pain (A) at rest and (B) during physiotherapy exercises as assessed on a VAS (0–10) on each postoperative day are shown.
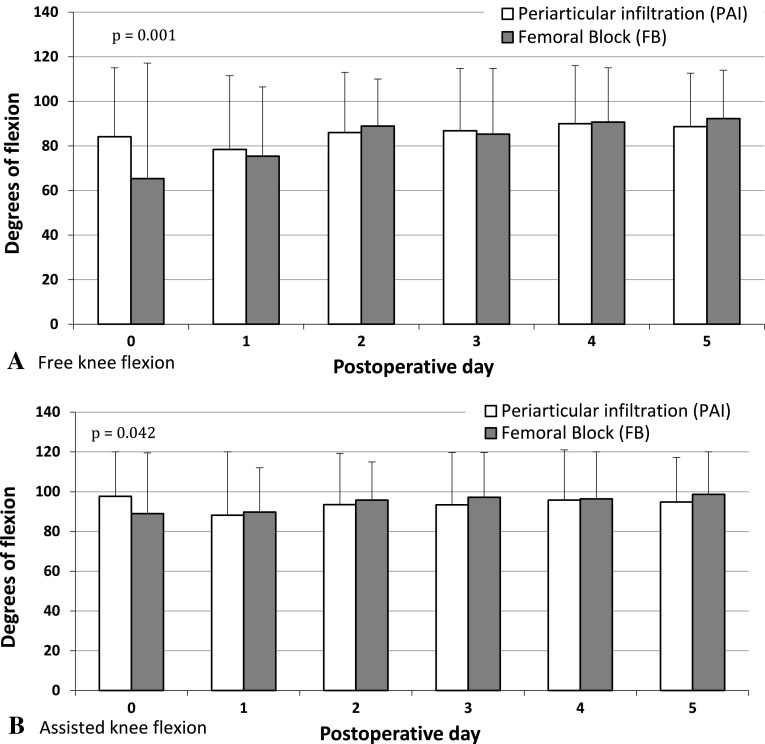
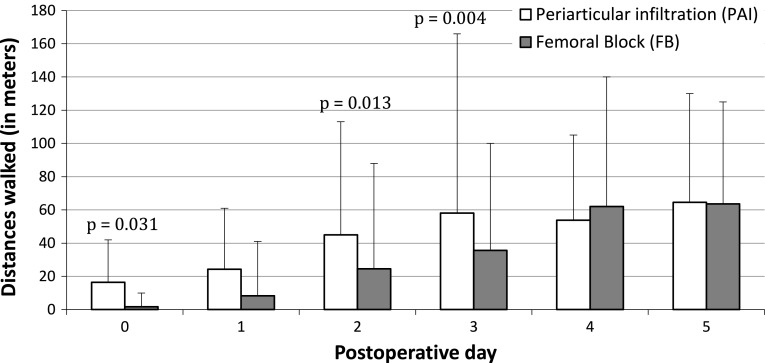
More (p < 0.001) subjects in the femoral nerve block group experienced motor block during the postoperative period (Table 3). The proportion of patients unable to perform a straight leg raise on postoperative Days 1 to 3 was greater (p < 0.001–0.002) in the femoral nerve block group (Table 3). Similarly, a higher proportion (p < 0.001–0.002) of patients who received a femoral nerve block were unable to perform active knee extension when sitting on Days 1 to 3 (Fig. 4A). On postoperative Day 1, more (p = 0.018) patients in the femoral nerve block group were unable to stand (Table 3). The pattern of postoperative knee-assisted flexion between groups differed (p = 0.020) over Days 1 through 5 (Fig. 4A). We observed greater (p = 0.001) knee-assisted flexion on the day of surgery in the PAI group but none (p = 0.228–0.988) at other followups. The pattern of free knee flexion between groups differed (p < 0.001) over Days 1 through 5 (Fig. 4B). Again, the PAI group had increased (p = 0.042) free knee flexion on the day of surgery but none (p = 0.131–0.549) at other followups. The distances walked by patients during the postoperative period differed (p = 0.0169) between the two groups. Patients in the PAI group walked longer (p = 0.004–0.031) distances on Days 0, 2, and 3 (Fig. 5).
Fig. 4A–B.
The (A) free and (B) assisted knee flexion on each postoperative day are shown.
Fig. 5.
The distances in meters walked on different postoperative days are shown.
Similar (p = 0.228) numbers of complications occurred in both groups (Table 3). According to the classification of Dindo et al. [12], there were eight versus six Grade 1, two versus seven Grade 2, and zero versus two Grade 3a complications in the PAI and femoral nerve block groups, respectively. One patient in the femoral nerve block group fell secondary to quadriceps weakness and the forceful knee flexion disrupted the wound and arthrotomy closures. In the femoral nerve block group, one deep and one superficial infection were treated with debridement and lavage and/or intravenous antibiotics. One patient who received PAI had a Pseudomonas infection at the sham femoral catheter site and was treated with intravenous antibiotics. The last day of wound discharge was similar (p = 0.395) in the PAI and femoral nerve block groups at 4.2 and 4.0 days, respectively. Surgical time, operating room time, and hospitalization durations were similar (p = 0.152–0.919) between groups (Table 1).
Discussion
A femoral nerve block is commonly administered to reduce the side effects and complications related to self-administered analgesia in patients who have had a TKA [2, 9, 28]. Nevertheless, diminished muscle control, nerve damage, and local infection are recognized complications, ranging from 0.1% to 2.5% [7, 14, 15, 23], and 15% of femoral nerve blocks are unsuccessful [20]. PAI offers the benefits of blocking pain influx at its origin and maximizing muscle control. Our randomized, double-blind, controlled study was designed to determine whether PAI, in comparison to femoral nerve block, reduces postoperative pain and morphine consumption, improves postoperative lower limb function, is associated with fewer postoperative complications, and results in decreased operating room time and shorter hospital stays.
We caution readers to limitations of our study. First, we compared our PAI protocol with a single nerve block. To cover all the sensitive areas of the knee, it may be necessary to obstruct the sciatic, femoral, and obturator nerves [20]. Our decision to block only the femoral nerve was made to compare the PAI procedure with the most common practice in our area. Second, the decision to prolong PCA over 48 hours was not based on any standardized guidelines. However, the anesthetist was blinded to the treatment group so this should not have polarized the results. Moreover, narcotics consumption, either from PCA, subcutaneous or oral, was added and converted into morphine equivalents. Third, the femoral nerve block group had slightly more females who might experience pain differently from males. We do not believe this jeopardized our results, because the number of females was not statistically greater than the number of males. Fourth, some patients received an evacuation drain and some did not. Pain levels may have been confounded by drainage or removal of the drain. Similar numbers of evacuation drains were used in both groups (16 versus 16) so it should not affect our results. Fourth, our study cohort was insufficient for some secondary outcomes such as DVT rate comparison (our study power 33%). However, the number of participants was appropriate to power the analysis of our primary objectives. Finally, perfect patient blinding would have required intraarticular saline injection via the infiltration catheter instead of an injection simulation. The potential risk of infection associated with this unessential procedure was not approved by our ethics committee. The occurrence of a Pseudomonas infection in one sham femoral block (PAI group) supports this assumption.
We showed the PAI group manifested better pain control during the first 8 hours and obtained equivalent results from 8 to 120 hours postoperatively (Table 3; Fig. 2). Some authors have investigated the benefits of local analgesia after TKA (Table 4) [3, 19, 22]. Except for the study by Badner et al. [3], studies using intraarticular injections alone failed to show a reduction of narcotic consumption after surgery [5, 16, 19, 22]. Comparing PAI with narcotics alone, two studies [17, 27] found decreased early postoperative pain and morphine consumption (Table 4). Comparing PAI with continuous femoral nerve block, Toftdahl et al. [26] reported lower pain and morphine consumption in the PAI group on the first day postsurgery. Carli et al. [8] showed that combining posterior knee capsule local anesthetic infiltration and femoral nerve block reduced morphine consumption and pain at rest during the first 48 hours postsurgery in comparison to PAI alone. These results confirm that the sensitive sciatic nerve territory of the knee is not covered by a femoral nerve block alone. To encompass entire knee territories, lumbar plexus block [18], or femoral [9], sciatic [4], and obturator block could be combined [20]. However, a combination of these procedures adds complexity to patient care. Many factors may explain the success of our protocol. PAI under direct vision provided local anesthetic tissue entrapment and reduced medication discharge through the evacuation drain or skin incision. Furthermore, two injections were given, and the anesthetic doses were higher than in other studies [3, 5, 17, 19]. Moreover, the addition of adrenaline to local anesthetics could slow ropivacaine release in the vascular system and prolong its local action.
Table 4.
Description of the scientific publications of PAI in TKA.
| Study | Groups | Study type | Length of followup | Results |
|---|---|---|---|---|
| Badner et al. [3] | Group 1: intraarticular 150 mg bupivacaine and 1:200,000 epinephrine before incision and 30 mL of saline after wound closure (N = 28) | Randomized, double-blind, controlled study | 24 hours | Intraarticular injection of bupivacaine and epinephrine after wound closure decreased the need for narcotics (59 ± 27 mg versus 81 ± 30 mg morphine, respectively) and increased the ROM motion. |
| Group 2: intraarticular 30 mL saline before incision and 150 mg bupivacaine and 1:200,000 epinephrine after wound closure (N = 27) | ||||
| Group 3: 30 mL saline for both intraarticular injections (N = 27) | ||||
| Mauerhan et al. [19] | Group 1: intraarticular saline (N = 27) | Randomized, double-blind, controlled study | 48 hours | The total amount of postoperative pain medication used in the first 24 hours after surgery was not different between the 4 treatment groups. |
| Group 2: intraarticular 5 mg morphine sulfate (N = 26) | ||||
| Group 3: intraarticular 50 mg bupivacaine (N = 24) | ||||
| Group 4: intraarticular 5 mg morphine sulfate and 50 mg bupivacaine (N = 28) | ||||
| All injections given immediately after wound closure | ||||
| Klasen et a. [16] | Group 1: postoperative epidural boluses of 2.5 mg of morphine, as requested by the patient (N = 10) | Randomized, controlled | 24 hours | No differences in VAS were observed between the three groups. |
| Group 2: intraarticular 1 mg morphine after wound closure, followed with PCA with morphine (N = 10) | ||||
| Group 3: epidural saline and intraarticular saline, followed by PCA with morphine (N = 10) | ||||
| Ritter et al. [22] | Group 1: intraarticular 10 mg morphine (N = 109) | Randomized, double-blind, controlled study | 24 hours | There were no differences between the groups in use of Demerol® and/or Toradol®, length of stay in hospital, or pain rating. Groups 1 and 4, whose injections included morphine, used more morphine in the first 24 postoperative hours than Group 2 or Group 3. |
| Group 2: intraarticular 25 mg bupivacaine (N = 114) | ||||
| Group 3: intraarticular saline (N = 97) | ||||
| Group 4: intraarticular 10 mg morphine 22.5 mg bupivacaine (N = 117) | ||||
| All injections given immediately after wound closure | ||||
| Browne et al. [5] | Group 1: intraarticular 100 mg bupivacaine (N = 30) | Randomized, double-blind, controlled study | 24 hours | The group receiving bupivacaine had a shorter time to discharge from the postanesthesia care unit than the group receiving saline. |
| Group 2: intraarticular 20 mL saline (N = 30) | Although bupivacaine resulted in lower pain scores and reduced narcotics during the 24-hour period, the difference was not significant between groups. | |||
| All injections given immediately after wound closure | ||||
| Lombardi et al. [17] | Group 1: narcotics alone (N = 138 patients) | Retrospective analysis | 48 hours | Patients receiving soft tissue and intraarticular injection had improved pain control in the immediate postoperative period, decreased blood loss, and decreased need for rescue narcotics and reversal agents. |
| Group 2: intraoperative injection 200 mg bupivacaine with 1:200,000 epinephrine and 10 mg morphine, 2/3 injected into the soft tissues and 1/3 injected into the joint (N = 171 patients) | ||||
| Vendittoli et al. [27] | Group 1: narcotics alone (N = 20) | Randomized, double-blind, controlled study | 120 hours | Morphine consumption was significantly lower in the local analgesia group than in the control group up to 40 hours after surgery. Both groups achieved a similar amount of knee flexion on the fifth postoperative day. |
| Group 2: PAI with 275 mg ropivacaine, 30 mg ketorolac, and 0.5 mL of adrenaline (1/1,000) before component implantation, followed with an additional 125 mg ropivacaine before wound closure (N = 22) | ||||
| Toftdahl et al. [26] | Group 1: continuous femoral nerve block (N = 37) | Randomized, controlled | 48 hours | More patients receiving periarticular and intraarticular infiltration could walk > 3 m, had lower pain scores during activity, and had lower consumption of opioids on the first postoperative day. |
| Group 2: periarticular and intraarticular infiltration with 300 mg ropivacaine, 30 mg ketorolac, and 0.5 mg epinephrine at the end of surgery, and 2 postoperative injections of these substances through an intraarticular catheter (N = 39) | ||||
| Carli et al. [8] | Group 1: continuous femoral nerve block (N = 20) | Randomized, double-blind, controlled study | 6 weeks | Patients in the femoral nerve block group had lower opioid consumption in the 48-hour postoperative period and better recovery at 6 weeks than patients in the PAI group. |
| Group 2: periarticular and intraarticular infiltration with 200 mg ropivacaine, 30 mg ketorolac, and 0.5 mg epinephrine (N = 20) | ||||
| Current study | Group 1: continuous femoral nerve block (N = 30) | Randomized, double-blind, controlled study | 120 hours | Patients in the PAI group had lower opioid consumption and lower pain at rest during the first 8 hours postoperative. More patients in the femoral nerve block group experienced quadriceps motor block. Patients in the PAI group experienced better capacity to perform the straight leg raise test and active knee extension and had longer walking distances in the postoperative period. |
| Group 2: periarticular infiltration (PAI) with 275 mg ropivacaine, 30 mg ketorolac, and 0.5 mL adrenaline (1/1,000) before component implantation, followed with an additional 125 mg ropivacaine before wound closure (N = 29) |
PAI = periarticular anesthetic infiltration.
Regarding lower limb function, we found a femoral nerve block was associated with loss of quadriceps motor control in the first 3 postoperative days in comparison to the PAI group (Table 3; Figs. 4, 5). Mullaji et al. [21] conducted a study of bilateral TKAs in which one knee received PAI and the other knee did not. Patients had greater active flexion up to 4 weeks and superior quadriceps recovery up to 2 weeks after surgery in the PAI knee [21]. Only Toftdahl et al. [26] compared the early functional benefits of PAI with those from femoral nerve block and reported that more patients in the PAI group could walk greater than 3 m on the first postoperative day. Aiming to improve recovery and shorten hospital length of stay, femoral nerve block affect on muscle function and the early rehabilitation protocol may be important. We found that PAI alone, preserving quadriceps muscle function, while providing similar pain control is favorable.
Major complications did not occur in the PAI group, but one patient in the femoral nerve block group with poor quadriceps control experienced a fall resulting in opening of the surgical wound. Although we did not observe permanent nerve damage in the patients in the femoral nerve block group, the rates in two series ranged from 0.1% to 0.4% [10, 24]. Even if our study was not adequately powered (210 patients were needed) to compare DVT rates, earlier and improved mobilization with PAI may reduce venous stasis and thrombosis. No patients in our study experienced any ropivacaine-related side effects. Similar large doses of ropivacaine reportedly do not lead to toxic blood levels [6, 27].
Our PAI protocol showed pain control similar to a femoral nerve block while avoiding motor block with its functional impact. Its functional benefit could be advantageous during the rehabilitation period. Easy to perform, our PAI protocol did not increase operating time and may decrease preoperative anesthetic procedures. The PAI protocol presented in this article is an interesting alternative to continuous femoral nerve block.
Acknowledgments
We thank Marie-Êve Lacasse and Charles Rivière, both research students; Lucie Grondin, physiotherapist, who helped gather the data; and Michel Fallaha, MD, FRCS (C) and Alain Roy, MD, FRCS (C) who performed some of the TKAs.
Footnotes
One of the authors (ML) has received payments or benefits not related to this work, during the study period, an amount of USD 10,000–USD 100,000 from Wright Medical Technology (Memphis, TN, USA) and Zimmer (Warsaw, IN, USA). One of the authors (P-AV) has received payments or benefits not related to this work, during the study period, an amount of USD 10,000–USD 100,000 from Wright Medical, Biomet (Warsaw, IN, USA), and Stryker Orthopaedics (Mahwah, NJ, USA). The institution of one or more of the authors (PAV, ML, DA, PD) has received, during the study period, unrestricted research funding not related to this work from Biomet, Zimmer, Stryker, Smith and Nephew (Memphis, TN, USA), and DePuy Orthopaedics (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Randomization, surgical procedures, and followup of the subjects were performed at Maisonneuve-Rosemont Hospital, Montreal, Quebec, Canada.
References
- 1.Albert TJ, Cohn JC, Rothman JS, Springstead J, Rothman RH, Booth RE., Jr Patient-controlled analgesia in a postoperative total joint arthroplasty population. J Arthroplasty. 1991;6(suppl):S23–28. doi: 10.1016/S0883-5403(08)80052-6. [DOI] [PubMed] [Google Scholar]
- 2.Allen HW, Liu SS, Ware PD, Nairn CS, Owens BD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesth Analg. 1998;87:93–97. doi: 10.1097/00000539-199807000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Badner NH, Bourne RB, Rorabeck CH, MacDonald SJ, Doyle JA. Intra-articular injection of bupivacaine in knee-replacement operations: results of use for analgesia and for preemptive blockade. J Bone Joint Surg Am. 1996;78:734–738. doi: 10.2106/00004623-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Ben-David B, Schmalenberger K, Chelly JE. Analgesia after total knee arthroplasty: is continuous sciatic blockade needed in addition to continuous femoral blockade? Anesth Analg. 2004;98:747–749. doi: 10.1213/01.ANE.0000096186.89230.56. [DOI] [PubMed] [Google Scholar]
- 5.Browne C, Copp S, Reden L, Pulido P, Colwell C., Jr Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19:377–380. doi: 10.1016/j.arth.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: a randomized trial. J Bone Joint Surg Am. 2006;88:959–963. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 7.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Carli F, Clemente A, Asenjo JF, Kim DJ, Mistraletti G, Gomarasca M, Morabito A, Tanzer M. Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous FB. Br J Anaesth. 2010;105:185–195. doi: 10.1093/bja/aeq112. [DOI] [PubMed] [Google Scholar]
- 9.Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, Criswell A. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty. 2001;16:436–445. doi: 10.1054/arth.2001.23622. [DOI] [PubMed] [Google Scholar]
- 10.Cuvillon P, Ripart J, Lalourcey L, Veyrat E, L’Hermite J, Boisson C, Thouabtia E, Eledjam JJ. The continuous FB catheter for postoperative analgesia: bacterial colonization, infectious rate and adverse effects. Anesth Analg. 2001;93:1045–1049. doi: 10.1097/00000539-200110000-00050. [DOI] [PubMed] [Google Scholar]
- 11.DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392:226–231. doi: 10.1097/00003086-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forst J, Wolff S, Thamm P, Forst R. Pain therapy following joint replacement: a randomized study of patient-controlled analgesia versus conventional pain therapy. Arch Orthop Trauma Surg. 1999;119:267–270. doi: 10.1007/s004020050407. [DOI] [PubMed] [Google Scholar]
- 14.Horlocker TT, Cabanela ME, Wedel DJ. Does postoperative epidural analgesia increase the risk of peroneal nerve palsy after total knee arthroplasty? Anesth Analg. 1994;79:495–500. doi: 10.1213/00000539-199409000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Horlocker TT, Hebl JR, Kinney MA, Cabanela ME. Opioid-free analgesia following total knee arthroplasty: a multimodal approach using continuous lumbar plexus (psoas compartment) block, acetaminophen, and ketorolac. Reg Anesth Pain Med. 2002;27:105–108. doi: 10.1053/rapm.2002.27177. [DOI] [PubMed] [Google Scholar]
- 16.Klasen JA, Opitz SA, Melzer C, Thiel A, Hempelmann G. Intraarticular, epidural, and intravenous analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 1999;43:1021–1026. doi: 10.1034/j.1399-6576.1999.431009.x. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–130. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]
- 18.Luber MJ, Greengrass R, Vail TP. Patient satisfaction and effectiveness of lumbar plexus and sciatic nerve block for total knee arthroplasty. J Arthroplasty. 2001;16:17–21. doi: 10.1054/arth.2001.16488. [DOI] [PubMed] [Google Scholar]
- 19.Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty. 1997;12:546–552. doi: 10.1016/S0883-5403(97)90178-9. [DOI] [PubMed] [Google Scholar]
- 20.McNamee DA, Parks L, Milligan KR. Post-operative analgesia following total knee replacement: an evaluation of the addition of an obturator nerve block to combined femoral and sciatic nerve block. Acta Anaesthesiol Scand. 2002;46:95–99. doi: 10.1034/j.1399-6576.2002.460117.x. [DOI] [PubMed] [Google Scholar]
- 21.Mullaji A, Kanna R, Shetty GM, Chavda V, Singh DP. Efficacy of periarticular injection of bupivacaine, fentanyl, and methylprednisolone in total knee arthroplasty: a prospective, randomized trial. J Arthroplasty. 2010;25:851–857. doi: 10.1016/j.arth.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Ritter MA, Koehler M, Keating EM, Faris PM, Meding JB. Intra-articular morphine and/or bupivacaine after total knee replacement. J Bone Joint Surg Br. 1999;81:301–303. doi: 10.1302/0301-620X.81B2.9110. [DOI] [PubMed] [Google Scholar]
- 23.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Singelyn FJ, Gouverneur JM. Postoperative analgesia after total hip arthroplasty: i.v. PCA with morphine, patient-controlled epidural analgesia, or continuous “3-in-1” block? A prospective evaluation by our acute pain service in more than 1,300 patients. J Clin Anesth. 1999;11:550–554. doi: 10.1016/S0952-8180(99)00092-6. [DOI] [PubMed] [Google Scholar]
- 25.Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94:130–137. doi: 10.1097/00000539-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri- and intraarticular analgesia with FB after total knee arthroplasty: a randomized clinical trial. Acta Orthop. 2007;78:172–179. doi: 10.1080/17453670710013645. [DOI] [PubMed] [Google Scholar]
- 27.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Boctor B, Verner J. The effect of single-injection FB on rehabilitation and length of hospital stay after total knee replacement. Reg Anesth Pain Med. 2002;27:139–144. doi: 10.1053/rapm.2002.29253. [DOI] [PubMed] [Google Scholar]