Abstract
Background
Surgical approaches for cervical ossification of the posterior longitudinal ligament (OPLL) include anterior, posterior, or combined decompression with or without fusion. The goal of surgery is to decompress the spinal cord while maintaining the stability and sagittal alignment of the cervical spine. C5 palsy has been reported as a postoperative complication of cervical laminectomy or laminoplasty characterized as motor weakness of the muscles supplied with C5 nerve roots. Several studies have shown this phenomenon was partially attributable to posterior shift of spinal cord.
Description of Technique
The rationale for choosing hemilaminectomy is to control postoperative posterior shift of the spinal cord and afford more stability by preserving ligamentous attachments and posterior bony elements as much as possible. After a fixation system of lateral mass screws and rods is installed unilaterally, laminae are removed from the underlying dura using a high-speed burr and Kerrison laminectomy rongeur on the other side. The spinous processes are preserved.
Patients and Methods
Patients with multilevel continuous/mixed cervical OPLL are good candidates for this technique. We retrospectively reviewed 146 patients who had multilevel continuous/mixed cervical OPLL and underwent surgery from January 2006 to January 2010. Neurologic condition was evaluated using the improvement ratio (IR) of the Japanese Orthopaedic Association (JOA) score for cervical myelopathy.
Results
The mean JOA score increased from 10 points before surgery to 14 points at last followup. The mean IR of neurologic function (JOA score) was 59%. C5 palsy was not observed in any patient after decompression, and cervical lordosis changed from 8.7° preoperatively to 9.1° at last followup.
Conclusions
For patients with multilevel continuous/mixed cervical OPLL without fixed kyphosis, multilevel hemilaminectomy with unilateral lateral mass fixation is an effective alternative technique.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Various surgical approaches are used to treat cervical ossification of the posterior longitudinal ligament (OPLL), depending on the degree of compression, sagittal alignment, number of levels of compression, and the patient’s general status. For patients with compression limited to one or two levels and a fixed kyphotic deformity, the anterior approach, although invasive, is recommended because direct resection of ossific tissue, complete decompression of the spinal cord, and firm fixation of the vertebrae are required [11]. However, when it comes to multilevel (three or more involved vertebrae) severe continuous/mixed OPLL, anterior decompression becomes more technically demanding and risky, and the posterior approach becomes an attractive option [7].
Postoperative C5 palsy after cervical surgery for cervical spondylotic myelopathy (CSM) is a well-known complication but poorly understood. The mean incidence has been reported to be 8.3% in patients with cervical OPLL [13]. It may be caused by the combination of tethering of nerve roots and a postoperative or intraoperative shift of the spinal cord [17]. Laminectomy and laminoplasty are widely used surgical treatments, and good results have been published in many reports [6, 9, 11]. However, both techniques may be complicated by the combination of spinal cord shift from damage of posterior elements of the spinal column, along with tethering of nerve roots and C5 palsy.
Based on the hypothesis that tethering of nerve roots causes C5 palsy, multilevel hemilaminectomy and nerve decompression have been performed to treat multilevel cervical OPLL in our hospital. The rationale for choosing this technique is to preserve ligamentous attachments and bony posterior elements as much as possible to control posterior shift of the spinal cord after suitable decompression, instead of excessive decompression.
We are not aware of any other studies investigating this surgical technique for cervical OPLL. We therefore retrospectively reviewed our experience in patients who underwent this surgery for multilevel cervical OPLL and describe this technique with its indications, advantages, and disadvantages.
Surgical Technique
According to the symptoms, signs, and details of preoperative radiographic findings, a multilevel hemilaminectomy was performed on the side with more severe spinal cord compression, and lateral mass fixation was performed on the other side. We chose contralateral instrument fixation because it was convenient to perform bone grafting, and there was sufficient bone graft bed to afford adequate stabilization of the cervical spine.
Patients were administered general anesthesia and were placed prone. Before surgery, the proper position of the cervical spine was confirmed by intraoperative radiography. After exposure of the posterior elements, holes were drilled in the lateral masses unilaterally with the Magerl technique [1, 8]. A fixation system of screws and rods (SUMMIT™ SI; Depuy Spine, Inc, Raynham, MA, USA) was installed and the axis rods were bent to match the cervical curvature (Fig. 1). Hemilaminectomy margins should be created from the junction of the lamina and lateral mass to basilar part of spinous process. The spinous process was preserved (Fig. 2). After thinning the lamina down to a thin cortical shell over the dura using a high-speed burr, a 1-mm Kerrison laminectomy rongeur was used to remove lamina from a caudal to cranial direction, but it was safer to use a small angled curette to finish the cut, and then lift the lamina away from the spinal cord. The facet joints at the involved levels were fused at the side of fixation with the bone grafts taken from the lamina. After the operation, all patients were asked to wear a Philadelphia collar for 3 weeks.
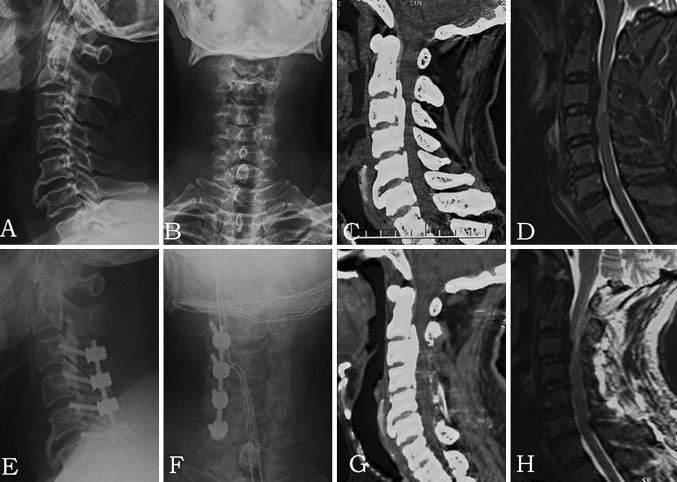
Fig. 1A–H.
The images illustrate the case of a 55-year-old man who experienced numbness of the left arm and bilateral progressive lower-extremity weakness for 2 years and was diagnosed as having mixed OPLL (C2–C5). The JOA score recovered from 11 points preoperatively to 15 points postoperatively (IR = 66.7%). Preoperative (A) lateral and (B) AP radiographs, (C) CT scan, and (D) MR image show mixed OPLL from C2 to C5, and the spinal cord was compressed. Postoperative (E) lateral and (F) AP radiographs, (G) CT scan, and (H) MR image show the lateral mass screw fixation on the right side from C3 to C6. A hemilaminectomy was performed from the inferior part of C2 to C5 on the left side.
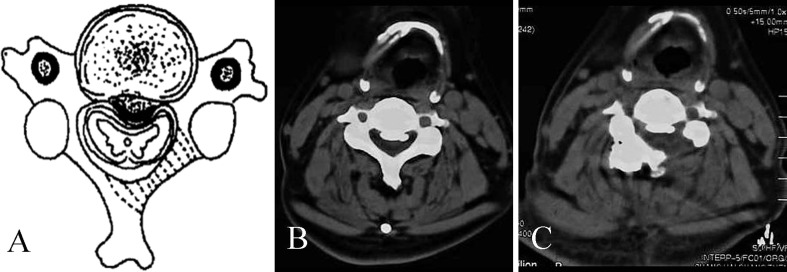
Fig. 2A–C.
The images illustrate nerve decompression used in multilevel hemilaminectomy. (A) A schematic diagram shows OPLL with a bias to the left, and the shaded part of the lamina requires resection. (B) A preoperative CT scan in the transverse view at the C4 level shows OPLL with a bias to the left. (C) A postoperative CT scan in the transverse view at the C4 level shows the spinal canal was enlarged after hemilaminectomy on the left.
Patients and Methods
From January 2006 to January 2010, a total of 169 patients with cervical OPLL were seen by our team, and 146 of them were diagnosed with multilevel continuous/mixed cervical OPLL and underwent multilevel hemilaminectomy with unilateral lateral mass fixation by the same senior surgeon (LJ). We included patients with (1) clearly documented physical examination findings consistent with progressive myelopathy for whom nonoperative measures failed, (2) radiographic confirmation of spinal cord compression involving three or more vertebrae caused by severe continuous/mixed OPLL, and (3) the absence of fixed kyphotic deformities and contraindications for surgery. Patients with focal cervical OPLL or fixed kyphotic deformities were not good candidates for this approach.
Of the patients, there were 105 men (72%) and 41 women (28%), with a mean age at surgery of 62 years (range, 39–75 years) and a mean duration of symptoms of 42 months (range, 1–240 months). Nine patients (6.2%) had diabetes mellitus.
Preoperative radiographic evaluation found multilevel (three or more involved vertebrae) continuous/mixed OPLL in all patients. All patients, while under general anesthesia, underwent multilevel hemilaminectomy on the affected levels by the same senior surgeon and coworkers. Twenty-three patients (15.8%) underwent surgery on level C2–C5, 106 (72.6%) on C3–C6, and 17 (11.6%) on C4–C7. Four partial laminae were removed in all patients. If C2 or C7 was affected, only the inferior part of the C2 lamina or the superior part of the C7 lamina was removed, so instrument fixation was performed on the C3–C6 levels in all patients. Hemilaminectomy was performed on the left side in 69 patients and on the right side in 77 patients.
Patients stayed in the hospital for a mean of 3.5 days (range, 3–5 days) after surgery. The minimum followup was 2 years (mean, 3.9 years; range, 2–5 years). The neurologic status of all patients was assessed preoperatively and postoperatively, at 3 months, 6 months, and then yearly until last followup.
Information collected included patients’ age at the time of surgery, sex, duration of symptoms, presence or absence of diabetes mellitus, radiographic findings, preoperative and postoperative and last followup Japanese Orthopaedic Association (JOA) scores [3], levels and side of decompression, intraoperative blood loss and transfusion, postoperative complications, and duration of hospital stay.
For the radiographic evaluation, we investigated the parameters of plain radiographs, CT, and MRI. Cervical lordosis was measured as the angle between a line vertical to the superior aspect of the C3 vertebral body and the inferior aspect of the C7 body (Cobb angle of C3–C7). Occupying rate was defined as the thickness of the OPLL divided by the AP diameter of the bony spinal canal on the axial CT image. Fusion was defined as the presence of the following features: (1) absence of radiolucent lines/area across the fusion site or around any of the screw sites; (2) presence of bridging trabeculae across the fusion site; and (3) absence of motion between the spinous processes on flexion–extension x-rays [5]. If the fusion was questionable, it was confirmed by a sagittal reconstructive CT scan. We measured the position of the central line of the spinal cord on the postoperative sagittal MRI. If the central line of the spinal cord was located more posterior than the central line of the spinal canal, we regarded it as posterior shift of the spinal cord.
The JOA scoring system [3] was used to evaluate the neurologic status. An improvement rate (IR) was calculated as IR = (postoperative JOA score − preoperative JOA score)/(17 − preoperative JOA score) × 100%. The neurologic status of the patients at latest followup was used for defining clinical prognosis. Good recovery was defined as an IR of 50% or greater and poor recovery was defined as an IR less than 50%. A VAS scoring system was used to measure postoperative neck pain.
The JOA score and cervical lordosis were compared between preoperatively and last followup. We analyzed the following possible predictor variables for poor recovery: age, sex, duration of symptoms, preoperative JOA score, diabetes mellitus, radiographic findings, side of partial laminae removed, intraoperative blood loss, and postoperative complications. For nonparametric analysis we used the Mann-Whitney U test, and for parametric analysis we used Fisher’s exact probability test. A multivariate logistic regression analysis using stepwise selection also was performed. Probability values less than 0.05 were considered statistically significant. We used SPSS® (Version 16.0; SPSS Inc, Chicago, IL, USA) for all analyses.
Results
The preoperative cervical lordosis averaged 8.7° (range, 5.9°–12.6°); at last followup, it was 9.1° (range, 5.5°–12.9°, p = 0.6245). The mean occupying rate was 41.6% (range, 30.1%–49.2%) on the axial CT image.
The mean intraoperative blood loss was 430 mL (range, 150–800 mL), and 19 patients (13.0%) received an average of 474 mL (range, 400–600 mL) of packed red blood cells.
No patients had iatrogenic neurologic deterioration postoperatively. The only postoperative complication was posterior hematoma 2 days after surgery in one patient who recovered neurologic function after an emergency operation. There was no instance of infection, spinal fluid leak, nonunion, rod breakage, or pullout of screws or other complications. Spinal fusion was noted in 100% of patients at the last followup.
The mean JOA score increased from 10 points preoperatively to 14 points postoperatively and 14 points at latest followup. The neurologic status was improved at last followup (p < 0.01), and the IR of neurologic function averaged 59% (range, 27%–80%). Among the 146 patients, 115 (78.8%) patients had a good recovery (IR ≥ 50%) and 31 (21.2%) had a poor recovery (IR < 50%). The mean postoperative neck pain VAS score was 2.5 (range, 0–4), and it was 2.7 (range, 0–4) at the 6-month followup.
The postoperative MRI showed no obvious posterior shift of the spinal cord.
Among the possible predictive variables for a poor result that we analyzed, only duration of symptoms (p = 0.0042) and preoperative JOA score (p < 0.0001) were found to have an influence on the prognosis (Table 1). Multivariate logistic regression analysis showed duration of symptoms (OR = 0.191, p = 0.0019), preoperative JOA score (OR = 3.716, p < 0.0001), and occupying rate (OR = 0.585, p = 0.0159) were significant factors (Table 2).
Table 1.
Comparison of factors for patients stratified by recovery status
| Factor | Good recovery | Poor recovery | p value |
|---|---|---|---|
| Age (years) | 63 | 60 | 0.5317* |
| Sex (male/female) | 85:30 | 20:11 | 0.3681† |
| Duration of symptoms (months) | 35 | 69 | 0.0024* |
| Preoperative JOA score (points) | 10.8 | 9.2 | < 0.0001* |
| Diabetes mellitus (yes:no) | 7:108 | 2:29 | 1† |
| Side of partial laminae removed (left:right) | 50:65 | 19:12 | 0.1046† |
| Intraoperative blood loss (mL) | 419 | 471 | 0.2516* |
| Postoperative complications (yes:no) | 1:114 | 0:31 | 1† |
| Cervical lordosis (°) | |||
| Preoperative | 8.7 | 8.8 | 0.5246* |
| At latest followup | 9.1 | 9.0 | 0.5735* |
| Occupying rate | 0.41 | 0.45 | 0.0732* |
* Mann-Whitney U test; †Fisher’s exact test; JOA = Japanese Orthopaedic Association.
Table 2.
Multivariate logistic regression for patients stratified by recovery status
| Factor | p value | Odds ratio | 95% CI |
|---|---|---|---|
| Duration of symptoms (6 months) | 0.0019* | 0.191 | 0.067–0.542 |
| Preoperative JOA score (2 points) | < 0.0001* | 3.716 | 1.955–7.065 |
| Occupying rate (5%) | 0.0159* | 0.585 | 0.378–0.904 |
* Multivariate logistic regression using stepwise selection; sle = 0.10, sls = 0.15; age, duration of symptoms, preoperative JOA score, intraoperative blood loss, cervical lordosis, and occupying rate were stratified by respectively 10 years, 6 months, 2 score, 200 mL, 1° and 5%; JOA = Japanese Orthopaedic Association.
Discussion
Surgical approaches for cervical OPLL include anterior, posterior, or combined decompression with or without fusion. C5 palsy has been reported as a postoperative complication after cervical decompression procedures [13]. In our hospital, multilevel hemilaminectomy and nerve decompression have been performed for the treatment of multilevel cervical OPLL to try to minimize the likelihood that this complication would occur.
There are numerous limitations to this retrospective review. First, our study is relatively short and longer followup will be required to evaluate long-term outcomes. Second, this is an observational retrospective study, which may give limited useful information and can be subject to a false positive statistical error from comparing many variables. Third, only patients with multilevel continuous/mixed OPLL were treated by this surgical technique, so there is the possibility that selection bias might have affected the results of this nonrandomized study. Fourth, this study has no control group. Does this technique decrease the degree of neurologic function owing to inadequate decompression compared with laminectomy, or can a lesser opening of a laminoplasty achieve the same goal of restricting posterior shift of the spinal cord? To prove this technique has merit, it has to be compared with the standard. Therefore, prospective randomized control trials comparing multilevel hemilaminectomy with laminectomy or laminoplasty are necessary to determine whether one procedure is superior to the others and whether there are any differences among these surgical techniques in short- and long-term outcomes for multilevel cervical OPLL.
Some controversies still exist regarding the surgical options for OPLL. For patients with anterior compression limited to one or two levels, fixed kyphotic deformity, and no significant developmental narrowing of the canal, anterior decompression and stabilization are favored [6, 11]. In contrast, patients with compression extending to more than two levels, developmental narrowing of the canal, and lordotic alignment are candidates for the posterior approach [7, 9].
In general, the indications and contraindications for multilevel hemilaminectomy are similar to those for other posterior approaches. Fixed kyphotic deformities are an absolute contraindication to this posterior approach. We limit use of this approach to patients with multilevel continuous/mixed OPLL with the absence of kyphosis.
Lateral mass screw fixation is a safe and effective stabilization technique [15]. Because the cervical spine in our patients was not unstable, we tried unilateral fixation after multilevel hemilaminectomy and found it was enough for stabilization of the cervical spine. After an average of 3.9 years followup, the spinal fusion rate was 100%. There was no instance of pseudarthrosis, rod breakage, or pullout of screws.
C5 palsy has been reported as a neurologic deficit after cervical decompression procedures [13]. The main symptom of this postoperative complication is motor weakness of the muscles supplied with C5 nerve roots. The incidence of C5 nerve palsy after posterior cervical decompression for OPLL is similar to that of cervical spondylotic myelopathy. The incidence ranges from 0% to 30%, with an average of 4.7% [4]. The etiology of C5 palsy has not been fully established. One influential hypothesis was that traction or tethering effect on the nerve roots owing to spinal cord shift occurring after spinal canal decompression results in postoperative C5 palsy [14, 17, 18]. One study showed patients with preoperative foraminal stenosis, posterior shift of the spinal cord, and additional iatrogenic foraminal stenosis attributable to cervical alignment correction were more likely to have postoperative C5 palsy develop after posterior instrumentation with fusion [12]. We did not observe C5 palsy in any patient after decompression in our series, and postoperative MRI showed no obvious posterior shift of the spinal cord. We are unable to conclude that multilevel hemilaminectomy is associated with a decreased incidence of postoperative C5 palsy based on the 146 cases, because we did not have a control group in this series.
The incidence of kyphosis after dorsal cervical procedures has been reported to be as much as 21% [16]. Release of the posterior tension band increases the risk of postoperative kyphotic deformity. The potential for this complication is influenced by numerous factors, including the underlying pathologic process, the number of spinal levels involved, the extent of the decompression, and the patient’s age and underlying medical condition (eg, presence of osteoporosis) [10]. At latest followup, we did not observe any patient with a kyphotic deformity developing. The stabilization of lateral mass fixation and advantage of hemilaminectomy in preserving sagittal alignment are significant factors to be considered [2]. Laminectomy and laminoplasty carry the risk of a late kyphotic deformity resulting from damage to musculoligamentous attachments and posterior bony elements, whereas hemilaminectomy is advantageous in preserving posterior spinal structures, which may decrease the incidence of kyphosis.
However, the hemilaminectomy approach provides a relatively narrow exposure of the spinal canal. Thus, decompression may be limited, and patients may not achieve satisfactory improvement. Under these circumstances, an anterior approach is recommended several months later depending on the patient’s symptoms, although it may be still risky and technically demanding. However, we have not encountered a patient whose condition has called for this.
We did not find any study about the possible predictive variables for a poor outcome regarding surgical treatment for cervical OPLL. Our statistical analysis showed duration of symptoms and preoperative JOA score had an influence on the prognosis. This indicated that patients should undergo surgery before their neurologic conditions were too bad to improve.
Under appropriate conditions, a multilevel cervical hemilaminectomy with unilateral lateral mass fixation is effective for the treatment of multilevel, severe cervical OPLL. We believe that patients undergoing surgery for multilevel continuous/mixed cervical OPLL are the best candidates for this technique. It appears to control postoperative spinal cord shift, afford adequate stability, and result in symptom relief at short term.
Acknowledgments
We thank Yuan Wang MD and Ning Liu MD, orthopaedic surgeons at Chang Zheng Hospital, for help with clinical data collection in this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Anderson PA, Henley MB, Grady MS, Montesano PX, Winn HR. Posterior cervical arthrodesis with AO reconstruction plates and bone graft. Spine (Phila Pa 1976). 1991;16(3 suppl):S72–S79. [DOI] [PubMed]
- 2.Asazuma T, Nakamura M, Matsumoto M, Chibo K, Toyama Y. Postoperative changes of spinal curvature and range of motion in adult patients with cervical spinal cord tumors: analysis of 51 cases and review of the literature. J Spinal Disord Tech. 2004;17:178–182. doi: 10.1097/00024720-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–295. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso MJ, Koski TR, Ganju A, Liu JC. Approach-related complications after decompression for cervical ossification of the posterior longitudinal ligament. Neurosurg Focus. 2011;30:E12. doi: 10.3171/2011.1.FOCUS10278. [DOI] [PubMed] [Google Scholar]
- 5.Gao R, Yang L, Chen H, Liu Y, Liang L, Yuan W. Long term results of anterior corpectomy and fusion for cervical spondylotic myelopathy. PLoS One. 2012;7:e34811. doi: 10.1371/journal.pone.0034811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geck MJ, Eismont FJ. Surgical options for the treatment of cervical spondylotic myelopathy. Orthop Clin North Am. 2002;33:329–348. doi: 10.1016/S0030-5898(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 7.Hee HT, Majd ME, Holt RT, Whitecloud TS 3rd, Pienkowski D. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16:1–8; discussion 8–9. [DOI] [PubMed]
- 8.Jeanneret B, Magerl F, Ward EH, Ward JC. Posterior stabilization of the cervical spine with hook plates. Spine (Phila Pa 1976). 1991;16(3 suppl):S56–S63. [DOI] [PubMed]
- 9.Komotar RJ, Mocco J, Kaiser MG. Surgical management of cervical myelopathy: indications and techniques for laminectomy and fusion. Spine J. 2006;6(6 suppl):252S–267S. doi: 10.1016/j.spinee.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Mehdorn HM, Fritsch MJ, Stiller RU. Treatment options and results in cervical myelopathy. Acta Neurochir Suppl. 2005;93:177–182. doi: 10.1007/3-211-27577-0_31. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno J, Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J. 2006;6(6 suppl):282S–288S. doi: 10.1016/j.spinee.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima H, Imagama S, Yukawa Y, Kanemura T, Kamiya M, Yanase M, Ito K, Machino M, Yoshida G, Ishikawa Y, Matsuyama Y, Hamajima N, Ishiguro N, Kato F. Multivariate analysis of C-5 palsy incidence after cervical posterior fusion with instrumentation. J Neurosurg Spine. 2012;17:103–110. doi: 10.3171/2012.4.SPINE11255. [DOI] [PubMed] [Google Scholar]
- 13.Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976). 2003;28:2447–2451. [DOI] [PubMed]
- 14.Saunders RL. On the pathogenesis of the radiculopathy complicating multilevel corpectomy. Neurosurgery. 1995;37:408–412; discussion 412–413. [DOI] [PubMed]
- 15.Sekhon LH. Posterior cervical lateral mass screw fixation: analysis of 1026 consecutive screws in 143 patients. J Spinal Disord Tech. 2005;18:297–303. doi: 10.1097/01.bsd.0000166640.23448.09. [DOI] [PubMed] [Google Scholar]
- 16.Steinmetz MP, Kager CD, Benzel EC. Ventral correction of postsurgical cervical kyphosis. J Neurosurg. 2003;98(1 suppl):1–7. doi: 10.3171/spi.2003.98.1.0001. [DOI] [PubMed] [Google Scholar]
- 17.Tsuzuki N, Abe R, Saiki K, Zhongshi L. Extradural tethering effect as one mechanism of radiculopathy complicating posterior decompression of the cervical spinal cord. Spine (Phila Pa 1976). 1996;21:203–211. [DOI] [PubMed]
- 18.Yonenobu K, Hosono N, Iwasaki M, Asano M, Ono K. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;16:1277–1282. [DOI] [PubMed]