Abstract
Background
Traditionally arthrotomy has rarely been performed during surgery for slipped capital femoral epiphysis (SCFE). As a result, most pathophysiological information about the articular surfaces was derived clinically and radiographically. Novel insights regarding deformity-induced damage and epiphyseal perfusion became available with surgical hip dislocation.
Questions/purposes
We (1) determined the influence of chronicity of prodromal symptoms and severity of SCFE deformity on severity of cartilage damage. (2) In surgically confirmed disconnected epiphyses, we determined the influence of injury and time to surgery on epiphyseal perfusion; and (3) the frequency of new bone at the posterior neck potentially reducing perfusion during epimetaphyseal reduction.
Methods
We reviewed 116 patients with 119 SCFE and available records treated between 1996 and 2011. Acetabular cartilage damage was graded as +/++/+++ in 109 of the 119 hips. Epiphyseal perfusion was determined with laser-Doppler flowmetry at capsulotomy and after reduction. Information about bone at the posterior neck was retrieved from operative reports.
Results
Ninety-seven of 109 hips (89%) had documented cartilage damage; severity was not associated with higher slip angle or chronicity; disconnected epiphyses had less damage. Temporary or definitive cessation of perfusion in disconnected epiphyses increased with time to surgery; posterior bone resection improved the perfusion. In one necrosis, the retinaculum was ruptured; two were in the group with the longest time interval. Posterior bone formation is frequent in disconnected epiphyses, even without prodromal periods.
Conclusions
Addressing the cause of cartilage damage (cam impingement) should become an integral part of SCFE surgery. Early surgery for disconnected epiphyses appears to reduce the risk of necrosis. Slip reduction without resection of posterior bone apposition may jeopardize epiphyseal perfusion.
Level of Evidence
Level IV, retrospective case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The primary focus of contemporary treatment concepts of slipped capital femoral epiphysis (SCFE) is to prevent further slippage and/or iatrogenic epiphyseal necrosis [2, 21]. Clinically stable slips, according to the classification of Loder et al. [22], are considered to have a rather benign long-term clinical outcome as illustrated based on the cohort of Carney et al. [4] in which the Iowa hip rating dropped from 93 when patients were in their mid-20s to 87 in their mid-40s. Today, the concept of SCFE as a benign process is questioned as a result of observations made at intraarticular surgery suggesting that even mild slips after pinning in situ cause femoroacetabular impingement with early acetabular cartilage damage [6, 10, 17, 32].
Unstable slips according to Loder et al. [22] have a high risk for epiphyseal necrosis, which is believed to be most likely secondary to the vascular injury/disruption caused at the initial displacement [2, 15, 22, 27, 29]. The timing of surgery and reduction of the epiphysis as well as need for capsular decompression are controversial [21, 25, 30] although some authors recommend emergent treatment and gentle reduction [20, 25, 37]. On the other hand, the rate of necrosis may be as high as 58% when total or substantial reduction is obtained [34]. This could be the result of new bone formation at the epimetaphyseal level of the posterior neck, a pathomorphology that is rarely addressed in the literature [7, 21, 28] but may be responsible for overstretching of the retinacular vessels during reduction of the epiphysis [7].
Traditionally, capsulotomy and intraarticular inspection of a diseased hip were rarely performed and most information and interpretation about pathophysiological aspects of SCFE were derived indirectly from clinical findings, radiographic assessment, and clinical outcome studies. More recently new observations have been made with the routine use of arthrotomy and surgical dislocation approaches [8, 9]. Two smaller series of 13 and 36 patients using these approaches have described cartilage and labrum damage of the acetabulum in SCFE [17, 32]. More extensive observations may provide additional insights into the pathophysiology of cartilage damage in SCFE, especially in slips with intraoperatively verified disconnection of the epiphysis.
We therefore (1) determined the influence of prodromal symptoms and severity of SCFE deformity on cartilage damage. (2) In surgically confirmed disconnected epiphyses, we determined the influence of injury and time to surgery on reduced epiphyseal perfusion; and (3) the frequency of new bone at the posterior neck and its role in jeopardizing epiphyseal perfusion during reduction.
Patients and Methods
We retrospectively reviewed the medical history of 125 patients with 128 SCFE treated at three institutions (Pediatric Surgery, Children’s Hospital, University of Berne, Berne, Switzerland; Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA; and the Hip and Pediatric Service, Schulthess Clinic, Zurich, Switzerland) between 1996 and 2011. In the two European hospitals, the indication for surgical hip dislocation and subcapital reduction was all slips with a slip angle higher than 30° [8, 9], whereas in the Boston hospital the indication for that procedure was mainly for clinically unstable slips with slip angle greater than 30°. Data from a subcohort of the cohort presented here were used in prior publications. Forty of the patients were reported in earlier publications dealing with treatment results [19, 30, 33, 36], 82 patients were used to compare clinical and intraoperative stability [35], and 13 patients were used to report early acetabular cartilage damage in SCFE [17].
For this study, clinical and intraoperative data were available from 116 of the 125 patients (119 hips) with a mean age of 12 years at surgery (range, 7–16 years). Sixty-four patients were boys (55%) and 52 were girls (45%). Duration of preoperative symptoms was 16 weeks (range, 0–156 weeks). The slip angle measured in a frog lateral or crosstable lateral view averaged 53° (range, 15°–90°). Thirty-five hips had a disconnected epiphysis as determined at the time of intraoperative inspection. This diagnosis was made at capsulotomy when motion between the epiphysis and metaphysis could be visualized. Minimum followup was 12 months (mean, 31 months; range, 12–108 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. We obtained institutional review board approval at all institutions for this retrospective study.
The technique of surgical dislocation of the hip has been described in detail previously [8]. Briefly, the patient is in a lateral decubitus position. After trochanteric flip osteotomy and Z-shaped capsulotomy, in doubt of physeal stability, the epiphysis is fixed with two Kirschner wires without attempt to reduce the epiphysis. For complete dislocation, the round ligament is sectioned. With the help of retractors, it is then possible to inspect 360° of the acetabulum and more than the anterior half of the circumference of the head and neck. The stable portion of the greater trochanter is trimmed in a piecemeal fashion down to the level of the neck, strictly staying in the subperiosteal layer. Retinaculum and periosteum of the neck are then freed from bone down to the lesser trochanter and around the accessible area of the posterior neck. Subperiosteal enucleation of the anteromedial and posteromedial neck is easier with the head again dislocated. The result of medial and lateral dissection is 360° access to the neck. The flap is at least twice as long as when using the original Dunn procedure with subperiosteal tunneling only at the neck proximal to the greater trochanter [7]. The longer flap allows more elongation before rupture and it creates a larger space around the neck for manipulations. Together with posterior bone resection, this decreases the tension on the retinacular vessels during epiphyseal reduction. Mobilization of the epiphysis is performed using curved chisels inserted in the growth plate while the head is out of the socket. The separated head together with the periosteal tube is placed medially and the metaphyseal stump is presented with external rotation of the leg. New bone formation at the posterior neck should be completely resected under visual and manual control. Remaining tissue of the growth plate is removed with a curette under manual fixation of the epiphysis. Repositioning of the head is performed manually and should be possible effortlessly; otherwise, shortening of the neck by trimming of the proximal surface should be considered; it was necessary in overall 33 hips. One hundred five hips were treated using the described approach; 14 hips had fixation in situ and open or arthroscopic osteochondroplasty [9, 18].
Two of us (KZ, RG) reviewed the wording of the descriptions of acetabular cartilage damage in the operative reports to categorize the severity of damage by consensus. The cartilage damage was deemed mild (+) when the reported damage conveyed the impression as superficial or small, severe (+++) when the descriptors suggested severe or substantial damage, and moderate (++) when damage was noted without further qualification.
For the hips with a stable physis at the time of surgical inspection, the duration of prodromal symptoms was categorized into less than 4 weeks, 4 to 8 weeks, and more than 8 weeks. For the hips with the disconnected epiphysis, the time from acute incidence to surgery was subdivided into less than 8 hours, 8 to 24 hours, 2 to 8 days, and more than 8 days.
Perfusion of the epiphysis was monitored in 23 hips using laser-Doppler flowmetry (LDF) at capsulotomy and after epiphyseal reduction [26]. In four additional hips with severe deformity, the epiphysis was accessible for placing the probe only after subluxation or dislocation. When LDF was not available, 2-mm drill holes in the epiphysis (32 hips) or of the cut surface of the round ligament (39 hips) were observed for bright red bleeding [11]. For seven hips no information was available.
New bone formation at the proximal end of the posterior neck is a sign of chronicity of the slip process and best visible on lateral radiographies (Fig. 1). It is of special interest in hips with disconnected epiphysis because it may increase the tension on the retinacular vessels during unintended or deliberate reduction. We intended to define it on the preoperative lateral radiographs as bone posterior to a line along the posterior border of the neck (Fig. 2); however, we were unable to reliably determine the presence of posterior callus as a result of a variety of lateral radiographic projections used and more importantly as a result of the poor quality radiographs in most of the hips with disconnected epiphyses. Information about new bone formation at the posterior neck was therefore only retrieved from the surgeon’s records from observations about the bone to be resected from the posterosuperior neck flush with the more distal neck surface.
Fig. 1.
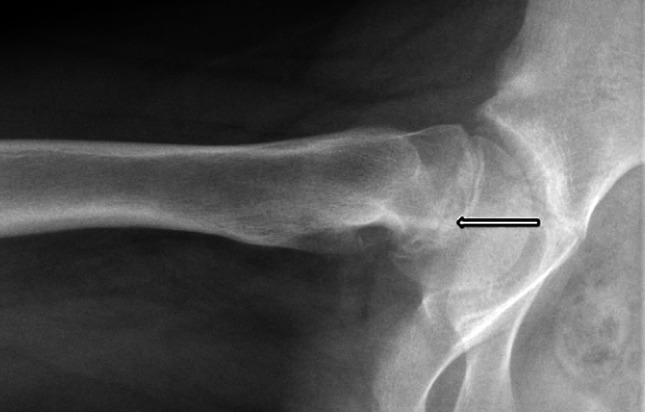
A 12-year-old girl reported symptoms for 4 weeks. The lateral radiography with a stable epiphysis at capsulotomy and a slip angle of 50° had substantial bone formation at the posterior neck posterior to the sclerotic line (arrow), indicating the process started earlier than the reported symptoms.
Fig. 2.
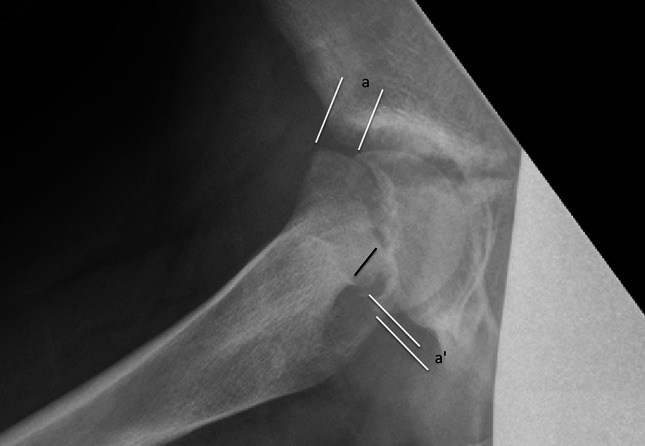
An 11-year-old girl had symptoms for 3 weeks and inability to walk for 1 week. The epiphysis was disconnected at capsulotomy. The lateral radiograph shows new bone formation at the superior end of the posterior border of the neck (black line), also indicated by the difference of the epimetaphyseal displacement a and a′. The black line along the neck shows the amount of bone to be resected for anatomical reduction of the epiphysis to avoid overstretching of the retinaculum.
Descriptive data have been reported as absolute frequency (number of cases) using the contingency tables. Using the Fisher’s exact test, we determined whether (1) cartilage damage was more likely with greater severity (Table 1) and chronicity (Table 2) of a stable SCFE. Additionally, we determined if physeal stability was associated with more cartilage damage (Table 3). Using the Fisher’s exact test, we determined whether delay from injury to surgery in the disconnected epiphyses (Table 4) was associated with disruption of the epiphyseal perfusion. The analysis was performed using STATA (STATA, College Station, TX, USA).
Table 1.
Severity of cartilage damage versus slip deformity severity in stable epiphysis at capsulotomy (n = 84; p = 0.440)
| Severity of cartilage damage | No information | < 30° | 30°–60° | > 60° |
|---|---|---|---|---|
| 0 | 1 | 2 | 1 | |
| + | 1 | 4 | 8 | 6 |
| ++ | 1 | 2 | 9 | 1 |
| +++ | 1 | 5 | 28 | 8 |
| No information | 2 | 4 |
Table 2.
Severity of cartilage damage versus duration of prodromal symptoms in stable epiphysis at capsulotomy (n = 84; p = 0.669)
| Severity of cartilage damage | No information | Less than 4 weeks | 4–8 weeks | More than 8 weeks |
|---|---|---|---|---|
| 0 | 1 | 1 | 1 | 1 |
| + | 7 | 1 | 1 | 8 |
| ++ | 2 | 1 | 2 | 9 |
| +++ | 3 | 4 | 6 | 29 |
| No information | 2 | 0 | 2 | 3 |
Table 3.
Severity of cartilage damage in stable versus disconnected epiphysis at capsulotomy (p < 0.001)
| Severity of cartilage damage | Stable (n = 84) | Unstable (n = 35) |
|---|---|---|
| 0 | 4 | 8 |
| + | 16 | 5 |
| ++ | 15 | 11 |
| +++ | 45 | 5 |
| No information | 4 | 6 |
Table 4.
Influence of timing of surgery on epiphyseal perfusion in disconnected epiphyses at capsulotomy (n = 27; p = 0.010)
| ≤ 8 hours | > 8 to 24 hours or less | > 1 to 8 days or less | > 8 to 90 days or less | |
|---|---|---|---|---|
| Number of hips | 6 | 4 | 13 | 4 |
| Perfusion before and after reduction | 6 | 0 | 9 | 2 |
| No perfusion before but after reduction | 0 | 3 | 3 | 0 |
| No perfusion before and after reduction | 0 | 1* | 1† | 2‡ |
*In one case with no perfusion at initial surgery, revision surgery for broken hardware showed perfusion; in three cases, avascular necrosis was found, †one treated between 1 and 8 days who had a ruptured reticnaculum and the other ‡two treated between 8 and 90 days.
Results
Ninety-seven hips (89%) had damage from superficial cartilage abrasion to full-thickness destruction in the anterosuperior acetabulum. There was no association (p = 0.440) between slips with more severe deformity and more extensive joint damage (Table 1) and no associations (p = 0.669) between longer duration prodromal symptoms and greater cartilage damage (Table 2). Severe damage could be observed in small slip angles and/or periods of symptoms less than 4 weeks. Hips with disconnected epiphysis compared with stable epiphysis had less severe damage and the highest number of nonaffected acetabula (Table 3) (p = 0.001). The duration of symptoms in the group with disconnected epiphysis was nearly half compared with epiphyses diagnosed as stable at capsulotomy (10 weeks; range, 0–48 weeks; n = 29 versus 19 weeks; range, 1–144 weeks; n = 57).
Only one disconnected epiphysis had nearly complete avulsion of the retinaculum from the epiphysis; all other 34 disconnected epiphyses were associated with rupture of the anterior periosteum only. For two disconnected epiphyses, the records did not contain information about the head perfusion; however, their postoperative course was uneventful without the development of avascular necrosis. Twenty-three hips showed epiphyseal perfusion before and after reduction; in 12 of these hips, the neck was shortened 2 to 3 mm to allow gentle reduction. Six hips had no perfusion before but clear signs after epiphyseal reduction, which, together with removal of bone formation at the posterior neck, allowed an unfolded configuration of the retinaculum without stretching. Four hips had no perfusion at capsulotomy or after reduction. Three of these four, including the one with ruptured retinaculum, went on to necrosis; however, one had an apparently vital and spherical head 7 years after surgery [31] (Table 4). The time interval between injury and surgery varied substantially from hours to 3 months. The seven hips treated within 8 hours all had intact perfusion at capsulotomy and after reduction. Greater than an 8-hour delay in treatment for the disconnected epiphysis was (p = 0.01) associated with lack of perfusion of the epiphysis (Table 4). Active bleeding occurred in all 66 hips with a stable epiphysis at capsulotomy where the femoral head bleeding was assessed.
Additional bone formation at the posterior neck was resected in 30 of 32 (93%) with disconnected epiphysis that had adequate intraoperative documentation of the posterior neck bone formation. In two hips, no posterior bone could be identified and for three hips, no information is given. Meaningful radiographic determination of posterior bone failed, mainly as a result of the small number and inferior quality of preoperative lateral films in this group. Reduction of the epiphysis after resection of the posterior bone reestablished the perfusion in six disconnected epiphyses. The importance of the bone resection is further demonstrated in one case in which demonstrated perfusion disappeared during reduction after allegedly sufficient posterior bone resection and was reestablished after resection of further bone found to be left accidentally in place (Table 5).
Table 5.
Epiphyseal perfusion in SCFE with disconnected epiphysis at capsulotomy (n = 33)
| Perfusion at capsulotomy and after epiphyseal reduction 11 with 2- to 3-mm shortening femoral neck 3 with increased pulsation after reduction 1 after resection of a part of posterior bone formation left in place |
23 |
| Perfusion only after epiphyseal reduction 1 only after 5- to 8-mm shortening of the neck |
6 |
| No perfusion at capsulotomy and after epiphyseal reduction 1 reperfused at revision 6 weeks after surgery |
4 |
| Definitive necrosis 1 with ruptured retinaculum at capsulotomy |
3 |
SCFE = slipped capital femoral epiphysis.
Discussion
The prominent metaphysis in SCFE can produce cam impingement and subsequent acetabular cartilage damage, an observation hitherto verified on small case series [6, 10, 17, 32]. A recent article [16] on 176 hips pinned in situ reported 12% reoperations, 33% pain, and a Marx activity score [24] of five of 16 after 16 years (range, 2–43 years). Risk factors for epiphyseal necrosis, with incidence ranging from 4.7% to 58% [3, 13, 23, 34] in clinically unstable slips, are still unclear [30] although the prevailing opinion is vascular injury at the initial displacement [1, 15, 22, 27, 29]. Further risk factors are timing of surgery, intracapsular pressure [25, 30, 37], applied reduction force, amount of reduction [34], but also younger patient age [30]. However, traditional studies based on clinical and radiographic data have not been able to identify the exact cause of avascular necrosis [21]. With the use of the surgical dislocation approach [8, 9] and a larger cohort of hips, more direct insight into the cause of avascular necrosis and joint damage was possible. We therefore (1) determined the influence of prodromal symptoms and severity of SCFE deformity on cartilage damage. (2) In surgically confirmed disconnected epiphyses, we determined the influence of injury and time to surgery on reduced epiphyseal perfusion; and (3) the frequency of new bone at the posterior neck jeopardizing epiphyseal perfusion during reduction.
Our study has limitations. First, we graded cartilage damage by systemizing the wording in the operative reports and cannot say whether the damage was properly recorded or whether the wording accurately reflects what was recorded. This limitation relates to the retrospective and multicenter nature of this study; a more rigorous prospective study design might have revealed differences we did not find. Second, the hips come from three institutions with possible differences in surgical technique and in acquisition and valuation of the intraoperative findings. Third, the indications for the surgical approaches varied. One institution executed epiphyseal reduction mainly in clinically unstable hips, whereas the two others indicated the technique for all slips over 30°. This may explain why the incidence of unstable SCFE in this cohort is 29%, clearly higher than the 5% in the literature [20]. Fourth, our cohort includes few hips with a followup of only 1 year. This will not influence our findings and conclusions regarding epiphyseal perfusion; however, it is too early to know whether some patients would develop necrosis.
Acetabular cartilage damage is typical for cam impingement [10] and SCFE with its prominent anterior metaphysis is a prototype. After Rab’s theoretical considerations [28], Leunig et al. [17] observed impingement mechanism and damage intraoperatively. In our cohort, only 4% of the stable epiphyses at capsulotomy had no acetabular rim damage. This is slightly less compared with the groups of Leunig et al. [17] (7%) and Sink et al. [32] (13%). On the other hand, 75% of our stable SCFEs at capsulotomy had substantial to severe damage. Severity of damage tended to increase with longer duration of symptoms and with higher slip angles; however, it was also seen in hips with small slip angles. Based on personal observations, we suspect activity level can also have an influence on the extent of cartilage damage. In contrast, patients with disconnected epiphyses had a seven times higher incidence of undamaged acetabular cartilage and labrum (27.5%) and substantial damage in only 55%. However, the disease period was 50% shorter compared with stable slips at capsulotomy, as observed by Sankar et al. [30].
We believe monitoring of the femoral head perfusion is important in joint-preserving hip surgery. In SCFE, a first step was inspection of the integrity of the retinaculum containing the main blood supply to the femoral head [14]. Observation of bleeding from drill holes has been successfully used during open fixation of femoral neck fractures [11]. LDF, a more elaborate technique, was an essential instrument in the implementation of surgical dislocation of the hip [8, 26]. Both techniques and observation of active bleeding from bone were used to control epiphyseal perfusion. We found rupture of the retinaculum in one patient with epiphyseal disconnection; the patient had no perfusion, which resulted in avascular necrosis. This appears to be an unusual case of a disconnected epiphysis in which the necrosis could be related to the injury. We therefore suspect vascular injury at initial displacement is probably not the main cause of avascular necrosis in unstable SCFE as frequently discussed [1, 15, 22, 23, 27, 29]. Although Loder et al. [21] found no influence of the interval between injury and surgery on the rate of necrosis, most surgeons agree with urgent surgery [21, 25] and some favor immediate decompression of a capsular hematoma [25]. In our series of 35 disconnected epiphyses, perfusion remained intact even with intervals longer than 1 week. On the other hand, the two patients with necrosis and intact retinacula were in the group with the longest interval between injury and surgery. The observation in six hips that perfusion could be documented only after epiphyseal reduction may be interpreted as reperfusion after being compromised by kinking or stretching of the retinacular vessels [11, 12] with varying positions of the mobile epiphysis. Longer periods of the epiphysis in the unreduced position may increase the risk of interruption of the femoral head blood flow and perhaps microdamage to the retinacular vessels such that even with realignment of the epiphysis onto the femoral neck, normal flow through the retinacular vessels may not be restored. Just like in any fracture of the femoral neck in which the malpositioned limb may be compromising blood flow, our observations would imply that urgent surgery would be ideal.
Bone formation at the posterior neck in SCFE is rarely mentioned [7, 21, 28]. Recommendations for gentle reduction [5, 23] or only to the chronic position [21] are more substantiated by the association that synovitis [20] reduces the retinacular elasticity and increases the risk of overstretching during reduction. In fact, the rate of epiphyseal necrosis increased with higher angular corrections and reached 58% when anatomic position was achieved [34]. Reduced tissue elasticity may indeed hamper the reduction and in 12 disconnected epiphyses, we addressed this concern with slight shortening of the neck before final reduction. Dunn’s publication [7], describing resection of bone at the posterior neck to release retinacular tension, led us to concentrate on this bone formation. Our data suggest posterior bone formation, exceeding the normal cup-shaped top of the metaphysis, occurs not only in chronic slips with stable epiphysis, but also in disconnected epiphyses and even in those without prodromal periods. This is indirectly documented by posterior bone resection before reduction in 30 of the 35 disconnected epiphyses; we also have anecdotal evidence about how important complete resection can be for epiphyseal perfusion. Preoperative radiographic evaluation of such bone formation was difficult because lateral projections were not routinely made in these painful hips and when performed, the quality of the films was reduced. Nevertheless, we conclude that clinically unstable slips may frequently have an unknown chronic phase producing bone at the posterior neck. Therefore, even gentle and incomplete closed reduction can contribute to disturbance of epiphyseal perfusion.
In summary, our intraoperative observations on SCFE confirm the high incidence and severity of cartilage damage of the acetabulum. It is produced by anterior cam impingement and may be of influence on the longevity of the hip. We therefore recommend correcting the cam deformity when surgery for SCFE is undertaken. Hips with complete disconnection of the epiphysis from the metaphysis have less cartilage damage, probably as a result of shorter time of disease. The trauma of epiphyseal disconnection per se is an infrequent reason for necrosis. The interval between trauma and surgery can indirectly influence the perfusion of the epiphysis; disturbance of perfusion is more frequently seen with longer intervals. Even clinically unstable slips without prodromal periods can have bone apposition at the posterior neck, which can jeopardize epiphyseal perfusion if not resected before epimetaphyseal reduction.
Acknowledgments
We thank Franco Impellizeri, PhD, from the Research Group at Schulthess Clinic for his statistical support in this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Inselspital, Berne, Switzerland; Schulthess Clinic, Zürich, Switzerland; and the Children’s Hospital, Harvard Medical School, Boston, MA, USA.
References
- 1.Aronsson DD, Loder RT. Treatment of the unstable (acute) slipped capital femoral epiphysis. Clin Orthop Relat Res. 1996;322:99–110. doi: 10.1097/00003086-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Aronsson DD, Loder RT, Breur GJ, Weinstein SL. Slipped capital femoral epiphysis: current concepts. J Am Acad Orthop Surg. 2006;14:666–679. doi: 10.5435/00124635-200611000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ballard J, Cossgrove AP. Anterior physeal separation: a sign indicating a high risk for avascular necrosis after slipped capital femoral epiphysis. J Bone Joint Surg Br. 2002;84:1176–1179. doi: 10.1302/0301-620X.84B8.12904. [DOI] [PubMed] [Google Scholar]
- 4.Carney BT, Weinstein SL, Noble J. Long-term follow-up of slipped capital femoral epiphysis. J Bone Joint Surg Am. 1991;73:667–674. [PubMed] [Google Scholar]
- 5.Casey BH, Hamilton HW, Bobechko WP. Reduction of acutely slipped upper femoral epiphysis. J Bone Joint Surg Br. 1972;54:607–614. [PubMed] [Google Scholar]
- 6.Dodds MK, McCormack D, Mulhall KJ. Femoroacetabular impingement after slipped capital femoral epiphysis: does slip severity predict clinical symptoms? J Pediatr Orthop. 2009;29:535–539. doi: 10.1097/BPO.0b013e3181b2b3a3. [DOI] [PubMed] [Google Scholar]
- 7.Dunn DM. Replacement of the femoral head by open operation in severe adolescent slipping of the upper femoral epiphysis. J Bone Joint Surg Br. 1978;60:394–403. doi: 10.1302/0301-620X.60B3.681417. [DOI] [PubMed] [Google Scholar]
- 8.Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83:1119–1124. doi: 10.1302/0301-620X.83B8.11964. [DOI] [PubMed] [Google Scholar]
- 9.Ganz R, Huff TW, Leunig M. Extended retinacular soft tissue flap for intraarticular hip surgery: surgical technique, indications, and results of application. Instr Course Lect. 2009;58:241–255. [PubMed] [Google Scholar]
- 10.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 11.Gill TJ, Sledge JB, Ekkernkamp A, Ganz R. Intraoperative assessment of femoral head vascularity after femoral neck fracture. J Orthop Trauma. 1998;12:474–478. doi: 10.1097/00005131-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Gordon JE, Abrahams MS, Dobbs MB, Luhmann SJ, Schonecker PL. Early reduction, arthrotomy and cannulated screw fixation in unstable slipped capital femoral epiphysis treatment. J Pediatr Orthop. 2002;22:352–358. [PubMed] [Google Scholar]
- 13.Herman MJ, Dormans JP, Davidson RS, Drummond DS, Gregg JR. Screw fixation of grade III slipped capital femoral epiphysis. Clin Orthop Relat Res. 1996;322:77–85. doi: 10.1097/00003086-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kalhor M, Horowitz K, Gharehdaghi J, Beck M, Ganz R. Anatomic variations in femoral head circulation. Hip Int. 2012;22:307–312. doi: 10.5301/HIP.2012.9242. [DOI] [PubMed] [Google Scholar]
- 15.Kallio PE, Mah ET, Foster BK, Paterson DC, LeQuesne GW. Slipped capital femoral epiphysis, Incidence and assessment of physeal instability. J Bone Joint Surg Br. 1995;77:752–755. [PubMed] [Google Scholar]
- 16.Larson AN, Sierra RJ, Yu EM, Trousdale RT, Stans AA. Outcomes of slipped capital femoral epiphysis treated with in situ pinning. J Pediatr Orthop. 2012;32:125–130. doi: 10.1097/BPO.0b013e318246efcb. [DOI] [PubMed] [Google Scholar]
- 17.Leunig M, Casillas MM, Hamlet M, Hersche O, Nötzli H, Slongo T, Ganz R. Slipped capital femoral epiphysis, Early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Orthop Scand. 2000;71:370–375. doi: 10.1080/000164700317393367. [DOI] [PubMed] [Google Scholar]
- 18.Leunig M, Horowitz K, Manner H, Ganz R. In situ pinning with arthroscopic osteoplasty for mild SCFE: a preliminary technical report. Clin Orthop Relat Res. 2010;468:3160–3167. doi: 10.1007/s11999-010-1408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leunig M, Slongo T, Ganz R. Subcapital realignment in slipped capital femoral epiphysis: surgical hip dislocation and trimming of the stable trochanter to protect the perfusion of the epiphysis. Instr Course Lect. 2008;57:499–507. [PubMed] [Google Scholar]
- 20.Loder RT. Unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2001;21:694–699. [PubMed] [Google Scholar]
- 21.Loder RT, Aronsson DD, Weinstein SL, Breur GJ, Ganz R, Leunig M. Slipped capital femoral epiphysis. Instr Course Lect. 2008;57:473–498. [PubMed] [Google Scholar]
- 22.Loder RT, Richards BS, Shapiro PS, Reznik LR, Aronsson DD. Acute slipped capital femoral epiphysis: the importance of physeal stability. J Bone Joint Surg Am. 1993;75:1134–1140. doi: 10.2106/00004623-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lubicky JP. Chondrolysis and avascular necrosis: complications of slipped capital femoral epiphysis. J Pediatr Orthop B. 1996;5:162–167. doi: 10.1097/01202412-199605030-00005. [DOI] [PubMed] [Google Scholar]
- 24.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 25.Mooney JF, III, Sanders JO, Browne RH, Anderson DJ, Jofe M, Feldman D, Raney EM. Management of unstable/acute slipped capital femoral epiphysis: results of a survey of the POSNA membership. J Pediatr Orthop. 2005;25:162–166. doi: 10.1097/01.bpo.0000151058.47109.fe. [DOI] [PubMed] [Google Scholar]
- 26.Noetzli HP, Siebenrock KA, Hempfing A, Ramseier LE, Ganz R. Perfusion of the femoral heads during surgical dislocation of the hip: monitoring by laser Doppler flowmetry. J Bone Joint Surg Br. 2002;84:300–304. doi: 10.1302/0301-620X.84B2.12146. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MD, Weiner DS, Green NE, Terry CL. Acute slipped capital femoral epiphysis: the value and safety of urgent manipulative reduction. J Pediatr Orthop. 1997;17:648–654. doi: 10.1097/00004694-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Rab GT. The geometry of slipped capital femoral epiphysis: implication for movement, impingement and corrective osteotomy. J Pediatr Orthop. 1999;19:419–424. doi: 10.1097/00004694-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Rhoad RC, Davidson RS, Heyman S, Dormans JP, Drummond DS. Pretreatment bone scan in SCFE: a predictor of ischemia and avascular necrosis. J Pediatr Orthop. 1999;19:164–168. doi: 10.1097/00004694-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Sankar WN, Partland TG, Millis MB, Kim YJ. The unstable slipped capital femoral epiphysis, Risk factors for osteonecrosis. J Pediatr Orthop. 2010;30:544–548. doi: 10.1097/BPO.0b013e3181e4f372. [DOI] [PubMed] [Google Scholar]
- 31.Schöniger R, Kain MSH, Ziebarth K, Ganz R. Epiphyseal reperfusion after subcapital realignment of an unstable SCFE. Hip Int. 2010;20:273–279. doi: 10.1177/112070001002000223. [DOI] [PubMed] [Google Scholar]
- 32.Sink EL, Zaltz I, Heare T, Dayton M. Acetabular cartilage and labral damage observed during surgical hip dislocation for stable slipped capital femoral epiphysis. J Pediatr Orthop. 2010;30:26–30. doi: 10.1097/BPO.0b013e3181c6b37a. [DOI] [PubMed] [Google Scholar]
- 33.Slongo T, Kakaty D, Krause F, Ziebarth K. Treatment of slipped capital femoral epiphysis with a modified Dunn procedure. J Bone Joint Surg Am. 2010;92:2898–2908. doi: 10.2106/JBJS.I.01385. [DOI] [PubMed] [Google Scholar]
- 34.Tokmakova KP, Stanton RP, Mason DE. Factors influencing the development of osteonecrosis in patients treated for slipped capital femoral epiphysis. J Bone Joint Surg Am. 2003;85:798–801. doi: 10.2106/00004623-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Ziebarth K, Domayer S, Slongo T, Kim YJ, Ganz R. Clinical stability of slipped capital femoral epiphysis does not correlate with intraoperative stability. Clin Orthop Relat Res. 2012;480:2274–2279. doi: 10.1007/s11999-012-2339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziebarth K, Zilkens C, Spencer S, Leunig M, Ganz R, Kim YJ. Capital realignment for moderate and severe SCFE using a modified Dunn procedure. Clin Orthop Relat Res. 2009;467:704–716. doi: 10.1007/s11999-008-0687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zilkens C, Jäger M, Bittersohl B, Kim YJ, Millis MB, Krauspe R. [Slipped capital femoral epiphysis] [in German] Orthopäde. 2010;39:1009–1021. doi: 10.1007/s00132-010-1659-4. [DOI] [PubMed] [Google Scholar]


