Abstract
Background
Avascular necrosis (AVN) of the capital femoral epiphysis (CFE) after an unstable slipped capital femoral epiphysis (SCFE), femoral neck fracture or traumatic hip dislocation can result in severe morbidity. Treatment options for immature patients with AVN are limited, including a closed bone graft epiphysiodesis (CBGE). However, it is unclear whether this procedure prevents AVN progression.
Questions/purposes
We investigated whether early MRI screening and CBGE prevented the development of advanced AVN changes in the CFE and the rates of complications with this approach.
Methods
We prospectively followed all 13 patients (seven boys, six girls) with unstable SCFEs (six patients), femoral neck fractures (five patients), and traumatic hip dislocations (two patients) and evidence of early AVN treated between 1984 and 2012. Mean age at initial injury was 12 years (range, 10–16 years). Nine of the 13 patients had followup of at least 2 years or until conversion to THA (mean, 4.5 years; range, 0.8–8.5 years), including two with unstable SCFEs, the five with femoral neck fractures, and the two with traumatic hip dislocations. All patients had technetium scans and/or MRI within 1 to 2 months of their initial injury (before CBGE) and all had evidence of early (Ficat 0) AVN. Patients were followed clinically and radiographically for AVN progression.
Results
Six of the nine hips did not develop typical clinical or radiographic evidence of AVN. These six patients have been followed 6.3 years (range, 4.3–9.1 years) from initial injury and 5.9 years (range, 3.8–8.5 years) from CBGE. The remaining three patients were diagnosed with AVN at periods ranging from 3 to 6 months after CBGE.
Conclusions
Early recognition and treatment of AVN with a CBGE may alter the natural history of this complication.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Avascular necrosis (AVN) of the capital femoral epiphysis (CFE) is one of the most devastating complications after a traumatic injury in older children and adolescents. It occurs in 15% to 58% of unstable slipped capital femoral epiphyses (SCFEs) [10, 16, 21, 30, 31, 37] and is common after pediatric femoral neck fractures, affecting 10% to 43% of involved hips [5, 18, 24, 26, 35, 36]. It occurs less commonly in patients with traumatic dislocations of the hip, affecting 3% to 15% of involved hips [2, 11, 13, 14, 24, 27, 41].
The blood supply to the CFE is particularly precarious during late childhood and adolescence, as the vascular flow provided by the artery of the ligamentum teres and the lateral femoral circumflex diminishes, while the presence of the proximal femoral physis serves as a block to intramedullary communication [38]. Disruption of the blood supply to the CFE provided by the medial femoral circumflex artery may occur either during the initial traumatic injury, secondary to intracapsular hematoma, or during aggressive reduction or posterosuperior instrumentation of the CFE intraoperatively [12, 22].
Once a vascular insult has occurred to the CFE, a recognized chain of events follows [17]. Initially, the devitalized bone is revascularized through both existing and new vascular channels. As revascularization progresses, there is simultaneous resorption of necrotic bone and deposition of new bone. This results in subchondral weakness and risk for a subchondral fracture. Until the subchondral fracture occurs, the hip is asymptomatic. After fracture, a second episode of ischemia occurs. Repeat revascularization is delayed due to the loss of subchondral stability. The repeat revascularization results in slow resorption of necrotic bone and new bone formation. This results in the potential for femoral head collapse and deformity leading to advanced osteoarthritic changes within the hip. A major problem in older children and adolescents is the prolonged healing process over 2 to 4 years [15], which further enhances the development of femoral head deformity and subsequent osteoarthritis.
Treatment protocols for AVN of the CFE in skeletally immature patients do not exist at this time. Medical treatment using bisphosphonates has resulted in favorable Harris hip scores and Stulberg ratings [9], while enoxaparin appeared to decrease the progression of Ficat I and II hips in patients with thrombophilic or hypofibronolytic disorders [28]. Surgical treatment has also been recommended. Gordon et al. [10] reported no cases of AVN in 16 patients with unstable SCFE treated with urgent closed reduction, arthrotomy and cannulated screw fixation with two screws.
Several surgical techniques have been used to treat early AVN in adults, including vascularized fibular grafts [39, 40], other vascularized grafts [3, 4], and core decompression of the femoral head [19, 20, 23, 25, 33, 34]. Late treatment of hips in older children and adolescents with AVN usually involves salvage procedures, such as proximal femoral derotational osteotomies, pelvic osteotomies, and arthrodeses.
For the past 28 years, we have utilized an early screening program for older children and adolescents with disorders that have a high risk for AVN. This consists of an initial technetium bone scan, MRI, or both. When AVN is detected, a closed bone graft epiphysiodesis (CBGE) is performed. The objectives of this procedure are to decompress the epiphysis and allow for rapid revascularization of the CFE before the development of a subchondral fracture, the hallmark of progressive collapse and deformity. Bone graft epiphysiodesis has been used as a primary treatment for SCFE, but to our knowledge, there is no description of its use as a treatment for AVN [1, 43]. When used in SCFE, including the unstable form, the incidence of AVN is reportedly less than 8% [1, 43], indicating a biologic effect. However, it is unclear whether CBGE will prevent progression in patients with evidence of early AVN.
We therefore investigated two key questions: (1) Does early MRI screening and CBGE prevent the development of advanced AVN changes in the immature hip? And (2) what are the rates of complications with this approach?
Patients and Methods
We prospectively followed nine older but skeletally immature patients with evidence of AVN of the CFE after a traumatic event treated by CBGE between 1984 and 2012. During this time, all older children or adolescents who sustained an unstable SCFE, femoral neck fracture, or traumatic dislocation were managed with the prospective protocol. Hips with unstable SCFE were treated with gentle reduction on a traction table before percutaneous titanium screw fixation. We included patients 10 years of age or older at the time of initial injury as the CFE epiphysiodesis would not result in a substantial lower-extremity length discrepancy (LELD); younger children were excluded from this treatment protocol. LELD was not evaluated preoperatively. All patients underwent a technetium bone scan, MRI of the pelvis, or both 1 to 2 months after treatment of their initial injury to assess for possible AVN. If evidence was detected, a CBGE was performed within the next 2 to 3 months. All hips were classified according to the Ficat classification [7, 8] at the time of CBGE. Of the 13 patients who underwent this prospective protocol, four were excluded from the study because they were lost to followup (two patients) or lacked the minimum 2 years of followup (two patients). One patient lost to followup was followed until 5 months, with no clinical symptoms and no evidence of AVN progression on MRI at that time point but did not return for followup. The second patient was followed until 4 months after surgery, with a slight decrease in internal rotation but no pain or decreased activities, and also did not return for followup. Thus, nine patients had the necessary minimum 2 years of followup for inclusion (mean, 4.5 years; range, 0.8–8.5 years) or developed advanced AVN (Table 1). There were five boys and four girls, with a mean age at time of initial injury of 12 years (range, 10–16 years) and a mean interval between initial treatment and CBGE of 4 months (range, 2–10 months). The delay between initial injury and CBGE allowed for healing of the initial injury. Two patients had unstable SCFEs, five femoral neck fractures, and two traumatic hip dislocations. Eight patients had positive technetium bone scans while all nine patients had MRI indicating AVN. A technetium bone scan was not performed in Patient 9 as MRI was strongly indicative of AVN. After the first patient, we routinely obtained histology from the removed core for the CFE; all eight patients had confirmed histologic evidence of AVN. All nine patients progressed to skeletal maturity within the followup period. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. The prospective protocol was approved by our institutional review board.
Table 1.
Patient demographics and results
| Patient | Sex | Mechanism | Age at injury (years) | Age at CBGE (years) | Followup from CBGE (years) | Bone scan | MRI | Biopsy | Ficat stage at CBGE | AVN |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | Femoral neck fracture | 14.1 | 14.6 | 8.5 | + | + | No | 0 | No |
| 2 | Male | Hip dislocation | 11.3 | 12.1 | 6.3 | + | + | + | 0 | No |
| 3 | Female | Hip dislocation | 11.3 | 11.4 | 4.2 | + | + | + | 0 | No |
| 4 | Female | Femoral neck fracture | 12.4 | 12.7 | 0.3 | + | + | + | 0 | Yes |
| 5 | Male | Femoral neck fracture | 13.8 | 14.2 | 3.8 | + | + | + | 0 | No |
| 6 | Female | Unstable SCFE | 11.7 | 11.9 | 1.8 | + | + | + | 0 | Yes |
| 7 | Male | Femoral neck fracture | 16.0 | 16.3 | 5.2 | + | + | + | 0 | No |
| 8 | Male | Femoral neck fracture | 10.3 | 10.7 | 7.3 | + | + | + | 0 | No |
| 9 | Male | Unstable SCFE | 9.8 | 10.00 | 2.4 | No | + | + | 0 | Yes |
CBGE = closed bone graft epiphysiodesis; SCFE = slipped capital femoral epiphysis; AVN = avascular necrosis.
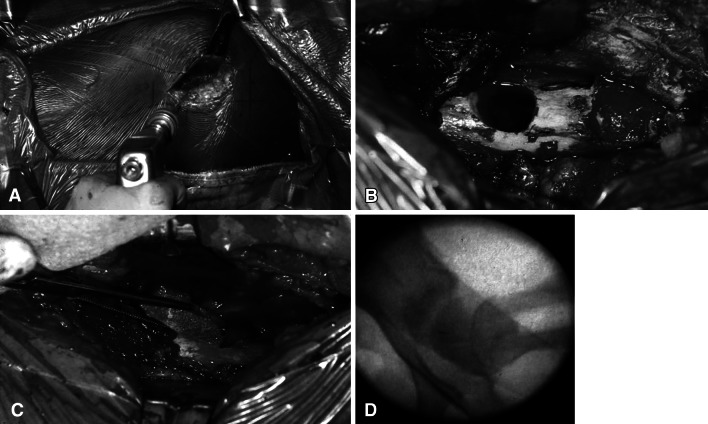
The procedure was performed on a radiolucent table. A standard lateral approach to the proximal femur was performed. Previously placed implants, if any, were identified and removed without difficulty given the short duration between placement and removal of the titanium cannulated screws. A guide wire was inserted under direct fluoroscopic visualization from the lateral cortex, through the middle of the femoral neck, and into the center of the CFE (Fig. 1A). The starting point along the lateral femoral shaft was superior to the level of the lesser trochanter. A 1.2-cm cannulated drill was then used to ream over the guide wire to approximately 5 mm from the proximal femoral physis. A biopsy including the metaphysis, physis, and epiphysis was then performed using a core biopsy reamer. The specimen was sent to pathology for histologic analysis to confirm the presence of AVN. A corticocancellous graft was harvested from the lateral aspect of the proximal femur extending across the greater trochanter, proximal to the drill hole (Fig. 1B). This graft measured approximately 1.2 × 1.2 × 4 cm. The corticocancellous graft was inserted into the reamed hole and into the femoral head (Fig. 1C). All cancellous reamings were packed behind the strut graft (Fig. 1D).
Fig. 1A–D.
(A) An intraoperative photograph illustrates the guide pin involved from the lateral cortex of the proximal femur through the middle of the femoral neck into the center of the CFE. (B) The corticocancellous bone graft is obtained proximal to the drill hole. It extends across the apophysis of the greater trochanter producing an apophysiodesis. (C) A photograph shows the graft before insertion into the drill hole. A metal clip can be applied to the end of the graft to aid visualization during insertion. (D) Air behind the graft can also aid in visualization during insertion. Reamings are packed into the hole after graft insertion.
Postoperatively, patients were restricted to partial weightbearing with crutches on the operated extremity for 6 weeks, until the graft was presumed to be incorporated. Patients underwent supervised physical therapy for 3 months for ROM and strengthening. Normal activities of daily living resumed 3 to 6 months postoperatively, based on symptoms and radiographic findings.
Patients were followed monthly for 2 to 4 months, then at 3-month intervals until 1 year, and then yearly. Clinical examinations included gait and ROM, and AP and frog lateral radiographs were obtained at each visit. We reviewed the patients’ charts for sex, age at injury, mechanism of initial injury, age at diagnosis of AVN, age at surgery, presenting signs and symptoms of AVN, preoperative imaging, length of followup, and long-term clinical and radiographic results. Any patient with suspected LELD on physical examination was evaluated with a scanogram. Patients were followed for development of complications, including surgical site infections, LELD, reslippage of the SCFE, and subtrochanteric fracture.
Results
Of the nine patients, six demonstrated no clinical or radiographic evidence of AVN during their followup (Fig. 2). These six patients were all Ficat 0 and have been followed for a mean of 6.3 years (range, 4.3–9.1 years) from initial injury and 5.9 years (range, 3.8–8.5 years) from their CBGE. The remaining three patients, also Ficat 0, developed clinical and radiographic evidence of advanced AVN. These three patients all went on to further surgical management of their hips. One patient (Patient 6) initially presented with an unstable SCFE after CBGE to progress to subchondral fracture with collapse of her femoral head. She was followed for 2.3 years from her initial injury and 1.8 years from the time of CBGE before she was referred for consideration of hip arthroplasty. The second patient (Patient 4) presented with a femoral neck fracture and went on to rapid progression of AVN after CBGE, ultimately requiring THA 1.2 years after her initial injury and 0.75 years after her CBGE. The final patient (Patient 9) who presented with an unstable SCFE sustained a fracture to his corticocancellous bone graft with reslippage after a traumatic fall postoperatively, indicating his unstable SCFE was not healed at the time of his CBGE.
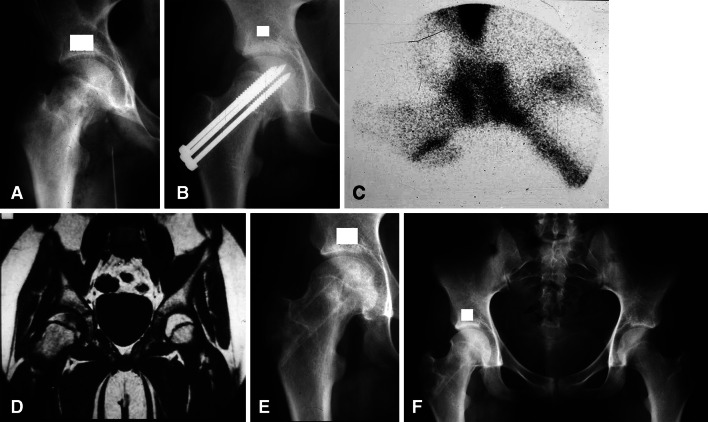
Fig. 2A–F.
(A) An AP radiograph shows the right hip of Patient 1, a 14.1-year-old girl who had sustained a gunshot wound to the groin with a minimally displaced femoral neck fracture and a femoral artery injury. (B) An AP radiograph shows the hip after in situ fixation with Knowles pins. The femoral artery was repaired with restoration of blood flow to the lower extremity. (C) A technetium bone scan at 1 month postoperatively demonstrates decreased isotope uptake indicating AVN. (D) Early-generation MRI at 2 months postoperatively demonstrates decreased signal intensity on T2 images, supporting the diagnosis of AVN. (E) An AP radiograph shows the hip after CBGE and 7 months after her initial injury. (F) An AP radiograph shows the hip 3 years and 9 months after CBGE at 19 years of age. She is asymptomatic and skeletally mature and there has been no clinical or radiographic evidence of AVN.
Two patients developed LELDs of 1.2 cm and 1.5 cm, presumably due to the premature closure of the CFE. The LELD did not require treatment in eight patients, while one (Patient 8) underwent a contralateral distal femoral epiphysiodesis at age 13 years and 6 months for a predicted 2.5-cm LELD and achieved equal lower-extremity lengths at skeletal maturity (Fig. 3). One patient (Patient 9, described above) sustained an injury from a fall after his CBGE and sustained further slippage of his previous unstable SCFE, requiring repeat pinning. He later underwent a valgus osteotomy of the proximal femur to improve the relationship between the femoral head and the acetabulum. He is currently rehabilitating from valgus osteotomy surgery with no complaints of hip pain but does have a persistent 1.2-cm LELD and limited internal rotation and abduction of the hip. There was no incidence of infection, intraoperative or postoperative fracture, neurologic injury, or vascular injury in any patient during CBGE or postoperatively. Based on the classification of Dindo et al. [6], there were two Grade IIIb complications (one LELD requiring contralateral epiphyseodesis and one reslippage of SCFE).
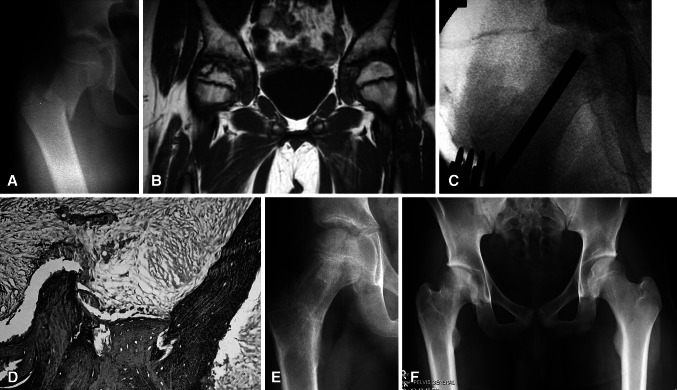
Fig. 3A–F.
(A) An AP radiograph shows the pelvis of Patient 8, a 10.3-year-old boy with a right basilar femoral neck fracture. (B) MRI 4 months after closed reduction and internal fixation demonstrates AVN of the CFE. (C) CBGE was performed shortly after the MRI had been performed. (D) Core biopsy at the time of CBGE also confirms AVN. The marrow space is fibrous and the lacunae are empty. (E) An AP radiograph 7 months after CBGE shows the hip is asymptomatic and there is no radiographic evidence of AVN. (F) An AP radiograph shows the hip 7 years postoperatively at 18 years of age. He is skeletally mature, his right hip is asymptomatic, and there has been no radiographic evidence of AVN. He did require a contralateral epiphysiodesis of the distal femoral epiphysis for a predicted 2.5-cm LELD at maturity.
Discussion
AVN of the CFE in older children and adolescents is a particularly serious complication. It can require 2 to 4 years to heal [15] and this is related to remaining growth, which is limited in these patients. The long healing process results in an increased risk for femoral head collapse and deformity. With limited growth after healing, the potential for remodeling is substantially reduced. All these factors result in an increased risk for hip deformity and early degenerative osteoarthritis, a potential lifelong disability. Developing a method for early diagnosis and intervention to alter the natural history of AVN in these older, skeletally immature patients is important. To accomplish this goal, we investigated two key questions: (1) Does early MRI screening and CBGE prevent the development of advanced AVN changes in the immature hip? And (2) what are the rates of complications with this approach?
Our study has several limitations. First, while a prospective series, there were relatively few patients owing to the infrequency of posttraumatic AVN in this age group. This prevents direct comparison between treated patients with untreated controls who could be followed to determine the outcome of the natural course of AVN when detected in this manner. Second, using this screening protocol raises the question of the natural course of this preclinical and preradiographic AVN if left untreated. We did confirm histologically that AVN was present. Therefore, we believe it reasonable to assume progression to subchondral fracture and the typical clinical and radiographic course of AVN would have occurred. We found six of nine patients with CFE and preclinical and preradiographic, asymptomatic femoral head AVN (Ficat Stage 0) treated by CBGE had no subsequent evidence of AVN at a minimum of 2 years’ followup.
Complications after this procedure were few. Recurrent or persistent slippage of the CFE has been described in other studies utilizing open bone graft epiphysiodesis [1, 29, 42]. This has usually been attributed to graft resorption or fracture. It is important that the corticocancellous bone graft be 1.2 cm in diameter to decrease the risk for this complication. It is our suspicion that the initial drilling allows vascular channels to form across the physis, while the bone graft provides the structural support required for a hip that has only recently been treated for unstable SCFE or other diagnosis.
Bone graft epiphysiodesis, primarily as an open procedure, has previously been used for treating SCFE [1, 32, 43]. The open procedure requires an intracapsular approach and grafting through the femoral neck into the center of the femoral head. The closed procedure is extracapsular, has little or no stress fracture risk, and is easier to perform.
In summary, this approach may offer a way to alter the natural progression of posttraumatic AVN in skeletally immature older children and adolescents. Based on our findings, we propose screening and CBGE are reasonable treatment options that may prevent preclinical and preradiographic AVN from progressing. Future larger, prospective studies will be necessary to elucidate the role for this protocol in the detection and treatment of adolescent AVN of the CFE after acute SCFEs, femoral neck fractures, and hip dislocations. Experimental animal studies are also necessary to define the histologic effects of this procedure. We presume there is rapid revascularization through the physis allowing callus formation to support the head and then subsequent healing similar to fracture repair.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Adamczyk MJ, Weiner DS, Hawk D. A 50-year experience with bone graft in the treatment of slipped capital femoral epiphysis. J Pediatr Orthop. 2003;23:578–583. doi: 10.1097/01241398-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Barquet A. Avascular necrosis following traumatic hip dislocation: factors of influence. Acta Orthop Scand. 1982;53:809–813. doi: 10.3109/17453678208992298. [DOI] [PubMed] [Google Scholar]
- 3.Baski DP. Treatment of osteonecrosis of the femoral head by drilling and muscle-pedicle bone grafting. J Bone Joint Surg Br. 1991;73:241–245. doi: 10.1302/0301-620X.73B2.2005147. [DOI] [PubMed] [Google Scholar]
- 4.Baski DP, Palak, Baski DD. Long-term results of decompression and muscle-pedicle bone grafting for osteonecrosis of the femoral head. Int Orthop. 2009;33:41–47. doi: 10.1007/s00264-007-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canale ST, Bourland WL. Fractures of the neck and intertrochanteric region of the femur in children. J Bone Joint Surg Am. 1977;59:431–443. [PubMed] [Google Scholar]
- 6.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 8.Ficat RP, Arlet J. Ischemia and Necrosis of Bone. Baltimore, MD: Williams & Wilkins; 1980. pp. 29–52. [Google Scholar]
- 9.Glueck CJ, Freibert RA, Sieve L, Wang P. Enoxaparin prevents progression of Stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;435:164–170. doi: 10.1097/01.blo.0000157539.67567.03. [DOI] [PubMed] [Google Scholar]
- 10.Gordon JE, Abrahams MS, Dobbs MB, Luhmann SJ, Schoenecker PL. Early reduction, arthrotomy and cannulated screw fixation in unstable slipped capital femoral epiphyses treatment. J Pediatr Orthop. 2002;22:352–358. [PubMed] [Google Scholar]
- 11.Hamilton PR, Broughton NS. Traumatic hip dislocation in childhood. J Pediatr Orthop. 1998;18:691–694. doi: 10.1097/00004694-199809000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressure after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28:723–728. doi: 10.1097/BPO.0b013e318186bda3. [DOI] [PubMed] [Google Scholar]
- 13.Herrera-Soto JA, Price CT. Traumatic hip dislocations in children and adolescents: pitfalls and complications. J Am Acad Orthop Surg. 2009;17:15–21. doi: 10.5435/00124635-200901000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hung NN. Traumatic hip dislocation in children. J Pediatr Orthop B. 2012;21:542–551. doi: 10.1097/BPB.0b013e328356371b. [DOI] [PubMed] [Google Scholar]
- 15.Joseph B. Natural history of early-onset and late-onset Legg-Calvé-Perthes disease. J Pediatr Orthop. 2011;31(2 suppl):S152–S155. doi: 10.1097/BPO.0b013e318223b423. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy JG, Hresko MT, Kasser JR, Shrock KB, Zurakowski D, Waters PM, Millis MD. Osteonecrosis of the femoral head associated with slipped capital femoral epiphysis. J Pediatr Orthop. 2001;21:189–193. [PubMed] [Google Scholar]
- 17.Kim HK. Pathophysiology and new strategies for the treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 2012;94:659–669. doi: 10.2106/JBJS.J.01834. [DOI] [PubMed] [Google Scholar]
- 18.Lam SF. Fractures of the neck of the femur in children. J Bone Joint Surg Am. 1971;53:1165–1179. [PubMed] [Google Scholar]
- 19.Lieberman JR. Core decompression for osteonecrosis of the hip joint. Clin Orthop Relat Res. 2004;418:29–33. doi: 10.1097/00003086-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–145. doi: 10.1097/01.blo.0000150312.53937.6f. [DOI] [PubMed] [Google Scholar]
- 21.Loder RT, Richards BS, Shapiro PS, Reznick LR, Aronson DD. Acute slipped capital femoral epiphysis: the importance of physeal stability. J Bone Joint Surg Am. 1993;75:1134–1140. doi: 10.2106/00004623-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Maeda S, Kita A, Funayama K, Kokobun S. Vascular supply to slipped capital femoral epiphyses. J Pediatr Orthop. 2001;21:664–667. [PubMed] [Google Scholar]
- 23.Maniwa S, Nishikori T, Furakawa S, Kajitani K, Iwata A, Nishikawa U, Ochi M. Evaluation of core decompression for early osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2000;120:241–244. doi: 10.1007/s004020050456. [DOI] [PubMed] [Google Scholar]
- 24.Mehlman CT, Hubbard GW, Crawford AH, Roy DR, Wall EJ. Traumatic hip dislocation in children: long-term follow-up of 42 patients. Clin Orthop Relat Res. 2000;376:68–79. doi: 10.1097/00003086-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Mont MA, Ragland PS, Parvizi J. Surgical treatment of osteonecrosis of the hip. Instr Course Lect. 2006;55:167–172. [PubMed] [Google Scholar]
- 26.Moon ES, Mehlman CT. Risk factors for avascular necrosis after femoral neck fractures in children: 25 Cincinnati cases and meta-analysis of 360 cases. J Orthop Trauma. 2006;20:323–329. doi: 10.1097/00005131-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Offierski CM. Traumatic dislocation of the hip in children. J Bone Joint Surg Br. 1981;63:194–197. doi: 10.1302/0301-620X.63B2.7217141. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am. 2007;89:1727–1734. doi: 10.2106/JBJS.F.00964. [DOI] [PubMed] [Google Scholar]
- 29.Rao SB, Crawford AH, Burger RR, Roy DR. Open bone peg epiphysiodesis for slipped capital femoral epiphysis. J Pediatr Orthop. 1996;16:37–48. doi: 10.1097/01241398-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Rattey T, Piehl F, Wright JG. Acute slipped capital femoral epiphysis: review of outcomes and rates of avascular necrosis. J Bone Joint Surg Am. 1996;78:398–402. doi: 10.2106/00004623-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Sankar WN, McPartland TG, Millis MB, Kim YJ. The unstable slipped capital femoral epiphysis: risk factors for osteonecrosis. J Pediatr Orthop. 2010;30:544–548. doi: 10.1097/BPO.0b013e3181e4f372. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt TL, Cimino WG, Seidel FG. Allograft epiphysiodesis for slipped capital femoral epiphysis. Clin Orthop Relat Res. 1996;322:61–76. doi: 10.1097/00003086-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg ME, Larcom PG, Strafford B, Hasick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. doi: 10.1097/00003086-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Stulberg BN, Davis AW, Bauer TW, Levine M, Easley K. Osteonecrosis of the femoral head: a prospective randomized treatment protocol. Clin Orthop Relat Res. 1991;268:140–151. [PubMed] [Google Scholar]
- 35.Swiontkowski MF, Winquist RA. Displaced hip fractures in children and adolescents. J Trauma. 1986;26:384–388. doi: 10.1097/00005373-198604000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Togrul E, Bayram H, Gulsen M, Kalaci A, Ozbarlas S. Fractures of the femoral neck in children: long-term follow-up in 62 hip fractures. Injury. 2005;36:123–130. doi: 10.1016/j.injury.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Tokmakova KP, Stanton RP, Mason DE. Factors influencing the development of osteonecrosis in patients treated for slipped capital femoral epiphysis. J Bone Joint Surg Am. 2003;85:798–801. doi: 10.2106/00004623-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br. 1957;39:358–394. doi: 10.1302/0301-620X.39B2.358. [DOI] [PubMed] [Google Scholar]
- 39.Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of osteonecrosis of the femoral head with vascularized fibular grafting: a long-term follow-up study of 103 hips. J Bone Joint Surg Am. 1995;77:681–694. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Urbaniak JR, Harvey EJ. Revascularization of the femoral head in osteonecrosis. J Am Acad Orthop Surg. 1998;6:44–54. doi: 10.5435/00124635-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Vialle R, Odent T, Pannier S, Paithier F, Laumonier F, Glorion C. Traumatic hip dislocation in childhood. J Pediatr Orthop. 2005;25:138–144. doi: 10.1097/01.bpo.0000151059.85227.ea. [DOI] [PubMed] [Google Scholar]
- 42.Ward WT, Wood K. Open bone graft for slipped capital femoral epiphysis. J Pediatr Orthop. 1990;10:14–20. [PubMed] [Google Scholar]
- 43.Weiner D, Weiner S, Melby A, Hoyt WA., Jr A 30-year experience with bone graft in the treatment of slipped capital femoral epiphysis. J Pediatr Orthop. 1984;4:145–152. doi: 10.1097/01241398-198403000-00001. [DOI] [PubMed] [Google Scholar]