Abstract
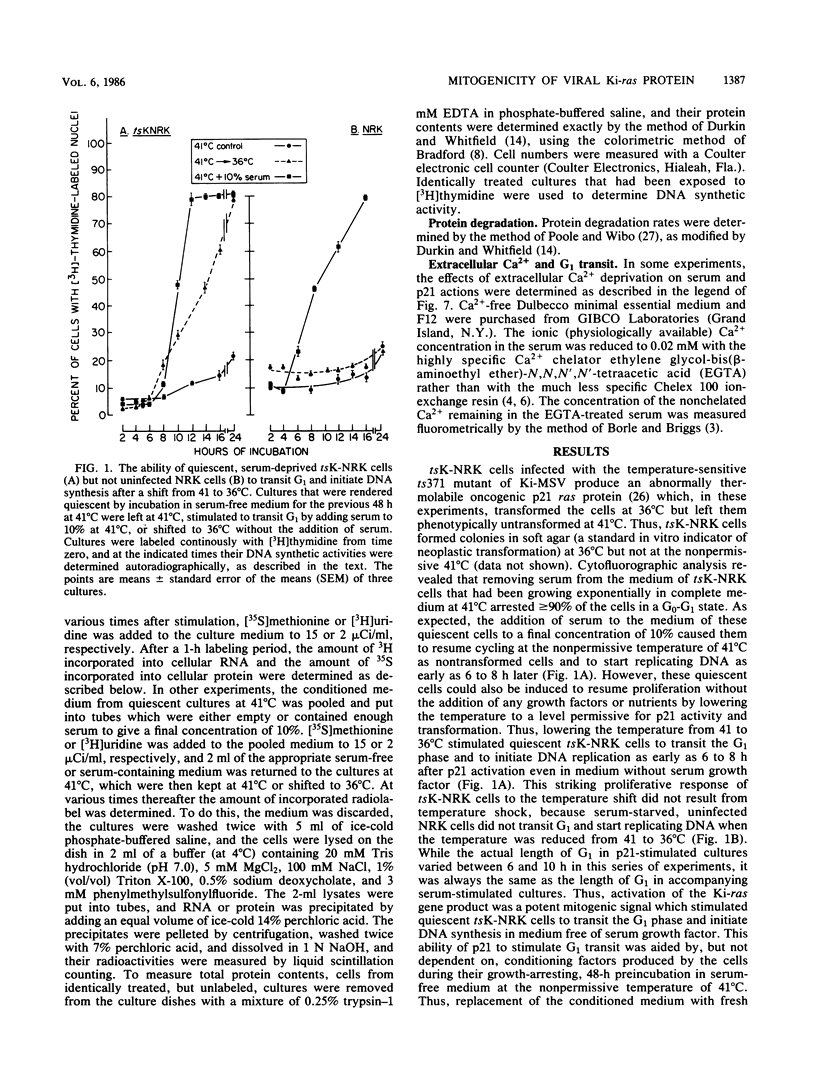
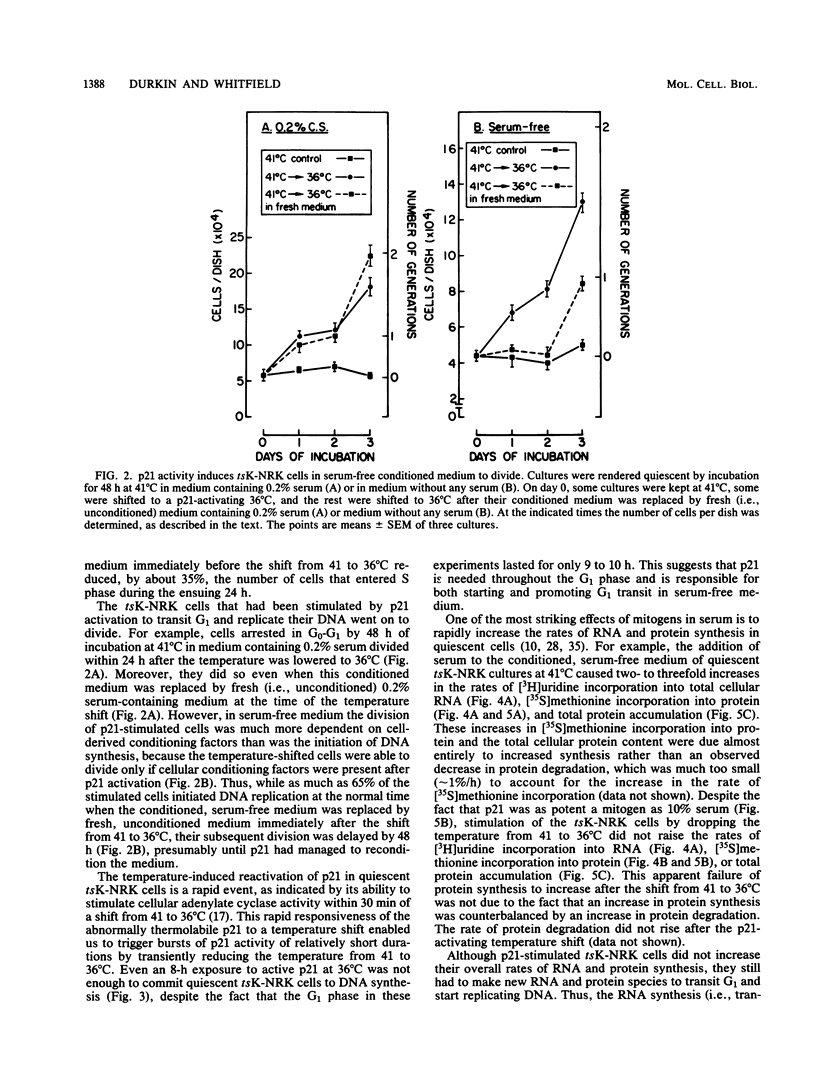
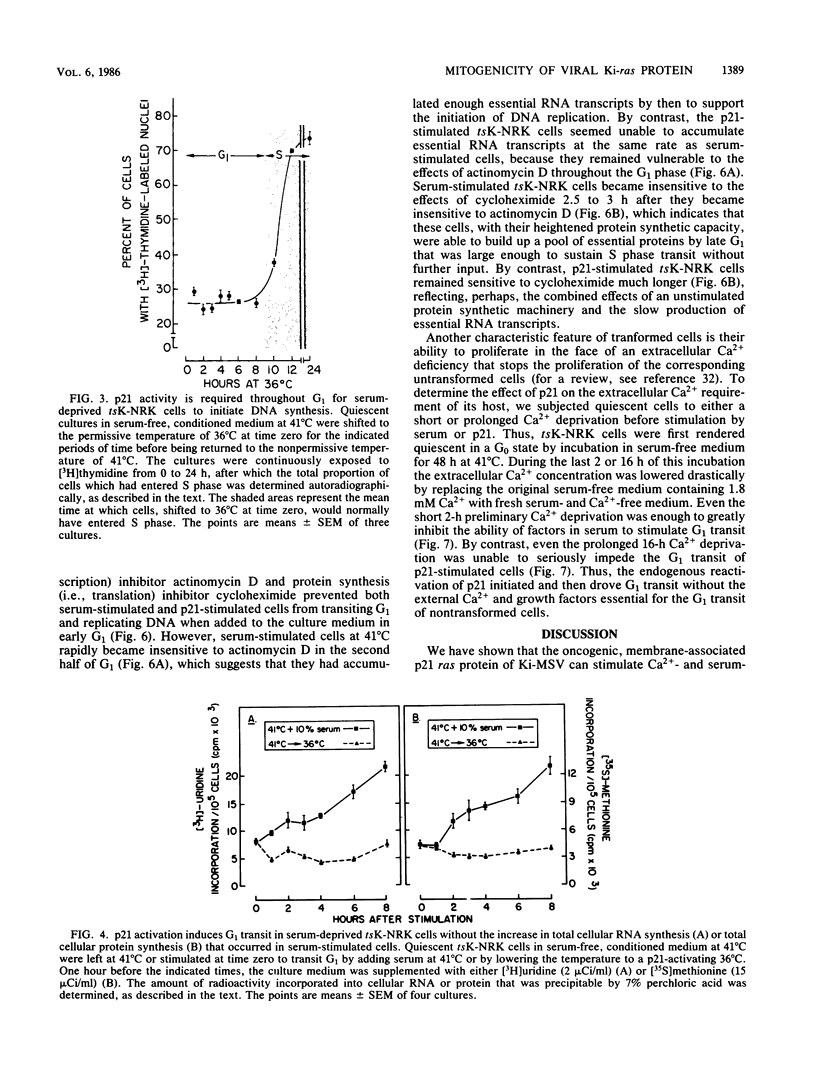
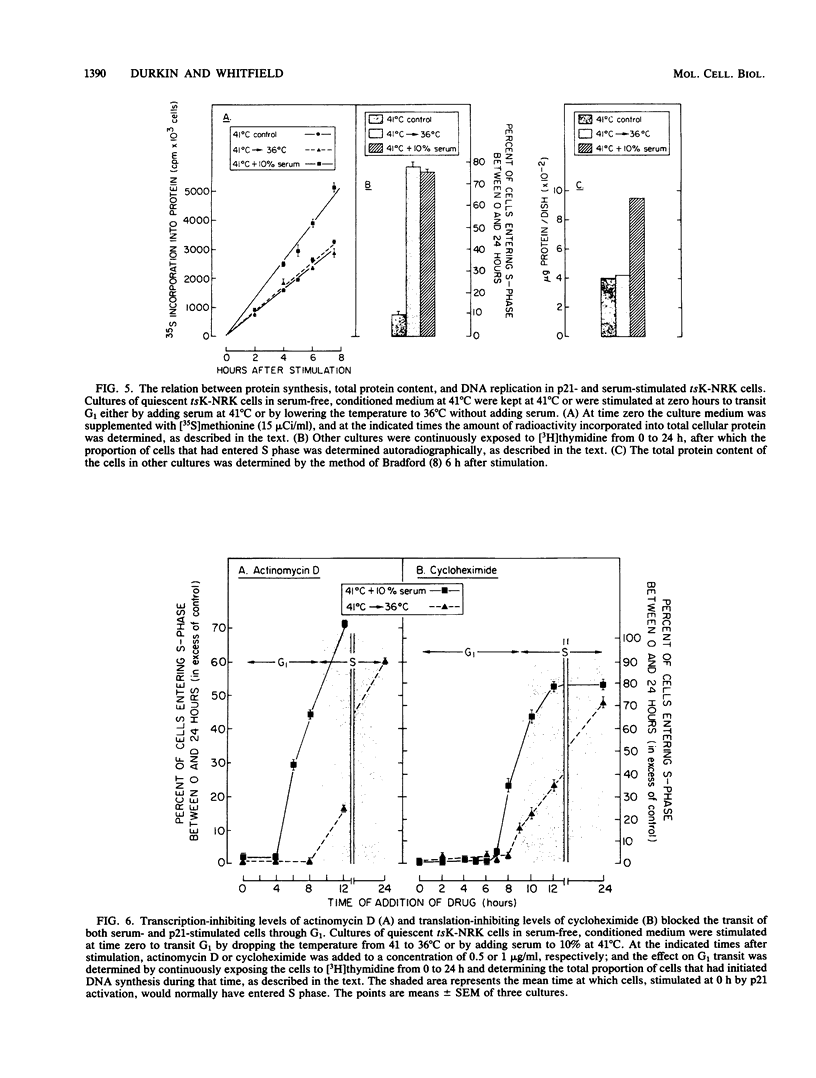
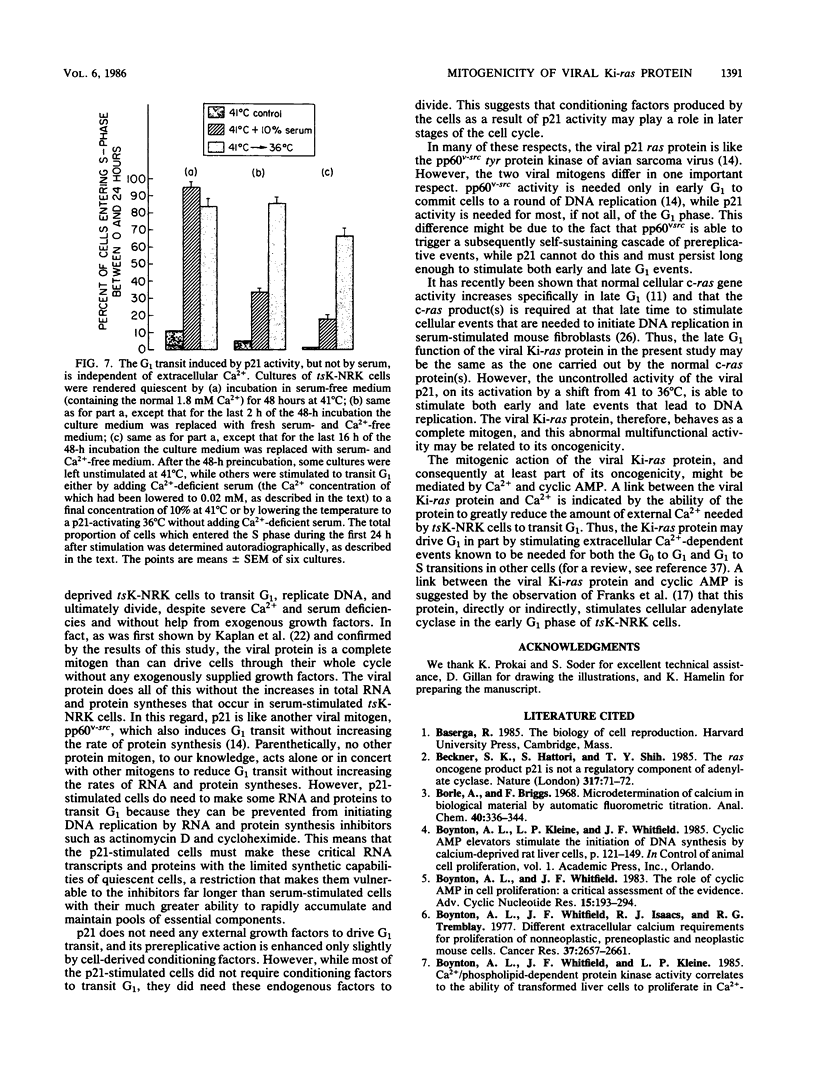
NRK rat kidney cells infected with a temperature-sensitive mutant of the Kirsten sarcoma virus (ts371) were transformed at 36 degrees C but were phenotypically nontransformed at 41 degrees C because of the abnormal thermolability of the oncogenic 21-kilodalton product of the viral Ki-ras gene. Thus tsK-NRK cells were rendered quiescent in a G0-G1 state by a 48-h incubation in serum-free medium at the nonpermissive, p21-inactivating temperature of 41 degrees C. The serum-starved cells could then be stimulated to transit G1 either as nontransformed cells by adding serum at 41 degrees C or as transformed cells by lowering the temperature to a p21-activating 36 degrees C. The viral p21 protein was as effective as serum in stimulating tsK-NRK cells to transit G1 and to start replicating DNA. While p21 effectively stimulated cells to transit G1 even in unconditioned, serum-free medium, they still needed cell-derived conditioning factors to subsequently divide. The p21 protein also enabled the cells to transit G1 in spite of an extracellular Ca2+ deficiency that inhibited the G1 transit of serum-stimulated cells. p21 activity was needed to stimulate both early and late G1 events. In contrast to serum, p21 did not stimulate total RNA or protein synthesis, but some RNA and protein synthesis must have been needed for the p21-driven G1 transit because it could be stopped by actinomycin D or cycloheximide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Briggs F. N. Microdetermination of calcium in biological material by automatic fluorometric titration. Anal Chem. 1968 Feb;40(2):339–344. doi: 10.1021/ac60258a056. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. G. Different extracellular calcium requirements for proliferation of nonneoplastic, preneoplastic, and neoplastic mouse cells. Cancer Res. 1977 Aug;37(8 Pt 1):2657–2661. [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Kleine L. P. Ca2+/phospholipid-dependent protein kinase activity correlates to the ability of transformed liver cells to proliferate in Ca2+-deficient medium. Biochem Biophys Res Commun. 1983 Aug 30;115(1):383–390. doi: 10.1016/0006-291x(83)91015-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. Partial characterization of the mitogenic action of pp60v-src, the oncogenic protein product of the src gene of avian sarcoma virus. J Cell Physiol. 1984 Aug;120(2):135–145. doi: 10.1002/jcp.1041200205. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. The mitogenic activity of pp60v-src, the oncogenic protein product of the src gene of avian sarcoma virus, is independent of external serum growth factors. Biochem Biophys Res Commun. 1984 Sep 17;123(2):411–417. doi: 10.1016/0006-291x(84)90245-6. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Franks D. J., Whitfield J. F., Durkin J. P. The mitogenic/oncogenic p21 Ki-RAS protein stimulates adenylate cyclase activity early in the G1 phase of NRK rat kidney cells. Biochem Biophys Res Commun. 1985 Oct 30;132(2):780–786. doi: 10.1016/0006-291x(85)91200-8. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp Cell Res. 1983 Jun;146(1):151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Poole B., Wibo M. Protein degradation in cultured cells. The effect of fresh medium, fluoride, and iodoacetate on the digestion of cellular protein of rat fibroblasts. J Biol Chem. 1973 Sep 10;248(17):6221–6226. [PubMed] [Google Scholar]
- Rudland P. S. Control of translation in cultured cells: continued synthesis and accumulation of messenger RNA in nondividing cultures. Proc Natl Acad Sci U S A. 1974 Mar;71(3):750–754. doi: 10.1073/pnas.71.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Swierenga S. H., Whitfield J. F., Boynton A. L., MacManus J. P., Rixon R. H., Sikorska M., Tsang B. K., Walker P. R. Regulation of proliferation of normal and neoplastic rat liver cells by calcium and cyclic AMP. Ann N Y Acad Sci. 1980;349:294–311. doi: 10.1111/j.1749-6632.1980.tb29534.x. [DOI] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Youdale T., Franks D. J. The roles of calcium and cyclic AMP in the stimulatory action of parathyroid hormone on thymic lymphocyte proliferation. J Cell Physiol. 1971 Dec;78(3):355–368. doi: 10.1002/jcp.1040780305. [DOI] [PubMed] [Google Scholar]