Abstract
Portal hypertension is caused by an increased intrahepatic resistance, a major consequence of cirrhosis. Endothelial dysfunction in liver sinusoidal endothelial cells (LSECs) decreases the production of vasodilators, such as nitric oxide (NO) and favors vasoconstriction. This contributes to an increased vascular resistance in the intrahepatic/sinusoidal microcirculation. Portal hypertension, once developed, causes endothelial cell (EC) dysfunction in the extrahepatic, i.e. splanchnic and systemic, circulation. Unlike LSEC dysfunction, EC dysfunction in the splanchnic and systemic circulation overproduces vasodilator molecules, leading to arterial vasodilatation. In addition, portal hypertension leads to the formation of portosystemic collateral vessels. Both arterial vasodilatation and portosystemic collateral vessel formation exacerbate portal hypertension by increasing the blood flow through the portal vein. Pathologic consequences, such as esophageal varices and ascites, result. While the sequence of pathological vascular events in cirrhosis and portal hypertension have been elucidated, the underlying cellular and molecular mechanisms causing EC dysfunctions are not yet fully understood. This review article summarizes the current cellular and molecular studies on EC dysfunctions found during the development of cirrhosis and portal hypertension with a focus on intra- and extrahepatic circulation. The article ends by discussing future directions of study for EC dysfunctions.
Keywords: liver cirrhosis, angiogenesis, vasodilatation, nitric oxide, portosystemic collateral circulation
INTRODUCTION
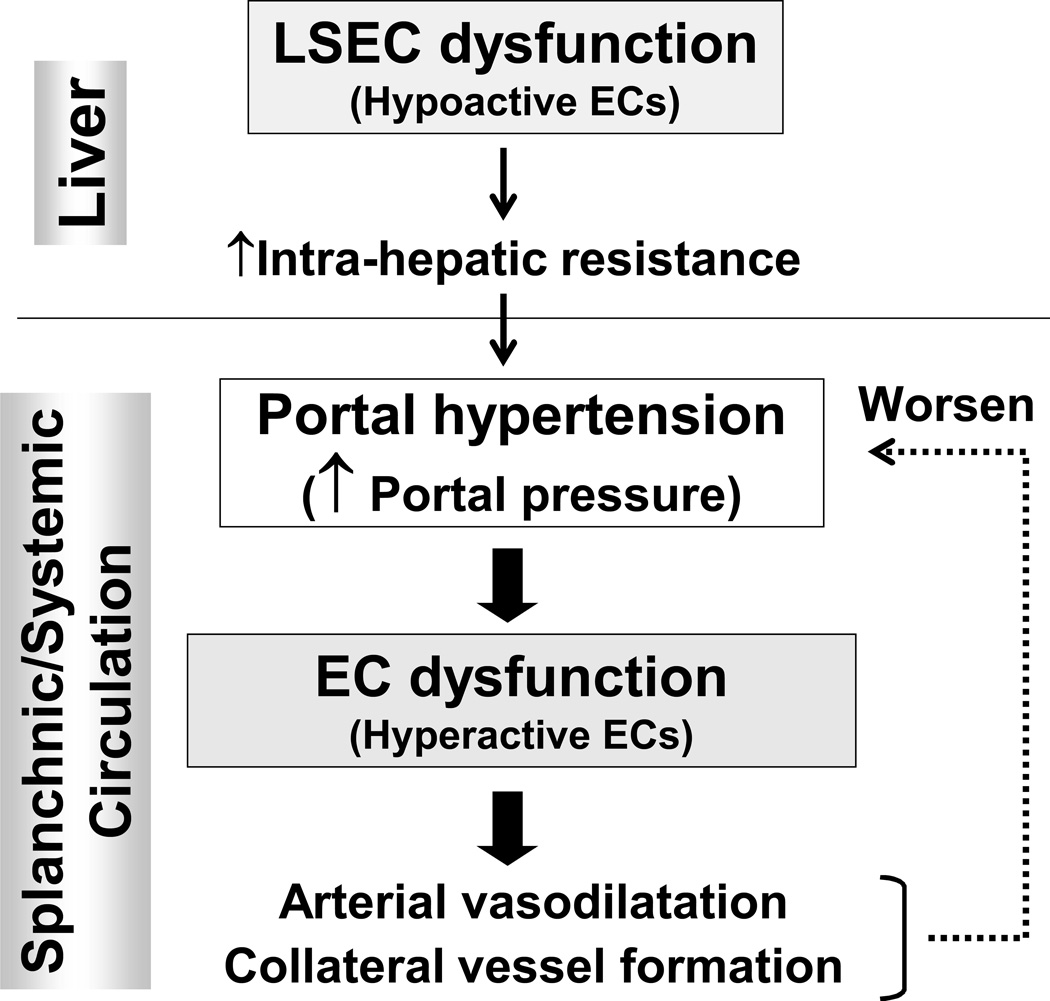
Portal hypertension is a detrimental complication resulting from liver cirrhosis (1, 2). Hepatic and non-hepatic endothelial dysfunction is a key factor that causes and worsens portal hypertension (3) (Figure 1). In the hepatic microcirculation, hypoactive endothelial cells (ECs), contribute to an increased intrahepatic resistance mainly by decreasing nitric oxide (NO) production, which in turn initiates portal hypertension. Portal hypertension, once it develops, affects extrahepatic vascular beds in the splanchnic and systemic circulation, leading to arterial vasodilatation and collateral vessel formation, resulting in greater blood flow into the portal vein. This increase in portal blood flow further exacerbates portal hypertension (1, 2). In contrast to hypoactive ECs in the hepatic microcirculation, ECs in the splanchnic and systemic circulation are hyperactive and increase NO production. Thus, ECs with opposing phenotypes are found in the intra- vs. extrahepatic circulation. Both contribute to the development and exacerbation of portal hypertension (3).
Figure 1. Overview of the development and consequences of portal hypertension in liver cirrhosis.
Endothelial cell (EC) dysfunction plays important roles in the pathophysiology of portal hypertension. LSEC; liver sinusoidal endothelial cell.
With knowledge of vascular biology, our understanding of the pathogenesis of portal hypertension has significantly advanced, revealing how vascular abnormalities both in and outside the liver contribute to portal hypertension, i.e. how these EC dysfunctions relate to those vascular abnormalities. However, how these EC dysfunctions occur in cirrhosis and portal hypertension still remains to be elucidated, particularly at the cellular and molecular levels.
This review article thus summarizes current cellular and molecular studies on EC dysfunctions in cirrhosis and portal hypertension, first in the area of the intrahepatic/sinusoidal microcirculation and second in the extrahepatic, i.e. splanchnic and systemic, circulation. This article concludes with a discussion of the future directions that the study of EC dysfunctions and the vascular abnormalities associated with them will take in relation to cirrhosis and portal hypertension.
1. Intrahepatic microcirculation
1-1. Overview - hypoactive ECs
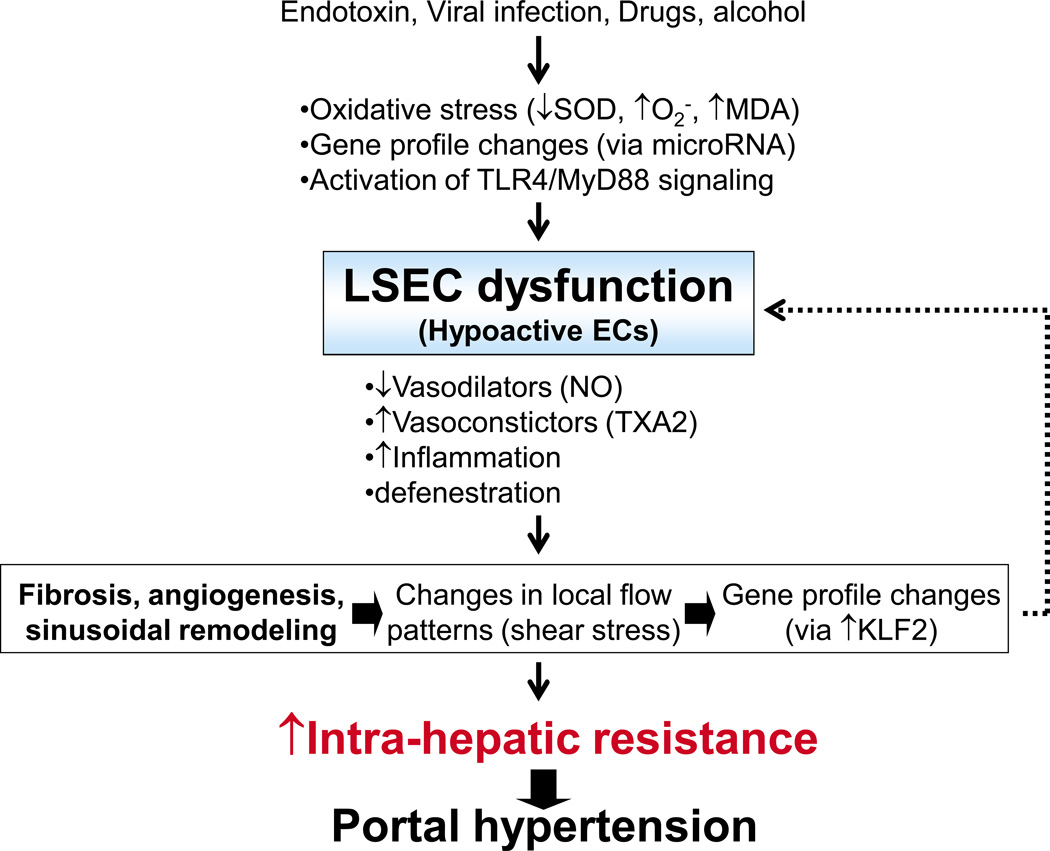
Endothelial cells are the first line of defense protecting the liver from injury (3). EC dysfunction seen in the intrahepatic/sinusoidal microcirculation is mainly due to hypoactive ECs, which contributes to an increased intrahepatic vascular resistance. This is the main cause of portal hypertension (1–3). The major hepatic ECs are liver sinusoidal ECs (LSECs), comprising ~98% of the total. Lymphatic and non-sinusoidal ECs comprise the remainder (4). Thus, the EC dysfunction observed in the intrahepatic circulation is apparently due to LSECs. LSECs have far-reaching effects regulating liver functions, including blood clearance, vascular tone, immunity, hepatocyte growth (4) and angiogenesis/sinusoidal remodeling (5). Thus, LSEC dysfunction results in a pathology contributing to impaired vasomotor control (primarily vasoconstrictive), inflammation, fibrosis, impaired liver regeneration (4) and pathological angiogenic/sinusoidal remodeling (5). These factors facilitate the development of cirrhosis and portal hypertension. Recent findings regarding the factors that cause LSEC dysfunction and its consequences are discussed (Figure 2).
Figure 2. Overview of liver sinusoidal endothelial cell (LSEC) dysfunction in intrahepatic/sinusoidal circulation, which leads to increased intrahepatic resistance and consequent portal hypertension.
SOD; superoxide dismutase, O2−; superoxide radical, MDA; malondialdehyde, TLR4; toll-like receptor 4, MyD88; myeloid differentiation protein, TXA2; thromboxine A2, NO; nitric oxide, H2S; hydrogen sulfide, KLF2; Kruppel-like factors.
1-2. Causes of LSEC functions
1-2-1. Oxidative stress attenuates NO bioavailability and eNOS activity in LSECs
Oxidative stress can cause EC dysfunction. In fact, patients with cirrhosis have significantly elevated levels of circulating malondialdehyde (MDA), an indicator of oxidative stress (6). It was demonstrated that administration of the antioxidant vitamin C to cirrhotic patients markedly attenuates postprandial increases in hepatic venous pressure, i.e., portal pressure, and significantly decreases MDA levels, suggesting increased oxidative stress in cirrhotic patients contributes to portal hypertension. In the LSECs of cirrhotic livers, there is increased production of the superoxide radical (O2−), which reacts with NO to form peroxynitrite (ONOO−). Consequently, NO bioavailability in the hepatic circulation is decreased(7), favoring vasoconstriction. Xanthine oxidase and cyclooxygenase (COX), but not NADPH oxide, are shown to be the primary cause of these elevated O2− levels. Furthermore, decreased activity of superoxide dismutase (SOD), an O2− scavenging enzyme, also contributes to elevated O2− levels in cirrhotic livers (7). Adenoviral delivery of SOD to cirrhotic livers attenuates O2− levels and can ameliorate portal hypertension (8). Collectively, these observations indicate that oxidative stress in LSECs results in EC dysfunction and that antioxidants such as vitamin C and SOD help to ameliorate portal hypertension.
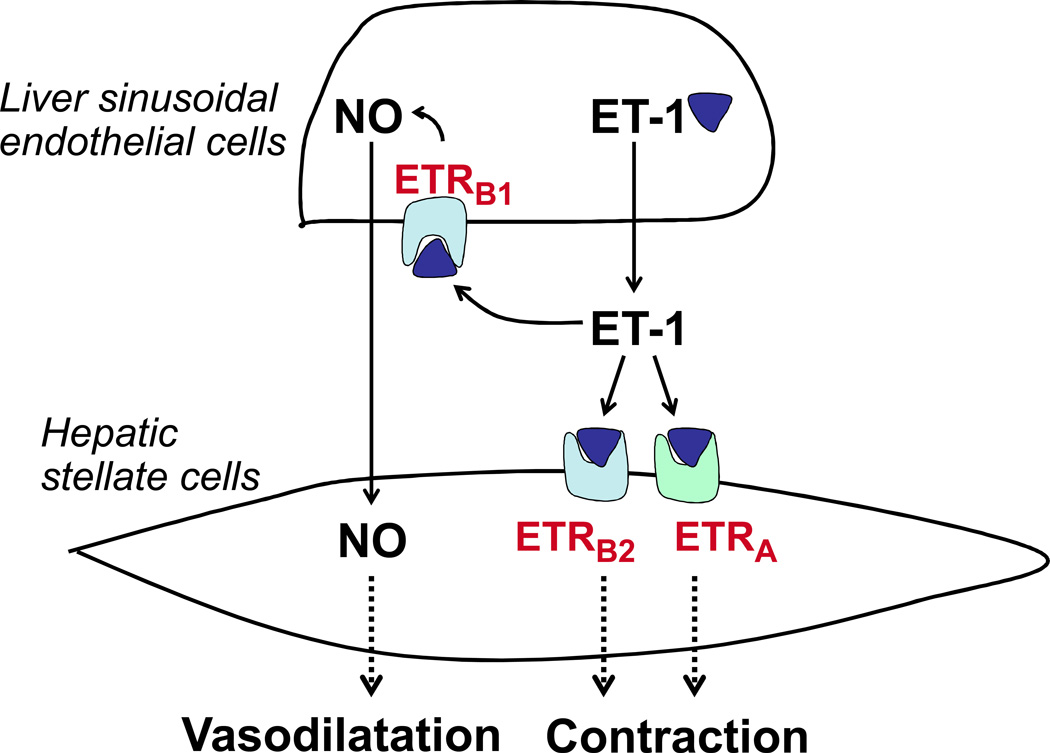
It is known that oxidative stress impairs endothelial NO synthase (eNOS) activity at least in three ways: 1) by increasing association of eNOS with caveolin-1, an inhibitor of eNOS activity, 2) by inhibiting endothelin-1 (ET-1)-induced eNOS phosphorylation, and 3) by increasing dissociation of eNOS from ET receptor B (ETRB), presumably the ETRB1 component. Taken together these lead to decreased NO production, a characteristic of EC dysfunction (9) (Figure 3).
Figure 3. Endothelin-1 (ET-1) mediates both vasodilatation and vasoconstriction through ET-1 receptors in the intrahepatic/sinusoidal microcirculation.
Endothelin type-A receptors (ETARs) reside in hepatic stellate cells (HSCs) and vascular smooth muscle cells, and mediate vasoconstriction. ET type-B receptors (ETBRs) induces both vasoconstriction and vasodilatation depending on cellular location. ETB receptors, residing on endothelial cells (known as ETB1 receptors), cause vasodilatation through nitric oxide (NO) production, whereas ETB2 receptors on HSCs and smooth muscle cells can induce vasoconstriction, similar to ETA receptors (27–29).
1-2-2. MicroRNAs regulate LSEC gene expression
MiRNAs are short non-coding RNAs that generally function as negative regulators of their target gene expression (10). While an increasing number of studies has demonstrated important regulatory roles of miRNAs in hepatic gene expression in many pathological conditions, little is known about miRNA regulation of LSEC gene expression in cirrhosis and portal hypertension. To date, one study carried out by Yeligar et al. (11) that miRNAs control gene expression in LSECs. Using an experimental model of alcohol-induced liver injury, they showed that elevated concentrations of ethanol can decrease miR-199 levels in LSECs. MiR-199 is known to bind to the untranslated region (UTR) of ET-1 mRNAs and decrease their levels. Thus, overexpression of miR-199 reduces ET-1 mRNA expression in LSECs. Conversely, blocking miR-199 increases ET-1 mRNA expression. Therefore, it is considered that ethanol increases intrahepatic resistance by suppressing miR-199, which increases expression of miR-199-target genes such as ET-1, an important regulator of vascular tone. Since ethanol increases portal pressure (12), it is possible that ethanol changes expression of miRNAs, such as miR-199, and causes LSEC dysfunction that facilitates an increase in intrahepatic resistance. These miRNAs can be a novel target for the attenuation of an increased intrahepatic resistance in cirrhosis, thereby ameliorating portal hypertension.
1-2-3. Toll-like receptors mediate LSEC signaling
LSECs express TLRs (13, 14). Toll-like receptor 4 (TLR4), a receptor of endotoxin, is expressed in LSECs and regulates angiogenic responses (13). Using TLR4-mutant (MT) mice, which express a spontaneous mutation conferring loss of TLR4 function, Jagavelu et al. (13) showed that LSECs isolated from TLR4-MT mice have diminished tubulogenesis, an indicator of angiogenic capacity of vascular cells in response to bacterial endotoxin, lipopolysaccharide (LPS). TLR4 conveys downstream signals through the adapter molecule, myeloid differentiation protein 88 (MyD88), and a MyD88-independent pathway (15). Overexpression of MyD88 in human LSECs results in enhanced tubulogenesis. The peptide IMG-2005-1 blocks MyD88 function by inhibiting homodimerization of MyD88 and suppresses tubulogenesis. Given that angiogenesis is postulated to contribute to portal hypertension by enhancing fibrogenesis (5, 16), collectively, these observations suggest that LSECs, being the first cells exposed to portal venous LPS, mediate angiogenesis through the TLR4/MyD88 signaling pathway and result in fibrosis and portal hypertension.
1-2-4. Mechanical stimulus such as shear stress changes gene expression in LSECs
Sinusoidal distortion resulting from fibrosis/cirrhosis alters the flow pattern in the intrahepatic microcirculation. LSECs sense these hemodynamic changes and influence cellular functions by changing their gene expression. For example, Kruppel-like factors (KLFs) are transcription factors that regulate cellular growth and tissue development. One of the KLFs, KLF2, is highly expressed in vascular ECs and protects the endothelium by upregulating the expression of a wide variety of vasoprotector genes (17, 18), including eNOS (19).
Shear-stress is the most potent known inducer of KLF2 expression (20–22). A study by Gracia-Sancho et al. (22) showed that LSECs express KLF2 in response to shear stress and that KLF2 expression is increased in cirrhotic liver tissue. The increased KLF2 then changes gene expression patterns in LSECs by increasing expression of its target vasoactive agents, such as eNOS, thrombomodulin (a blood coagulation inhibitor) and c-type natriuretic peptide (CNP). However, since there was no increase in eNOS and CNP protein levels, changes in the stability of eNOS mRNA and CNP mRNA may also play a role. In contrast, thrombomodulin protein levels remain significantly higher in cirrhotic compared to normal livers. Collectively, these observations indicate that changes in flow-mediated mechanical forces may worsen LSEC dysfunction by altering gene expression patterns.
1-3. Consequences of LSEC dysfunction
1-3-1. LSEC dysfunction impairs regulation of vascular tone
LSECs play an important role in the regulation of hepatic vascular tone by releasing vasoactive molecules (1, 2). However, as a consequence of LSEC dysfunction, LSECs lose their vasodilatory properties. They decrease the production/bioavailability of vasodilator molecules (such as NO) and increase vasoconstrictor molecules (such as thromboxane A2), thereby increasing intrahepatic resistance through the mechanisms described below.
Nitric oxide (NO) is probably the most important vasodilator regulating vascular tone in the intrahepatic microcirculation. It is produced primarily by eNOS (23, 24) in LSECs. In cirrhotic livers, NO levels decrease and contribute to an increased intrahepatic vascular resistance. Decreased NO levels result from at least two mechanisms. As mentioned earlier, one is the decreased bioavailability of NO due to increased superoxide radicals (O2−) (7), which quench NO. The other is due to a decreased activity of eNOS itself (3, 25, 26). In general, the level of eNOS is not different between normal and cirrhotic livers (22, 24). However, in cirrhotic livers, complex post-translational modifications of eNOS result in a decrease in its enzymatic activity (3, 25, 26). For example, the increased association of eNOS with caveolin-1, a negative regulator of eNOS, inhibits NO production (24). Another important regulatory pathway of eNOS is via endothelin-1 (ET-1). ET-1 has dual vasoactive effects (Figure 3). It promotes vasoconstriction by binding to cell surface receptors on hepatic stellate cells (HSCs): endothelin A receptor (ETRA) and endothelin B2 receptor (ETRB2) (27, 28). ET-1 also promotes vasodilation by binding to endothelin B1 receptor (ETRB1) receptors on LSECs and thus activating eNOS (29). However, for example, endotoxin increases caveolin-1 expression, which facilitates caveolin-1’s binding to eNOS and consequently blocks the ET-1-induced eNOS activation in LSECs (30, 31).
Thromboxane A2 (TXA2), a vasoconstrictor molecule, is generated by the action of cyclooxygenase-1 (COX-1) in LSECs. In cirrhotic liver, TXA2 production is increased due to LSEC dysfunction and contributes to an increased intrahepatic resistance. It has been shown that blocking TXA2 (by a prostaglandin H2/TXA2 receptor blocker, SQ-29548) or inhibiting COX-1 activity (by a COX-1 inhibitor, SC-560) can be beneficial for attenuating an increased intrahepatic resistance in fibrotic/cirrhotic livers (32, 33).
1-3-2. LSEC dysfunction facilitates hepatic inflammation
LSECs are primary mediators of hepatic immune tolerance. After injury from hepatotoxins, LSECs become defenestrated (34) and promote inflammation, secreting an array of cytokines and chemokines (35). Accordingly, LSEC dysfunction enhances capacity for antigen capture and induces T-cell proliferation. Furthermore, in fibrotic livers, as a result of their dysfunction, LSECs do not reject dendritic cell priming of T-cells and subsequently enhance immunogenicity. This results in increased inflammation in the intrahepatic circulation (36).
One recently proposed mechanism of regulation of immunogenicity involves a molecule expressed in LSECs called LSEC-specific lectin (LSECtin) (37). LSECtin recognizes activated T-cells and inhibits their immune response. Mice lacking LSECtin gene exhibit accelerated T-cell-mediated immune response and enhance liver injury. Conversely, exogenous administration of recombinant LSECtin protein or plasmid ameliorates liver injury by suppressing T-cell immune response. It is not known whether LSEC dysfunction decreases LSECtin expression in LSECs in liver cirrhosis. However, this study indicates that molecules such as LSECtin expressed in LSECs and ameliorating inflammation could provide a novel approach for treating cirrhosis and portal hypertension.
1-3-3. LSEC dysfunction exhibits defenestration
Pathophysiological alterations of LSEC structure result in a wide range of adverse effects on the liver and on metabolism (38). LSECs are perforated with fenestrations, pores that facilitate the transfer of lipoproteins and macromolecules between blood and hepatocytes. Loss of this pore structure, termed defenestration, is caused by exposure to various pathologic endogenous and exogenous agents resulting in significantly impaired hepatic function.
Defenestration entails endothelial thickening and the deposition of excessive extracellular matrix in the subendothelial space of Disse. Altogether these changes are referred to as cirrhotic capillarization and impede the transfer of many substrates from the sinusoidal lumen to hepatocytes through the space of Disse. This capillarization is considered to contribute to an increase in intrahepatic resistance (1). The review article by Cheluvappa et al. (38) details LSECs and fenestration.
1-4. Summary – intrahepatic microcirculation
LSECs regulate a wide range of liver functions. In response to various agents, such as bacterial endotoxin, viruses, drug, and ethanol, LSECs experience oxidative stress (6–8), alteration in gene expression via miRNAs, such MiR199-regulation of ET-1 mRNA expression (11), and activation of pathways, such as TLR4/MyD88 signaling (13, 14), and become dysfunctional (Figure 2). Furthermore, mechanical stimuli such as shear stress changes gene expression pattern via a transcription factor KLF2 in cirrhotic livers and worsens LSEC dysfunction (22). LSEC dysfunction is characterized by vasoconstriction brought about by decreasing levels of vasodilators (NO) (2, 3, 23–25) or by increasing levels of vasoconstrictors (TXA2) (32, 33). Further, LSEC dysfunction results in increased inflammation due to impaired immune tolerance and defenestration (34–36, 38). LSEC dysfunction facilitates fibrosis/cirrhosis and angiogenesis/pathological sinusoidal remodeling, which worsens LSEC dysfunction, and increases intrahepatic resistance, leading to portal hypertension.
2. Splanchnic and systemic circulations
2-1. Overview – hyperactive ECs
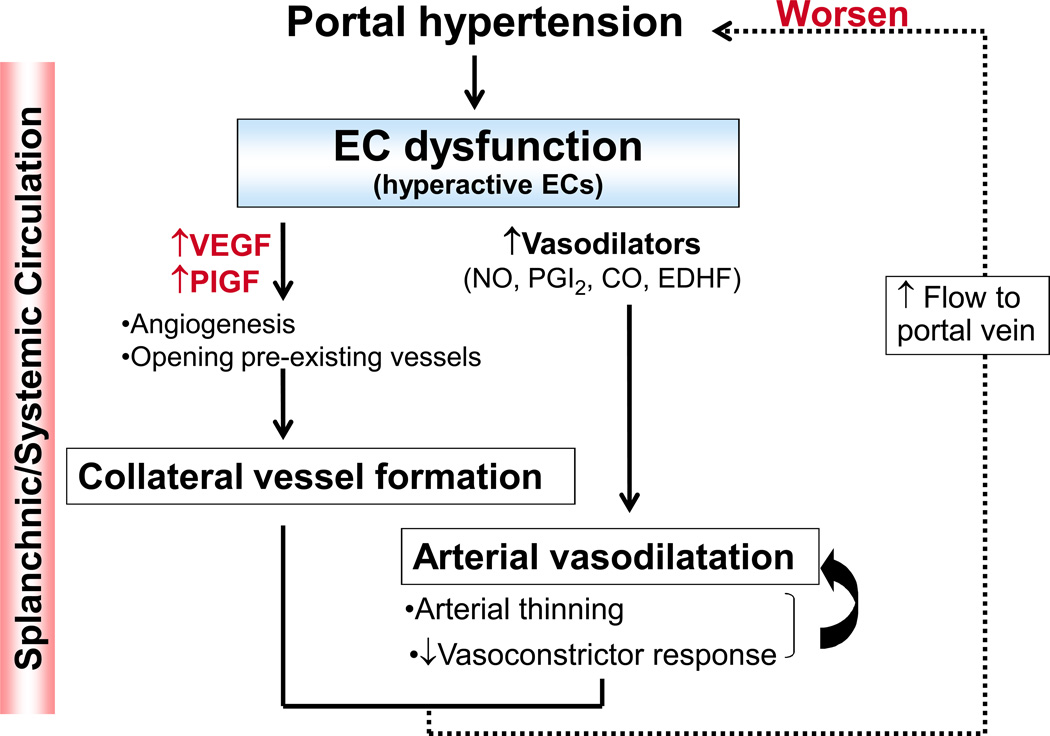
An increase in portal pressure triggers mechanical signals that induce diverse vascular changes in the splanchnic and systemic circulation (Figure 4). There are two major vascular changes that develop in response to an increase in portal pressure. One is arterial vasodilatation, and the other is formation of portosystemic collateral vessels. These vascular changes increase blood flow to the portal vein, thereby exacerbating portal hypertension, as reviewed elsewhere (1, 2, 39, 40). Alterations in EC functions are central to the vascular changes seen in the splanchnic and systemic circulation during portal hypertension. In contrast to hypoactive LSECs in the intrahepatic microcirculation, “hyperactive” ECs characterized by increased NO production play a critical role in the vascular changes in the splanchnic and systemic circulation (3, 41). Molecular and cellular mechanisms that cause hyperactive ECs and subsequent vascular changes that in turn worsens portal hypertension are discussed in this section.
Figure 4. Overview of endothelial cell (EC) dysfunction/hyperactivation in the arterial splanchnic and systemic circulation, leading to increased blood flow through the portal vein and a consequent worsening of portal hypertension.
VEGF; vascular endothelial growth factor, PIGF; placental growth factor, NO; nitric oxide, PGI2; prostacyclin, CO; carbon monoxide, EDHF; endothelium-derived hyperpolarizing factor.
2-2. Portal pressure triggers EC dysfunction/hyperactivation in the splanchnic and systemic circulations
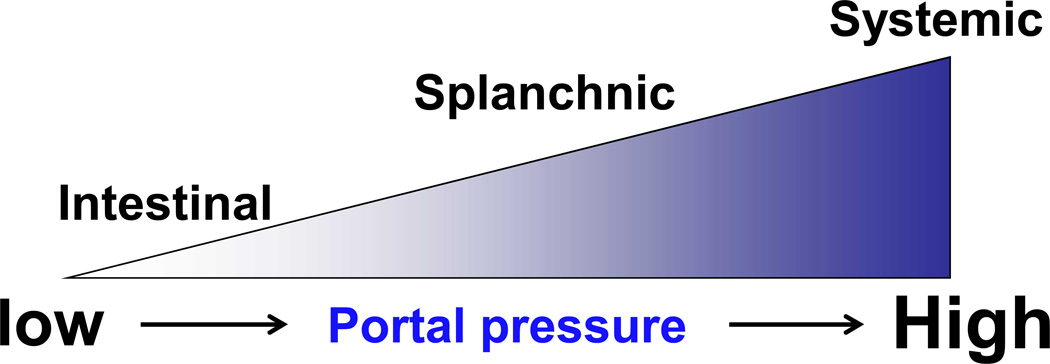
A study by Abraldes et al. (42) demonstrated that portal pressure is sensed at different vascular beds depending on the severity of portal hypertension (Figure 5). A small increase in portal pressure is first sensed by the intestinal microcirculation, followed by the arterial splanchnic circulation (e.g., the mesenteric arteries) and then arterial systemic circulation (e.g., the aorta). Thus, the intestinal microcirculation serves as a “sensing organ” to portal pressure (43). These observations were demonstrated using a surgical technique termed partial portal vein ligation (PVL) (2), which enables varying degrees of portal hypertension to be created in a rat model. The portal vein is ligated along with needles of different gauges. When the needle is removed at the end of surgery, luminal openings of varying diameters are created. In this way, different stages of portal hypertension are developed proportional to the degree of luminal diameter. This model allows us to determine how changes in portal pressure specifically affect local vascular beds/ECs without influencing liver functions (2).
Figure 5. Portal pressure is sensed at different vascular locations depending on the severity of portal hypertension.
Local nitric oxide (NO) production is affected by the severity of portal hypertension. The intestinal microcirculation may be the most sensitive to changes in portal pressure, followed by the arterial splanchnic circulation (e.g., the mesenteric arteries) and then arterial systemic circulation (e.g., the aorta). The diagram adapted from Iwakiri (43).
When mild portal hypertension is induced, the change in portal pressure is too low to induce splanchnic arterial vasodilatation. However, there is a significant increase in the production of intestinal vascular endothelial growth factor (VEGF) with a subsequent increase in the expression of eNOS in the intestinal microcirculation (42). This model of mild portal hypertension is most likely representative of the portal pressure changes observed in early-stage cirrhosis, during which portal hypertension generally progresses slowly. When a certain level of portal pressure is reached, vasodilatation in the arterial splanchnic circulation develops. It is postulated that mechanical forces generated as a result of increased portal pressure, presumably cyclic strains and shear stress generated by an increased blood flow, activate eNOS, leading to NO production (42–44). A study using rats with severe portal hypertension induced by PVL surgery supports this. Rats with severe portal hypertension showed eNOS up-regulation in both the intestinal microcirculation (45) and the splanchnic arterial circulation (46). Finally, the systemic circulatory abnormalities seem to occur (43).
2-3. Arterial vasodilatation causes increased blood flow to the portal vein
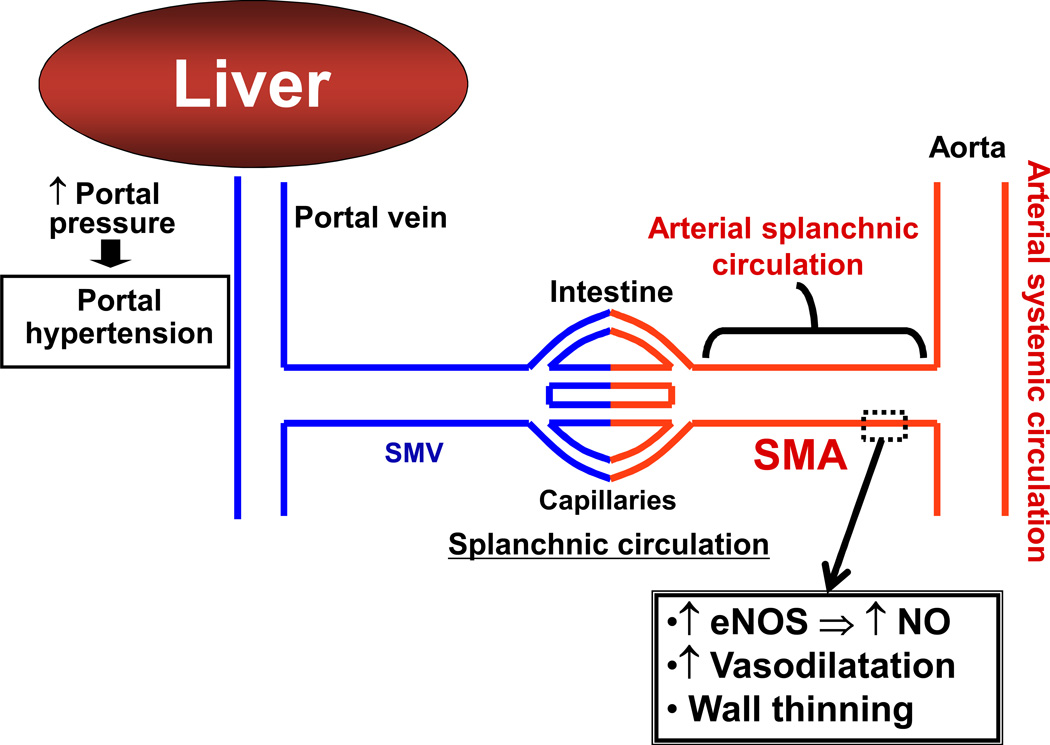
Arterial vasodilatation in the splanchnic and systemic circulation is mediated by an increased production of vasodilator molecules in ECs. Subsequently, chronic overproduction of NO, for example, contributes to thinning of arterial walls, which may help to sustain arterial vasodilatation and to attenuate a response to vasoconstrictors (Figure 6). This decreased response to vasoconstrictors is also mediated by impaired signaling through smooth muscle cells [see a review article by Hennenberg et al. (47)]. Splanchnic arterial vasodilatation is a prerequisite for the development of the hyperdynamic circulatory syndrome observed in patients with cirrhosis and portal hypertension. This syndrome is characterized by decreased mean arterial pressure, increased cardiac output and decreased peripheral resistance. These changes can ultimately lead to life-threatening complications such as esophageal varices and ascites (48, 49).
Figure 6. Arterial vasodilatation in the splanchnic and systemic circulation in portal hypertension.
In response to increased portal pressure, raised levels of vasodilators, such as nitric oxide (NO), cause subsequent vasodilatation. NO overproduction causes arterial wall thinning, which may also contribute to sustained vasodilatation. SMA: superior mesenteric artery, SMV: superior mesenteric vein, eNOS: endothelial nitric oxide synthase.
2-3-1. Increased levels of vasodilators
eNOS-derived NO has been considered the most important vasodilator molecule leading to excessive arterial vasodilatation in the splanchnic and systemic circulation in portal hypertension. Other important vasodilator molecules involved in arterial vasodilatation include carbon monoxide (CO), prostacyclin (PGI2), endocannabinoids such as anandamide, and endothelium-derived hyperpolarizing factor (EDHF) (2, 3, 25). Hydrogen sulfide (H2S) is a vasodilator in the aorta (50, 51) and mesenteric arteries (52). However, the role of H2S in arterial vasodilatation in splanchnic and systemic circulations has not yet been explored. Considering its role in the regulation of vascular tone and arterial pressure (53), it is highly possible that this molecule may have a role as an arterial vasodilatator in both splanchnic and systemic circulation.
2-3-2. Arterial wall thinning
Endothelial-derived NO plays a central role in regulating the structure of the vessel wall (54). Using cirrhotic rats with ascites, studies showed intensive arterial thinning, as indicated by a decreased thickness of the vascular walls of the thoracic aorta, abdominal aorta, mesenteric arteries and renal artery (55, 56). Treatment with a NOS inhibitor significantly improved wall thickness and attenuated the degree of hyperdynamic circulatory syndrome by increasing arterial pressure and peripheral resistance (55). These observations suggest that increased endothelium-derived NO, at least in part, contributes to arterial wall thinning. Understanding the mechanisms of arterial wall thinning will be important in the development of useful treatments for patients with portal hypertension.
2-4. Portosystemic collateral vessel formation
In addition to arterial vasodilatation in the splanchnic and systemic circulation, the development of portosystemic collateral vessels is thought to worsen portal hypertension (1, 2). The development of collateral blood vessels is probably an adaptive response to an increased portal pressure, which may help to delay development of the pathology. However, the formation of these vessels can cause detrimental complications. Since the vessels are fragile, they rupture easily, causing esophageal and gastric variceal bleeding. Furthermore, since these vessels bypass the liver, portal blood carrying toxic substances, such as drugs, bacterial toxins and toxic metabolites, returns to the systemic circulation and can result in portal-systemic encephalopathy and sepsis (1, 2). These collateral vessels are formed by the enlargement of pre-existing vessels and by angiogenesis (40, 41). The process of angiogenesis is modulated by growth factors exhibiting vasodilatory activity, such as VEGF. This can contribute to an increase in blood flow through the portal vein, exacerbating portal hypertension (40). How are these angiogenic growth factors upregulated? One mechanism would be triggered by an increase in portal pressure. Studies using portal hypertensive rats indicated that a sudden increase in portal pressure is sensed at the intestinal microcirculation and induces VEGF expression as described previously (42, 45). This generates local mechanical forces, which can induce VEGF expression.
Anti-angiogenic agents, such as blockers of VEGF receptor-2 (SU5416, anti-VEGFR2 monoclonal antibody) (45, 57) and inhibitors of receptor tyrosine kinases (Sorafenib and Sunitinib) (58, 59), have been shown to decrease portosystemic collateral vessel formation and reduce portal pressure. Besides VEGF, another member of the VEGF family, placental growth factor (PIGF), has been shown to be up-regulated in the intestinal microcirculation of portal hypertensive mice (60). Portal hypertensive mice lacking PIGF or given anti-PIGF monoclonal antibody show a decrease of portal pressure and portosystemic collateral vessel formation. Collectively, these studies suggest that blocking angiogenic activity, thereby reducing collateral blood vessel formation, is beneficial for the treatment of portal hypertension.
2-5. Circulating endothelial cell levels are elevated in patients with cirrhosis and portal hypertension
It is not certain whether levels of circulating ECs (CECs) are related to EC dysfunction. Furthermore, CECs may come from not only the splanchnic and systemic circulation, but also from the intrahepatic circulation. However, the CEC is still an interesting topic that has received attention in recent years, and is discussed briefly. Elevated levels of CECs have been observed in a variety of disease conditions associated with vascular injury (61–63), and are considered to reflect the severity of vascular injury (64). A recent study by Abdelmoneim et al. (63) reported an interesting observation that CEC levels are significantly higher in patients with cirrhosis compared to healthy subjects. Cirrhosis and portal hypertension are characterized by prominent changes in the vascular endothelium of both the intrahepatic and splanchnic/systemic circulations (1). Furthermore, changes in the levels of inflammatory cytokines and vasoactive molecules, such as tumor necrosis factor (TNF) and NO, respectively, may also contribute to changes in the endothelium and increase peripheral CEC levels (65–67).
The origin of CECs in patients with cirrhosis and portal hypertension is not known. Originally, it was thought that CECs may derive from mature ECs shed from the vessel walls in response to vascular injury, or from endothelial progenitor cells (EPCs), which are bone marrow-derived and are assumed to contribute to vascular repair (68–70). However, the study by Abdelmoneim et al. (63) showed that CECs observed in patients with cirrhotic and portal hypertension are a mature EC population, not EPCs. This is indicated by the CECs being positive for CD146 and CD105 markers, but negative for CD34, a marker for EPCs (63). This may indicate these CECs arise from ECs released from the damaged vasculature of patients with cirrhosis and portal hypertension. The mechanism of their release, the degree to which they are still functional, and their eventual fate require further research.
2-6. Summary – splanchnic and systemic circulation
In portal hypertension, ECs in the splanchnic and systemic circulation are hyperactive, producing increased levels of NO derived from eNOS (1–3). This causes arterial vasodilatation and thinning of arterial walls in the splanchnic and systemic circulation (1–3, 55, 56). Hyperactive ECs contribute to angiogenic collateral vessel formation (42, 45). An increase in portal pressure is a key factor that causes hyperactive ECs in the splanchnic and systemic circulation (42, 43). Studies using surgically-induced portal hypertensive rats demonstrated that changes in portal pressure are first sensed by the vascular beds of the intestinal microcirculation, followed by arteries in the splanchnic and then systemic circulation(42). An increase in portal pressure induces production of VEGF and PIGF, potent angiogenic growth factors that facilitate collateral vessel formation (57–60). Blocking these angiogenic growth factors attenuates collateral vessel formation and ameliorates portal hypertension, suggesting potential therapeutic targets for the treatment of portal hypertension. Circulating mature ECs are increased in patients with cirrhosis and portal hypertension (63). The cause of increased levels of CECs in these patients, whether they are still functional, and their fate require further research.
3. Future direction
The basic pathology and mechanisms of portal hypertension can be considered to be closely related to those for cardiovascular disease (1, 2). Thus, a thorough familiarity with cardiovascular disease in particular and vascular biology in general will be of great advantage in the study of portal hypertension and our understanding of the cellular and molecular mechanisms of vascular change underlying it. It has become increasingly clear in the study of vascular biology that mediating factors, such as miRNAs and EC progenitor cells, involve the regulation of vascular changes, such as angiogenesis and vascular remodeling in vascular homeostasis and pathophysiological conditions. The roles of inflammation and endothelial to mesenchymal transition (EndoMT) in EC dysfunction are another significant areas in the study of portal hypertension. This section will briefly discuss potential roles of these mediators/factors in the vascular changes seen in cirrhosis and portal hypertension.
3-1. microRNAs
A growing body of evidence suggests that microRNAs (miRNAs) are crucial regulators of a wide range of the events occurring during gene expression that are associated with many biological processes, such as angiogenesis, vessel remodeling, fibrosis and apoptosis (71). Because of their involvement in various biological processes, dysregulation of miRNA expression leads to a number of human diseases (72). Identification of miRNAs and miRNA-target genes related to vascular changes in portal hypertension will provide a tremendous opportunity to elucidate the mechanisms of hepatic angiogenesis, collateral vessel formation and arterial wall thinning, seen in cirrhosis and portal hypertension. Since many miRNAs exhibit a strikingly specific pattern based on cell type (73–75), identification of relevant miRNAs would be more successful by performing it in a cell-specific manner, rather than in a procedure involving the whole vasculature. Recognizing this would make the identification of relevant miRNAs more efficient in the determination of new therapeutic targets for the amelioration of the pathologic vascular changes seen in cirrhosis and portal hypertension.
3-2. Endothelial progenitor cells (EPCs)
Circulating EPCs enhance angiogenesis and vascular repair. However, in recent years the definition of EPCs has become controversial (76). It has become increasingly clear that so-called “EPCs” are not genuine endothelial progenitors, but are predominantly of monocytic lineage (77). Accordingly, the name EPC may not be appropriate. Nevertheless, these so-called “EPCs” exhibit angiogenic activity and play a role in vascular repair (76). Therefore, it is possible that “EPCs” may contribute to both the newly formed vessels of the liver and the portosystemic collateral vessels that appear during the progression of cirrhosis and portal hypertension. Furthermore, circulating ECs (CECs), which do not seem to be EPCs and are found to be elevated in patients with cirrhosis and portal hypertension as mentioned previously (63), may play a role in this aspect. It is further possible that both CECs and “EPCs” may be the origin for ECs in the newly formed vessels in cirrhosis and portal hypertension.
3-3. Endothelial-mesenchymal/myofibroblast transition (EndoMT)
EndoMT has been implicated in the pathogenesis of fibrosis in various organs. Furthermore, EndoMT involves a process similar to that found in epithelial-mesenchymal transition (EMT). As its name implies, during EndoMT, ECs exhibit gene expression patterns similar to those of myofibroblasts, including gene expression of α-smooth muscle actin (α-SMA), fibroblast specific protein-1 (FSP-1) and collagen type I (78). It is tempting to speculate that the loss of fenestrae in LSECs during the development of cirrhosis could relate to EndoMT. Furthermore, EndoMT of LSECs, by gaining gene expression patterns similar to those of myofibroblasts, may contribute to an increased intrahepatic resistance found in cirrhosis and portal hypertension.
3-4. Inflammation
Inflammation is coupled with angiogenesis in many pathological conditions, such as atherosclerosis and diabetes. One of the consequences of inflammation in the vasculature is an increase in EC permeability, frequently induced by VEGF, NO or other mediators. As a result, increased permeability permits plasma components and immune cells to enter the subendothelial space from the bloodstream, initiating and sustaining the inflammatory response (79). In cirrhotic rats, it has reported that EC permeability significantly increases in the liver and mesenteric vascular beds, compared to normal rats (80). In this study, EC permeability was assessed by Evan’s Blue dye extravasation and colloidal carbon deposition at the basal lamina of ECs. This increase in EC permeability is accompanied by an elevated expression of VEGF-A and angiopoietin-2, and is attenuated by the administration of VEGFR2 blockers. These observations suggest that increased levels of VEGF-A and possibly angiopoietin-2, generated in response to inflammation occurring at the endothelium, may enhance EC permeability and facilitate angiogenesis observed in cirrhotic livers and in portosystemic collateral vessels during the development of cirrhosis and portal hypertension. It is tempting to speculate that increased inflammation and the subsequent increase in EC permeability lead to angiogenesis by destabilizing the EC junction.
CONCLUSION
Although portal hypertension leads to serious complications in the liver during the development of cirrhosis, there are few options for its treatment. Because of this there is a strong need for further study of the vascular changes associated with portal hypertension (81). Studying the role of miRNAs, EPCs, EndoMT and inflammation in the vascular changes associated with portal hypertension advances our understanding of the molecular and cellular mechanisms producing these changes. The practical benefit of this research enables the development of novel target molecules for the treatment of portal hypertension. Further, a cell-specific approach in vivo and in vitro (e.g., cell/tissue specific gene manipulation, drug delivery, etc.) will be important in understanding these mechanisms in detail. Finally, although this review article discussed mostly ECs, it is also relevant to understand the contribution of other cell types, such as HSCs, smooth muscle cells and immune cells, to EC dysfunctions and the subsequent vascular changes found in cirrhosis and portal hypertension.
Acknowledgment
I thank Mr. Jay Prendergast for editing and Dr. Teruo Utsumi for useful discussion.
Financial Support
This work was supported by grants from NIH/NIDDK (R01DK082600, K01DK067933) and Yale Liver Center Pilot Grant (P30-DK034987).
List of abbreviations
- ECs
endothelial cells
- MDA
malondialdehyde
- O2−
superoxide radical
- ONOO−
peroxynitrite
- SOD
superoxide dismutase
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- ETR
endothelin receptor
- CEC
circulating endothelial cell
- EPC
endothelial progenitor cell
- miRNA
microRNA
- EndoMT
endothelial-mesenchymal transition or endothelial-myofibroblast transition
- EMT
epithelial-mesenchymal transition
- α-SMA
alpha-smooth muscle actin
- FSP-1
fibroblast specific protein-1
REFERENCES
- 1.Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol. 2007;41(Suppl 3):S247–S253. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43(2) Suppl 1:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 3.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46(5):927–934. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53(5):976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Guerra M, Garcia-Pagan JC, Turnes J, et al. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43(3):485–491. doi: 10.1002/hep.21080. [DOI] [PubMed] [Google Scholar]
- 7.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, et al. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47(4):1248–1256. doi: 10.1002/hep.22166. [DOI] [PubMed] [Google Scholar]
- 8.Lavina B, Gracia-Sancho J, Rodriguez-Vilarrupla A, et al. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58(1):118–125. doi: 10.1136/gut.2008.149880. [DOI] [PubMed] [Google Scholar]
- 9.Karaa A, Kamoun WS, Xu H, Zhang J, Clemens MG. Differential effects of oxidative stress on hepatic endothelial and Kupffer cell eicosanoid release in response to endothelin-1. Microcirculation. 2006;13(6):457–466. doi: 10.1080/10739680600776278. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeligar S, Tsukamoto H, Kalra VK. Ethanol-induced expression of ET-1 and ET-BR in liver sinusoidal endothelial cells and human endothelial cells involves hypoxia-inducible factor-1alpha and microrNA-199. J Immunol. 2009;183(8):5232–5243. doi: 10.4049/jimmunol.0901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshita M, Takei Y, Kawano S, et al. Endogenous nitric oxide attenuates ethanol-induced perturbation of hepatic circulation in the isolated perfused rat liver. Hepatology. 1994;20(4 Pt 1):961–965. doi: 10.1002/hep.1840200427. [DOI] [PubMed] [Google Scholar]
- 13.Jagavelu K, Routray C, Shergill U, O'hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52(2):590–601. doi: 10.1002/hep.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Trippler M, Pei R, et al. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol. 2009;51(6):1037–1045. doi: 10.1016/j.jhep.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J Immunol. 2001;166(9):5688–5694. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 16.Taura K, De Minicis S, Seki E, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135(5):1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KP, Kern CB, Crable SC, Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol Cell Biol. 1995;15(11):5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11(22):2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116(1):49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dekker RJ, Van Thienen JV, Rohlena J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167(2):609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fledderus JO, Van Thienen JV, Boon RA, et al. Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood. 2007;109(10):4249–4257. doi: 10.1182/blood-2006-07-036020. [DOI] [PubMed] [Google Scholar]
- 22.Gracia-Sancho J, Russo L, Garcia-Caldero H, Garcia-Pagan JC, Garcia-Cardena G, Bosch J. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut. 2010 doi: 10.1136/gut.2010.220913. [DOI] [PubMed] [Google Scholar]
- 23.Shah V, Haddad FG, Garcia-Cardena G, et al. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. Journal of Clinical Investigation. 1997;100(11):2923–2930. doi: 10.1172/JCI119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117(5):1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 25.Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41(10) Suppl 3:S288–S294. doi: 10.1097/MCG.0b013e3181468b4c. [DOI] [PubMed] [Google Scholar]
- 26.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117(Pt 12):2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 27.Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213(2):815–823. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- 28.Bauer M, Bauer I, Sonin NV, et al. Functional significance of endothelin B receptors in mediating sinusoidal and extrasinusoidal effects of endothelins in the intact rat liver. Hepatology. 2000;31(4):937–947. doi: 10.1053/he.2000.5922. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Premont RT, Kontos CD, Huang J, Rockey DC. Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein betagamma subunit signaling to protein jinase B/Akt. J Biol Chem. 2003;278(50):49929–49935. doi: 10.1074/jbc.M306930200. [DOI] [PubMed] [Google Scholar]
- 30.Kwok W, Lee SH, Culberson C, Korneszczuk K, Clemens MG. Caveolin-1 mediates endotoxin inhibition of endothelin-1-induced endothelial nitric oxide synthase activity in liver sinusoidal endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G930–G939. doi: 10.1152/ajpgi.00106.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwok W, Clemens MG. Targeted mutation of Cav-1 alleviates the effect of endotoxin in the inhibition of ET-1-mediated eNOS activation in the liver. Shock. 2010;33(4):392–398. doi: 10.1097/SHK.0b013e3181be3e99. [DOI] [PubMed] [Google Scholar]
- 32.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47(2):220–227. doi: 10.1016/j.jhep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Graupera M, March S, Engel P, Rodes J, Bosch J, Garcia-Pagan JC. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;288(4):G763–G770. doi: 10.1152/ajpgi.00300.2004. [DOI] [PubMed] [Google Scholar]
- 34.Dobbs BR, Rogers GW, Xing HY, Fraser R. Endotoxin-induced defenestration of the hepatic sinusoidal endothelium: a factor in the pathogenesis of cirrhosis? Liver. 1994;14(5):230–233. doi: 10.1111/j.1600-0676.1994.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 35.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 36.Connolly MK, Bedrosian AS, Malhotra A, et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol. 2010;185(4):2200–2208. doi: 10.4049/jimmunol.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang L, Yang J, Liu W, et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology. 2009;137(4):1498–508. e1–e5. doi: 10.1053/j.gastro.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheluvappa R, Denning GM, Lau GW, Grimm MC, Hilmer SN, Le Couteur DG. Pathogenesis of the hyperlipidemia of Gram-negative bacterial sepsis may involve pathomorphological changes in liver sinusoidal endothelial cells. Int J Infect Dis. 2010;14(10):e857–e867. doi: 10.1016/j.ijid.2010.02.2263. [DOI] [PubMed] [Google Scholar]
- 39.Morales-Ruiz M, Jimenez W. Neovascularization, Angiogenesis, and Vascular Remodeling in Portal hypertension. Totowa, NJ: Humana Press; 2005. [Google Scholar]
- 40.Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006;44(1):209–216. doi: 10.1016/j.jhep.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Sumanovski LT, Battegay E, Stumm M, Van Der Kooij M, Sieber CC. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29(4):1044–1049. doi: 10.1002/hep.510290436. [DOI] [PubMed] [Google Scholar]
- 42.Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G980–G987. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 43.Iwakiri Y. The Systemic and Splanchnic Circulation. In: Gines P, Kamath PS, Arroyo V, editors. Chronic Liver Failure, Mechanisms and Management. New York, NY: Humana Press; 2011. [Google Scholar]
- 44.Tsai MH, Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125(5):1452–1461. doi: 10.1016/j.gastro.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodes J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43(1):98–103. doi: 10.1016/j.jhep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Iwakiri Y, Tsai MH, Mccabe TJ, et al. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol Heart Circ Physiol. 2002;282(6):H2084–H2090. doi: 10.1152/ajpheart.00675.2001. [DOI] [PubMed] [Google Scholar]
- 47.Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57(9):1300–1314. doi: 10.1136/gut.2007.144584. [DOI] [PubMed] [Google Scholar]
- 48.Colombato LA, Albillos A, Groszmann RJ. Temporal relationship of peripheral vasodilatation, plasma volume expansion and the hyperdynamic circulatory state in portal-hypertensive rats. Hepatology. 1992;15(2):323–328. doi: 10.1002/hep.1840150224. [DOI] [PubMed] [Google Scholar]
- 49.Wiest R, Shah V, Sessa WC, Groszmann RJ. NO overproduction by eNOS precedes hyperdynamic splanchnic circulation in portal hypertensive rats. American Journal of Physiology. 1999;276(4 Pt 1):G1043–G1051. doi: 10.1152/ajpgi.1999.276.4.G1043. [DOI] [PubMed] [Google Scholar]
- 50.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 51.Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283(2):H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 52.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287(5):H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 53.Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101(4):731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Varo G, Ros J, Morales-Ruiz M, et al. Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis. Am J Pathol. 2003;162(6):1985–1993. doi: 10.1016/S0002-9440(10)64331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Varo G, Morales-Ruiz M, Ros J, et al. Impaired extracellular matrix degradation in aortic vessels of cirrhotic rats. J Hepatol. 2007;46(3):440–446. doi: 10.1016/j.jhep.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126(3):886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49(4):1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 59.Tugues S, Fernandez-Varo G, Munoz-Luque J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46(6):1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 60.Van Steenkiste C, Geerts A, Vanheule E, et al. Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology. 2009;137(6):2112–24. e1–e6. doi: 10.1053/j.gastro.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 61.Clancy R, Marder G, Martin V, Belmont HM, Abramson SB, Buyon J. Circulating activated endothelial cells in systemic lupus erythematosus: further evidence for diffuse vasculopathy. Arthritis Rheum. 2001;44(5):1203–1208. doi: 10.1002/1529-0131(200105)44:5<1203::AID-ANR204>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 62.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23) Suppl 1:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 63.Abdelmoneim SS, Talwalkar J, Sethi S, et al. A prospective pilot study of circulating endothelial cells as a potential new biomarker in portal hypertension. Liver Int. 2010;30(2):191–197. doi: 10.1111/j.1478-3231.2009.02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erdbruegger U, Haubitz M, Woywodt A. Circulating endothelial cells: a novel marker of endothelial damage. Clin Chim Acta. 2006;373(1–2):17–26. doi: 10.1016/j.cca.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Llorent L, Richaud-Patin Y, Alcocer-Castillejos N, et al. Cytokine gene expression in cirrhotic and non-cirrhotic human liver. J Hepatol. 1996;24(5):555–563. doi: 10.1016/s0168-8278(96)80140-1. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 67.Ozuyaman B, Ebner P, Niesler U, et al. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost. 2005;94(4):770–772. doi: 10.1160/TH05-01-0038. [DOI] [PubMed] [Google Scholar]
- 68.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 69.Kim HK, Song KS, Kim HO, et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 2003;198(1):83–88. doi: 10.1016/s0304-3835(03)00268-4. [DOI] [PubMed] [Google Scholar]
- 70.Strijbos MH, Gratama JW, Kraan J, Lamers CH, Den Bakker MA, Sleijfer S. Circulating endothelial cells in oncology: pitfalls and promises. Br J Cancer. 2008;98(11):1731–1735. doi: 10.1038/sj.bjc.6604383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113(6):673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 72.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 73.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 74.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432(7014):226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 75.Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316(5824):575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 76.Pearson JD. Endothelial progenitor cells--an evolving story. Microvasc Res. 2010;79(3):162–168. doi: 10.1016/j.mvr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitao A, Sato Y, Sawada-Kitamura S, et al. Endothelial to mesenchymal transition via transforming growth factor-beta1/Smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am J Pathol. 2009;175(2):616–626. doi: 10.2353/ajpath.2009.081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 86(2):226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melgar-Lesmes P, Tugues S, Ros J, et al. Vascular endothelial growth factor and angiopoietin-2 play a major role in the pathogenesis of vascular leakage in cirrhotic rats. Gut. 2009;58(2):285–292. doi: 10.1136/gut.2008.155028. [DOI] [PubMed] [Google Scholar]
- 81.Shah V. Therapy for portal hypertension: What is our pipeline? Hepatology. 2009;49(1):4–5. doi: 10.1002/hep.22687. [DOI] [PubMed] [Google Scholar]