One of the earliest genes identified with stem and early progenitor cells is the RNA-binding protein, Musashi1 (Msi1). Through gene profiling of mammary epithelial cells transduced with Msi1, a unique autocrine signaling pathway was identified that activates both the Wnt and Notch pathways 1. This process was associated with increased secretion of the growth factor, PLF1 and inhibition of the secreted Wnt pathway inhibitor, DKK3. Identification of PLF1 as an effector of these pathways in the absence of the DKK3 tumor suppressor provides a new avenue for investigating differences between normal and malignant tissues, and potentially targeting tumor stem cells.
Introduction
Msi1 was first identified in Drosophila as a determinant of sensory organ development 2, and later as a cell fate determinant of neuroglial stem cells 3. Msi1 blocks translation of Numb, a negative regulator of Notch 4, as well as Ttk69, a transcriptional repressor downstream of Notch 5, and each gene is inherited asymmetrically and separately by the daughter cells. There are no studies of Ttk69 orthologs in mammalian cells, but it has been shown to alter signal transduction downstream of growth factor receptor activation in insect cells 6. Msi1 expression is also impacted by a second family of RNA-binding proteins related to Drosophila Elav that are involved in the development and maintenance of the nervous system in the fly and mouse 7, 8. Mammalian Elav orthologs HuB, HuC and HuD promote mRNA stabilization by binding to AU-rich elements in the 3’-UTR of several target mRNAs, including Msi19. Their activity has been linked to PKCα10, a protein kinase involved in multiple signaling pathways 11, and are localized to the nucleus of neuronal stem cells 12 similarly to Msi1 13.
Most studies of Msi1 have focused on regulation of the Notch pathway. Notch is activated by sequential proteolytic cleavage of its membrane-associated form to a constitutively active coactivator 14, whose expression is regulated by Msi1 and Numb 15. Numb promotes ubiquitination of intracellular Notch 16 and interferes with its nuclear translocation 17. Msi1 associates with the cis-acting repressor motif, GU3–5(G/AG), in the 3’-UTR of Numb and other targets to block translation 4.
Musashi1 and mammary progenitor cells
Msi1 maintains the proliferation of multipotential neural stem/progenitor cells15, and is rapidly downregulated in post-mitotic neurons3 and upregulated in central nervous system tumors originating from neural stem cells 18, 19. Mammary stem cells, like other stem cells, exhibit the ubiquitous feature of either remaining quiescent or undergoing self-renewal in response to their microenvironment 20, and retain the ability to pass on newly labeled DNA to their progeny by asymmetric cell division 21 (Fig. 1). “Label-retaining cells” are enriched in the “side population”, which express higher levels of ABC transporter proteins, Msi1, Notch1, CK19, ERα and a progenitor cell morphology22, as well as the CD24hi/CD133+ phenotype23. Human breast stem cells are enriched in Notch324, and Notch ligands promote the proliferation of epithelial and myoepithelial progenitor cells24. Since the mammary gland contains a population of multipotent stem/progenitor cells throughout development 25, it is an ideal tissue in which to assess the pathways regulating stem/progenitor cell proliferation, as well as those leading to malignant transformation 26.
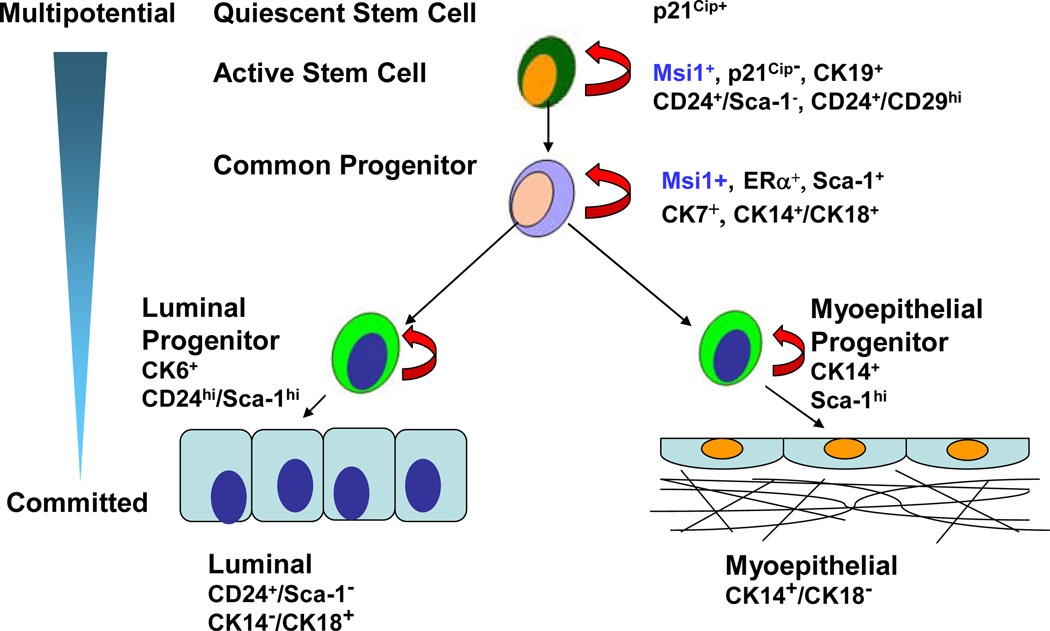
Figure 1.
Mammary gland differentiation. The degree of differentiation increases from top to bottom as cells become more committed. Stem cells are distinguished by their long label retention that reflects quiescence and nuclear expression of p21Cip. Stem cells undergo self-renewal within the end bud niche through interactions by integrins on their cell surface, eg. CD29 (integrinβ1), with the extracellular matrix. In response to various stimuli, stem cells exit the niche and actively divide into early progenitors that express Musashi1 (Msi1), CK19, CD24+/Sca-1− and CK24+/CD29hi. As cells become further committed, they differentiate into bipotential luminal and myoepithelial progenitor cells that express ERα, CK7, CK14+/CK18+ and Sca-1. These cells give rise to CD24hi/Sca-1hi/CK6+ luminal progenitor cells, and Sca-1+/CK14+ myoepithelial progenitor cells. Committed luminal cells are CD24+/Sca-1−/CK18+/CK14−, whereas myoepithelial cells are CK14+/CK18−.
We recently discovered that Msi1 regulated a unique autocrine signaling pathway in mammary epithelial cells1. Msi1-transduced cells expressed the CD24hi/Sca-1+ and CD24hi/CD29+ phenotypes. It remains controversial whether Sca-1 is a stem cell or progenitor cell marker since CD24hi/Sca-1+ cells had no mammary gland repopulating activity compared to CD24hi/Sca-1− cells 23, 27, whereas Sca-1+, but not Sca-1− cells, exhibited mammary outgrowth activity 28, 29. On the other hand, CD24+/CD29hi mammary cells were found to be multipotent self-renewing stem cells capable of reconstituting the mammary gland from a single cell30. Preneoplastic tissue from MMTV-Wnt1 mice exhibited an increased percentage of CD24+/CD29+ cells 30, and mammary outgrowth capacity segregated with CD24lo rather than CD24hi cells 27. The upregulation of CD24hi/CD29+ cells by Msi1 therefore appears to be more consistent with its ability to drive expansion of multipotent progenitor cells rather than pluripotent stem cells.
Msi1-transduced cells expressed a higher percentage of CK6, CK19 and double-positive CK14/CK18 cells, which are indicative of basal cells, a mixture of stem and progenitor cells 1. CK6 is abundant in stem and basal cells 29, 31–33 and has been linked to proliferation of alveolar epithelium and activation of the Wnt pathway 34. CK19 is expressed in luminal progenitor cells that give rise to CK14+ basal cells 35, 36, and double-positive CK14/CK18 cells are bipotential progenitor cells32. The Wnt pathway drives alveolar proliferation, as shown in MMTV-Wnt1 37 and MMTV-ΔN89β-catenin 38 transgenic mice. Activation of ΔN89β-catenin in mammary basal cells under the control of the CK5 promoter produced abundant end bud development 39. β-Catenin also participates in establishing the mitotic spindle 40, suggesting an additional role in stem cell self-renewal and progenitor cell proliferation. Expansion of mammary basal cells with characteristic CK6/CK14 expression has also been noted in mice with increased Notch pathway activation 32. These results are also consistent with Msi1 being a transducer of multipotential progenitor cell expansion rather than stem cell self-renewal.
An additional finding of our study was that p21Cip1 was absent in Msi1-expressing cells, which is in agreement with the ability of Msi1 to block its translation41. p21Cip1 is believed to function as a rheostat to maintain a balance between stem cell quiescence and stem cell exhaustion resulting from increased cell cycle entry42, 43. p21Cip1 is also associated with chromosome segregation during mitosis 44, as well as the negative regulation of Wnt4 transcription 45. Like β-catenin 40, p21Cip1 may play a role in mitosis to regulate progenitor cell expansion.
Msi1 regulates the proliferin signaling pathway
Gene profiling of Msi1-expressing cells presented the initial clue that Msi1 regulated a unique autocrine pathway. Msi1 produced an increase in the growth factor, PLF1, and an equally large reduction of the secreted Wnt pathway inhibitor, DKK3 1, and closely paralleled their respective changes in protein level in the conditioned medium of Msi1-transduced cells (Fig. 2). PLF1 is one of three highly homologous genes related to the prolactin gene family that map to a single locus on mouse chromosome 13 46. PLF1 is a ligand for the Gi-protein-coupled IGF2R 47, 48 that mediates prolactin-induced alveolar development in the mammary gland through activation of ERK and Jak2 49, 50. Receptor activation by PLF1 activates ERK 48, 51 and transcription factor AP-1 52, and is blocked by pertussis toxin 48, 51, which catalyzes ADP-ribosylation of the Giα subunit to prevent its interaction with the receptor 53. This mechanism was corroborated in Msi1-expressing cells by showing that pertussis toxin inhibited PLF1-mediated ERK activation by conditioned medium from Msi1-transduced cells, and that depletion of PLF1 from the medium or its downregulation by RNA interference inhibited ERK activation and Notch and Wnt signaling1. IGF2R activation is known to increase β-catenin nuclear localization and EMT 54, which are associated with growth and invasion 55. IGF2 increases the number of Msi1-positive intestinal stem/progenitor cells and their susceptibility to tumorigenesis56. Also pertinent to our findings is the identification of PLF2 and PLF3 as Wnt-1 target genes57. Since the three PLF1 genes are transcribed from a single locus, it is likely that they are all regulated in a similar manner. We previously found that PLF1 and PLF3 expression increased in primary mouse mammary tumors, particularly in those with basal cell characteristics58, 59. Interestingly, PLF2 has been shown to increase expansion of mouse hematopoietic stem cells ex vivo 60. These results therefore support a role for PLF1 in Msi1-mediated activation of the Wnt and Notch pathways and in mammary progenitor cell expansion.
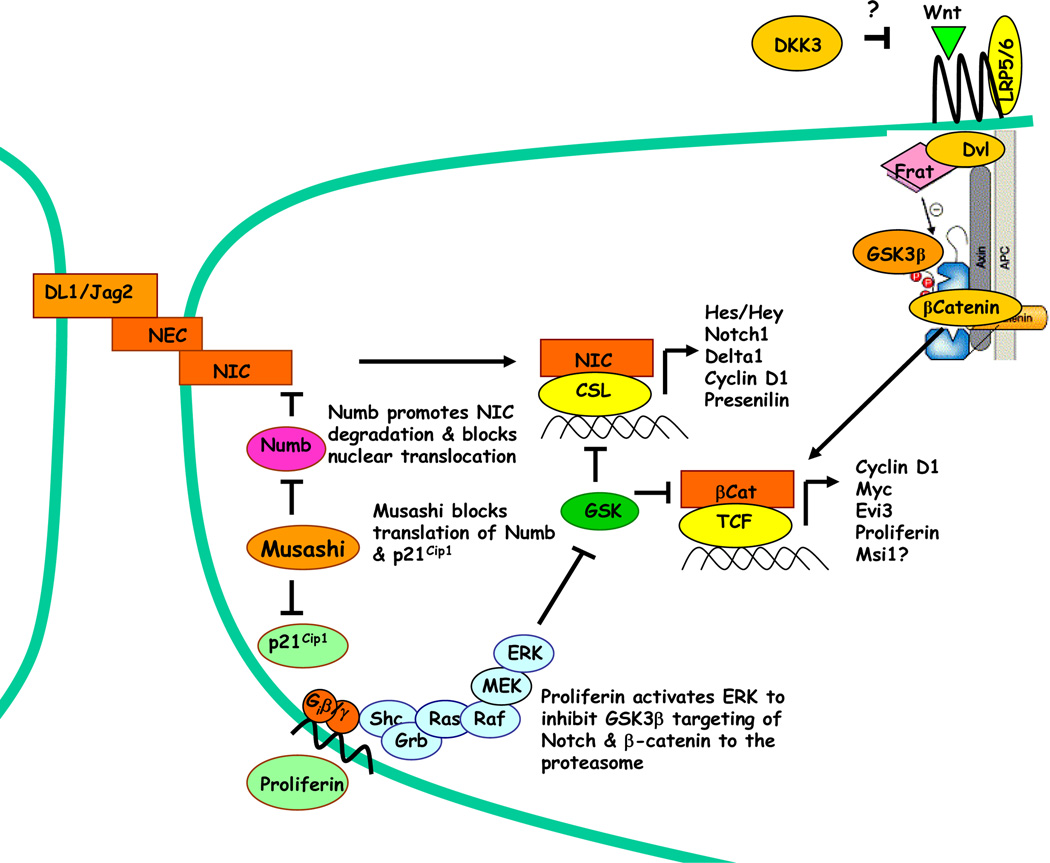
Figure 2.
Musashi signaling pathways associated with mammary progenitor cell expansion. Notch is processed proteolytically to an extracellular domain (NEC) and an intracellular domain (NIC). The Notch ligands Delta1 (DL1) and Jagged1 (Jag1) associate with NEC, and induce cleavage and release of membrane-bound NIC. NIC translocates to the nucleus, where it serves as a coactivator of CSL to activate transcription of Hes/Hey, Notch, DL1/Jag2 and cyclin D1. Musashi inhibits the translation of Numb and p21Cip1 by binding to a motif in the 3-UTR. Inhibition of Numb prevents NIC degradation and nuclear translocation, whereas inhibition of p21Cip1 prevents inhibition of CDK’s to promote G1/S transition. Preliminary studies indicate that Musashi increases secretion of the growth factor proliferin, which is known to mediate ERK activation through the Gi-coupled IGFII receptor and inhibit GSK3β activity. Msi1 also blocks expression of the Wnt pathway inhibitor, DKK3, to increase β-catenin/TCF activity by an unknown mechanism.
Downregulation of DKK3 was reciprocally related to PLF1 expression downstream of Msi1 signaling1(Fig. 2). DKK3 (also known as REIC or Reduced Expression in Immortalized Cells) is one of four homologous secreted proteins 61 that function as tumor suppressors 62. DKK1 and DKK2, but not DKK3, bind to the Wnt co-receptor, LRP5/6, to block Wnt pathway activation 63, but DKK3 similarly prevents nuclear localization of β-catenin through an as yet undefined mechanism64. Downregulation of DKK3 by RNA interference in control cells showed that it negatively regulated both β-catenin/TCF- and CBF1-dependent transcription, which resembled the phenotype resulting from Msi1 expression1. DKK3 expression in lung, prostate and liver tumor cells has been shown to induce apoptosis65–67 and disrupt acinar morphogenesis and growth of prostate tumor cells 68. In melanoma cells, reduction of DKK3 expression resulted in loss of cell adhesion, increased invasion, upregulation of the transcriptional repressor, Snail-1 69, and reduction of E-cadherin 70, all of which are associated with EMT. However, gene profiling and western analysis of Msi1-tranduced cells did not reveal reduction in E-cadherin, suggesting that this mechanism is not operative. Reduction of DKK3 therefore appears to work in concert with PLF1 to promote increased progenitor cell expansion upstream of Wnt and Notch signaling, but not EMT per se.
Notch-mediated transformation has been reported downstream of Ras and ERK activation 71, which is also in agreement with the dependence of Msi1-induced Notch and Wnt signaling on ERK activation1(Fig. 2). One mechanism common to activation of both pathways is inhibition of GSK3β by ERK, which is necessary to prime GSK3β for inactivation by other protein kinases 72(Fig. 2). Since GSK3β in its activated state phosphorylates and promotes ubiquitination and proteasomal degradation of β-catenin 73 and intracellular Notch 74, this mechanism provides a link between Msi1 and Wnt and Notch pathway activation.
PLF1 induced ERK signaling correlated with the CD24hi/CD29+ progenitor cell phenotype of Msi1-transduced cells1. CD24 is highly expressed in invasive tumor cells75, and mediates its effects through integrinβ1, the subunit expressed by CD29, and which itself is upregulated through the Ras/ERK pathway76. CD24 expression is linked to IGF2 signaling through IGF2R, the same receptor activated by PLF1. Importantly, deletion of the IGF2 gene reduced CD24 expression by 90% and suppressed invasion of glioblastoma cells77. Thus, increased PLF1 signaling through the IGF2R accounts for most, if not all, of the phenotypic changes occurring in Msi1-expressing cells. In summary, increased PLF1 secretion and reduced DKK3 expression by Msi1 leads to ERK activation and increased Notch and Wnt pathway activation. Inhibition of p21Cip1 and Numb work cooperatively with Notch and Wnt to promote cell cycle transit and progenitor cell expansion, but not terminal differentiation. Still undefined in this process is the role of the Msi1 target, Ttk69, which may function in the regulation of noncoding RNA. We have recently found that Msi1 induces a microRNA signature that resembles a breast cancer phenotype78 (X. Wang and R.I. Glazer, unpublished results). Thus, one role of Msi1 in mammary tissue is the expansion of an early multipotenital progenitor cell population during development. Whether this is a determinant of susceptibility to tumorigenesis will be the subject of future studies.
Acknowledgments
This work was supported by grant R01CA11482 and contract N01CN43309 from the National Institutes of Health, Bethesda, MD, and The Charlotte Geyer Foundation.
Abbreviations
- DKK3
Dickkopf3
- EMT
epithelial to mesenchymal transition
- Msi1
Musashi1
- PLF
proliferin
- Sca-1
stem cell antigen-1
- TCF
T-cell factor
- Ttk69
Tramtrack69
- UTR
untranslated region
References
- 1.Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008 doi: 10.1128/MCB.00040-08. published ahead of print on 24 March 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura M, Okano H, Blendy JA, Montell C. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron. 1994;13:67–81. doi: 10.1016/0896-6273(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 3.Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 4.Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–3900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okabe M, Imai T, Kurusu M, Hiromi Y, Okano H. Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature. 2001;411:94–98. doi: 10.1038/35075094. [DOI] [PubMed] [Google Scholar]
- 6.Baonza A, Murawsky CM, Travers AA, Freeman M. Pointed and Tramtrack69 establish an EGFR-dependent transcriptional switch to regulate mitosis. Nat Cell Biol. 2002;4:976–980. doi: 10.1038/ncb887. [DOI] [PubMed] [Google Scholar]
- 7.Antic D, Keene JD. Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am J Hum Genet. 1997;61:273–278. doi: 10.1086/514866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratti A, Fallini C, Cova L, Fantozzi R, Calzarossa C, Zennaro E, Pascale A, Quattrone A, Silani V. A role for the ELAV RNA-binding proteins in neural stem cells: stabilization of Msi1 mRNA. J Cell Sci. 2006;119:1442–1452. doi: 10.1242/jcs.02852. [DOI] [PubMed] [Google Scholar]
- 10.Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, Govoni S, Alkon DL, Quattrone A. Neuronal ELAV proteins enhance mRNA stability by a PKCalpha-dependent pathway. Proc Natl Acad Sci U S A. 2005;102:12065–12070. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez DE, Skilton G, Alonso DF, Kazanietz MG. The role of protein kinase C and novel phorbol ester receptors in tumor cell invasion and metastasis (Review) Oncol Rep. 1999;6:1363–1370. doi: 10.3892/or.6.6.1363. [DOI] [PubMed] [Google Scholar]
- 12.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991;67:325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 13.Akamatsu W, Fujihara H, Mitsuhashi T, Yano M, Shibata S, Hayakawa Y, Okano HJ, Sakakibara S, Takano H, Takano T, Takahashi T, Noda T, Okano H. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc Natl Acad Sci U S A. 2005;102:4625–4630. doi: 10.1073/pnas.0407523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–119. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 15.Okano H, Imai T, Okabe M. Musashi: a translational regulator of cell fate. J Cell Sci. 2002;115:1355–1359. doi: 10.1242/jcs.115.7.1355. [DOI] [PubMed] [Google Scholar]
- 16.McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003 doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- 17.Wakamatsu Y, Maynard TM, Jones SU, Weston JA. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron. 1999;23:71–81. doi: 10.1016/s0896-6273(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 18.Toda M, Iizuka Y, Yu W, Imai T, Ikeda E, Yoshida K, Kawase T, Kawakami Y, Okano H, Uyemura K. Expression of the neural RNA-binding protein Musashi1 in human gliomas. Glia. 2001;34:1–7. doi: 10.1002/glia.1034. [DOI] [PubMed] [Google Scholar]
- 19.Yokota N, Mainprize TG, Taylor MD, Kohata T, Loreto M, Ueda S, Dura W, Grajkowska W, Kuo JS, Rutka JT. Identification of differentially expressed and developmentally regulated genes in medulloblastoma using suppression subtraction hybridization. Oncogene. 2004;23:3444–3453. doi: 10.1038/sj.onc.1207475. [DOI] [PubMed] [Google Scholar]
- 20.Tsai RY. A molecular view of stem cell and cancer cell self-renewal. Int J Biochem Cell Biol. 2004;36:684–694. doi: 10.1016/j.biocel.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- 22.Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 23.Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chepko G, Smith GH. Mammary epithelial stem cells: our current understanding. J Mammary Gland Biol Neoplasia. 1999;4:35–52. doi: 10.1023/a:1018752519356. [DOI] [PubMed] [Google Scholar]
- 26.Glazer RI, Wang X, Yuan H, Yin Y. Mammary stem and progenitor cell regulation. Cancer Biomark. 2007;3:171–181. doi: 10.3233/cbm-2007-34-502. [DOI] [PubMed] [Google Scholar]
- 27.Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 29.Deugnier MA, Faraldo MM, Teuliere J, Thiery JP, Medina D, Glukhova MA. Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dbeta cell line. Dev Biol. 2006;293:414–425. doi: 10.1016/j.ydbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 31.Smith GH, Mehrel T, Roop DR. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1990;1:161–170. [PubMed] [Google Scholar]
- 32.Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Welm B, Behbod F, Goodell MA, Rosen JM. Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 2003;36(Suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol. 1999;206:88–99. doi: 10.1006/dbio.1998.9133. [DOI] [PubMed] [Google Scholar]
- 36.Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O'Hare MJ. Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J Histochem Cytochem. 1999;47:1513–1524. doi: 10.1177/002215549904701203. [DOI] [PubMed] [Google Scholar]
- 37.Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 1999;59:1869–1876. [PubMed] [Google Scholar]
- 38.Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze'ev A, Thiery JP, Glukhova MA. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan DD, Meigs TE, Kelly P, Casey PJ. Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem. 2004;279:10829–10832. doi: 10.1074/jbc.C400035200. [DOI] [PubMed] [Google Scholar]
- 41.Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21WAF-1. Mol Cell Neurosci. 2006;31:85–96. doi: 10.1016/j.mcn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 43.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461–466. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 45.Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005;19:1485–1495. doi: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiemers DO, Shao LJ, Ain R, Dai G, Soares MJ. The mouse prolactin gene family locus. Endocrinology. 2003;144:313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988;263:3521–3527. [PubMed] [Google Scholar]
- 48.Groskopf JC, Syu LJ, Saltiel AR, Linzer DI. Proliferin induces endothelial cell chemotaxis through a G protein-coupled, mitogen-activated protein kinase-dependent pathway. Endocrinology. 1997;138:2835–2840. doi: 10.1210/endo.138.7.5276. [DOI] [PubMed] [Google Scholar]
- 49.Hovey RC, Harris J, Hadsell DL, Lee AV, Ormandy CJ, Vonderhaar BK. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol. 2003;17:460–471. doi: 10.1210/me.2002-0214. [DOI] [PubMed] [Google Scholar]
- 50.Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA, Jan T. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- 51.El-Shewy HM, Lee MH, Obeid LM, Jaffa AA, Luttrell LM. The Insulin-like Growth Factor Type 1 and Insulin-like Growth Factor Type 2/Mannose-6-phosphate Receptors Independently Regulate ERK1/2 Activity in HEK293 Cells. J Biol Chem. 2007;282:26150–26157. doi: 10.1074/jbc.M703276200. [DOI] [PubMed] [Google Scholar]
- 52.Toft DJ, Rosenberg SB, Bergers G, Volpert O, Linzer DI. Reactivation of proliferin gene expression is associated with increased angiogenesis in a cell culture model of fibrosarcoma tumor progression. Proc Natl Acad Sci U S A. 2001;98:13055–13059. doi: 10.1073/pnas.231364798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns DL. Subunit structure and enzymic activity of pertussis toxin. Microbiol Sci. 1988;5:285–287. [PubMed] [Google Scholar]
- 54.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L. IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 55.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 56.Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, Ko MS, Ohlsson R, Longo DL, Feinberg AP. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 57.Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- 58.Yin Y, Bai R, Russell RG, Beildeck ME, Xie Z, Kopelovich L, Glazer RI. Characterization of medroxyprogesterone and DMBA-induced multilineage mammary tumors by gene expression profiling. Mol Carcinog. 2005;44:42–50. doi: 10.1002/mc.20119. [DOI] [PubMed] [Google Scholar]
- 59.Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP, Kopelovich L, Glazer RI. Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005;65:3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 60.Choong ML, Tan AC, Luo B, Lodish HF. A novel role for proliferin-2 in the ex vivo expansion of hematopoietic stem cells. FEBS Lett. 2003;550:155–162. doi: 10.1016/s0014-5793(03)00844-5. [DOI] [PubMed] [Google Scholar]
- 61.Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, Chang B, Duong T, Goodearl AD, Gearing DP, Sokol SY, McCarthy SA. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 62.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 63.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 64.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 65.Tsuji T, Nozaki I, Miyazaki M, Sakaguchi M, Pu H, Hamazaki Y, Iijima O, Namba M. Antiproliferative activity of REIC/Dkk-3 and its significant down-regulation in non-small-cell lung carcinomas. Biochem Biophys Res Commun. 2001;289:257–263. doi: 10.1006/bbrc.2001.5972. [DOI] [PubMed] [Google Scholar]
- 66.Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H, Huh NH. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005;65:9617–9622. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- 67.Hsieh SY, Hsieh PS, Chiu CT, Chen WY. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- 68.Kawano Y, Kitaoka M, Hamada Y, Walker MM, Waxman J, Kypta RM. Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene. 2006;25:6528–6537. doi: 10.1038/sj.onc.1209661. [DOI] [PubMed] [Google Scholar]
- 69.Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem. 2001;276:24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- 70.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- 71.Fitzgerald K, Harrington A, Leder P. Ras pathway signals are required for notch-mediated oncogenesis. Oncogene. 2000;19:4191–4198. doi: 10.1038/sj.onc.1203766. [DOI] [PubMed] [Google Scholar]
- 72.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 73.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 74.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 75.Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- 76.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. Embo J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukushima T, Tezuka T, Shimomura T, Nakano S, Kataoka H. Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem. 2007;282:18634–18644. doi: 10.1074/jbc.M609567200. [DOI] [PubMed] [Google Scholar]
- 78.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]