Abstract
Environmental factors, including viral infections, may explain an increasing and fluctuating incidence of childhood type 1 diabetes (T1D). Ljungan virus (LV) isolated from bank voles have been implicated, but it is unclear whether LV contributes to islet autoimmunity, progression to clinical onset, or both, of T1D. The aim was to test whether LV antibodies (LVAb) were related to HLA-DQ and islet autoantibodies in newly diagnosed T1D patients (n=676) and controls (n=309). Patients, 0–18 years of age, diagnosed with T1D in 1996–2005 were analyzed for LVAb, HLA-DQ genotypes, and all seven known islet autoantibodies (GADA, IA-2A, IAA, ICA, ZnT8RA, ZnT8WA, and ZnT8QA).
LVAb at 75th percentile, defined as cut off, was 90 (range 6–3936) U/mL and 4th quartile LVAb were found in 25% (170/676) of which 64% were <10 (n=108, p<0.0001), and 27% were<5 (n=45; p<0.0001) years old. The 4th quartile LVAb in children <10 years of age correlated to HLA DQ2/8, 8/8, and 8/X (p<0.0001). Furthermore, in the group with 4th quartile LVAb, 55% were IAA positive (p=0.01) and correlation was found between 4th quartile LVAb and IAA in children <10 years of age (p=0.035).
It is concluded that 1) LVAb were common among the young T1D patients and LVAb levels were higher in the younger age groups; 2) 4th quartile LVAb correlated with IAA; and 3) there was a correlation between 4th quartile LVAb and HLA-DQ8, particularly in the young patients. The presence of LVAb supports the notion that prior exposure to LV may be associated with T1D.
Introduction
Type 1 diabetes (T1D), a serious chronic autoimmune disease affecting a large number of young people, is known to have a multifactorial background with strong genetic components (32,35). Sweden has the highest incidence of T1D, next to neighboring Finland (9). The HLA-DQ locus on chromosome 6p21 confers the strongest genetic risk for T1D (24). The majority of individuals with these genetic risk factors do not develop T1D and several environmental factors have been considered including infections (6,13,34,40), climate (35), diet (3,21), and stress (16). The possible influence of viral infections as a trigger of islet autoimmunity or clinical onset of T1D has been reported in numerous studies (5,19).
An influence by infectious agents may explain the observations of temporal variations in T1D incidence (11,18). A fluctuating pattern of infectious diseases, including zoonoses (29), has been suggested to contribute to the appearance of either islet autoimmunity, T1D, or both (4,34). The Ljungan virus (LV), a parechovirus, possibly pathogenic to humans (8,23,28) has been proposed to contribute to T1D. The LV was defined (23,29) as a separate species in the family picornaviridae of the parechovirus genus, that formerly was classified as an enterovirus but later reclassified as a separate genus containing both human parechoviruses (HPeV) and the LV. Similar to LV, both HPeV, type 1 and 2, formerly named echovirus 22 and 23, respectively, have been considered for associations to T1D (10,36). The LV was first isolated in a bank vole, Myodes glareolus, from the valley of the river Ljungan in Northern Sweden (30). It was also reported that bank voles captured in the wild developed diabetes and had not only Ljungan virus antibodies (LVAb) but also increased levels of autoantibodies against glutamic acid decarboxylase 65 (GADA), islet antigen-2 (IA-2), and insulin (IAA) (28). The population of bank voles in Northern Sweden is known to fluctuate, with multiannual population cycles showing similarities with the T1D incidence fluctuations (29). Levels of LVAb were increased in young age at onset of T1D in children but a possible relationship to the incidence of T1D could either not be determined (28) or was suggestive (31). In our previous investigation, LVAb were studied in children from the Northern part of Sweden where the multiannual population cycles of bank voles now show a trend towards reduced fluctuation pattern (20). LV is thought to have a continuous worldwide presence in voles, although its possible role as a human pathogen remains unclear (22). Although voles are also common in Southern Sweden, they are not known to fluctuate in this part of the country. During 1996–2005, the children developing diabetes in Southern Sweden (i.e., the Skåne region), were registered for classification of diabetes with HLA genotyping and islet autoantibodies, including islet cell antibodies (ICA), GADA, IA-2A, IAA, and all three variants of the 325 Zinc transporter 8 autoantibody (ZnT8A) (2). We took advantage of this register to analyze these sera for LVAb.
The first aim of the present study was to test whether LVAb was related to age at clinical diagnosis as reported previously (28,31). Our second aim was to test if HLA types were associated with LVAb levels. The third aim was to test if LVAb was related to islet autoantibodies (GADA, IA2A, IAA, and the three ZnT8A to either one or all three amino acid variants at position 325 (ZnT8RWQ) along with ICA) in T1D. An attempt was also made to relate LVAb levels to the incidence of T1D in Southern Sweden.
Methods
Subjects
All children and adolescents (1–18 years of age) diagnosed with T1D between February 1996 and April 2005 in the province Skåne (1,200,000 inhabitants) in the very southern part of Sweden were included. Serum was obtained at the time of clinical diagnosis from 686 consecutive patients who were classified with T1D according to the recommendation by ADA (1). There were 373 (54%) boys and 313 (46%) girls in the study. The mean age of the children at T1D diagnosis was 9.8 years (range 1–18.8 years). Serum samples were stored at −20°C until analyzed for islet autoantibodies. A total of 676 samples were available to be analyzed for LVAb. As controls for evaluating the LVAb distribution, we used sera collected January–March 1989 from 309 healthy school children (11–13 years of age), also stored at −20°C (27) .
The study was approved by the Research Ethics Committee of the Faculty of Medicine, Lund University, Lund, Sweden.
Measurements
Ljungan virus cDNA
The Ljungan virus isolate 87-012 (23) was subcloned in the pCRII- TOPO® vector (Life technologies, Grand Island, NY) to contain nucleotide positions 301–2685 representing VP0 (VP2), VP3, and VP1(28). The resulting cDNA clone, pLV1, was verified by sequencing and used in coupled in vitro transcription translation to label the resulting polyprotein (Mr 97K) with 35S-methionine as described previously (15).
Coupled in vitro transcription translation
The pLV1 polyprotein (28) was labeled in a reaction mixture containing 2 μg pLV1, 50 μL TNT® rabbit reticulocyte lysate, 4 μL TNT®reaction buffer, 2 μL amino acid mixture without methionine, 2 μL RNasin® Ribonuclease inhibitor, 2 μL SP6 RNA Polymerase, (all from Promega Corporation, Madison, WI), 4 μL 35S-methionine (N1000 Ci/mmol from Amersham Int., Amersham, Bucks., UK), and nuclease-free water to a final volume of 100 μL. The reaction mixture was incubated for 90 min at 30°C with shaking (300 rpm in an Eppendorf Thermomixer comfort, Eppendorf, AG Hamburg, Germany). The translation product was immediately subjected to gel filtration on Illustra™ NAP-5 Columns (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and radioactivity incorporated into protein was determined (1450 MicroBeta TriLuxMicroplate Scintillation- Luminescence Counter, PerkinElmer Life and Analytical Sciences, Shelton, CT). SDS gel electrophoresis combined with autoradiography was used to confirm the presence of a major product of labeled antigen representing Mr 97K (data not shown).
Radiobinding assay (RBA) of Ljungan virus antibodies (LVAb)
The RBA for LVAb was carried out overnight at 4°C in duplicate samples of 2.5 μL serum incubated with 60 μL labeled antigen at a final concentration of 425±25 cpm/μL after dilution in antigen buffer (150 mmol/L NaCl, 20 mmol/L Tris, pH 7.4, 0.15% (v/v) Tween 20, 0.1% (w/v) BSA). V-formed 96-well plates (catalog nr 442587, Micro Well™ plates, Nunc A/S, Roskilde, Denmark) were used. A total of 50 μL reaction mixture was then incubated for 1 h at 4°C with 50 μL 20% Protein A-Sepharose (PAS) (Invitrogen Corporation, Camarillo, CA), washed five times at 4°C by sedimentation in antigen buffer) in a 96-well MultiScreen-DV filtration plate (Merck Millipore Corporation, Billerica, MA). The plate was then washed 8 times with wash buffer (150 mM NaCl, 20 mM Tris, 0.15% Tween 20, pH 7.4), using a Multiscreen vacuum manifold (Multi-ScreenHTS, Vacuum Manifold, Millipore, Merck KGaA, Darmstadt, Germany). Antibody-bound radioactivity was counted in a β-counter (1450 MicroBeta TriLux Microplate Scintillation-Luminescence Counter, PerkinElmer Inc, San Jose, CA). PAS bound radioactivity was converted into in-house units (U) using individual standard curves generated by six step doubling dilutions of high-titer guinea pig antisera (Antiserum 174 F #4) (31) in all runs. Patient and control sera were analyzed intermixed. Intra-assay CV for duplicate determinations was 4% and the inter-assay CV was 10%.
Autoantibodies to glutamic acid decarboxylase (GADA)
GADA were analyzed in a commercially available kit (RSR Limited, Cardif, UK) validated in the Diabetes Autoantibody Standardization Program (DASP) with 74% study sensitivity and 96% study specificity. The CV was 8.9% at level 2.0 U/mL and 14.2% at level 44.6 U/mL.
Insulin autoantibodies (IAA) Non-competitive method
Serum samples (7 μL) were added to duplicate wells in 96-well microplates followed by 36 μL of 125I-insulin (60 000 cpm/well). The plates were incubated at +4°C for 48 h on a shaker. PAS (40% slurry; 50 μL) was added to a filter plate, previously saturated with coating buffer, and washed three times (Platewasher ELX50/8FMW, BioTek Instruments Inc, Winooski, VT) with 200 μL Tris buffer. After incubation with 125I-insulin, 25 μL was transferred to the filter plate with PAS and incubated at +4°C for 1.5 h on shaker. The plates were washed 10 times with 200 μL Tris buffer and then dried for 15 min before adding 50 μL scintillation solution. Radioactivity was measured in a β-counter (Wallac MicroBeta Trilux, Perkin Elmer Life and Analytical Sciences, Shelton, CT). Competitive method: Positive serum samples (7 μL) were added to four wells on a 96-well plate followed by 36 μL 125I-insulin (60,000 cpm/well) and 0.072 U (2 IU/mL) unlabeled insulin (Actrapid, Novo Nordisk, Denmark) in the last two wells. The plates were incubated and processed as in the noncompetitive method. IAA levels were calculated as relative units (RU) and were related to positive controls. Positivity for IAA was set to 1.0 RU. The inter-assay CV was 6.0% for precision and 13.2% for reproducibility. Validated in DASP study sensitivity was 26% and study specificity 100%.
Autoantibodies to islet-antigen-2 (IA-2A)
were analyzed in a commercially available kit (RSR Limited, Cardiff, UK) validated in DASP with 68% study sensitivity and 100% study specificity. The CV was 7.7% at level 2.6 U/mL and 5.8% at level 25.6 U/mL.
Autoantibodies to the zinc transporter variants
RBA for all three variants, Arginin ZnT8 (ZnT8R), Tryptophan ZnT8 (ZnT8W), and Glutamine ZnT8 (ZnT8Q) of human ZnT8 (Slc30A8) were performed separately with 5 μL of human sera, as described in detail (39). Briefly, duplicate serum samples were incubated with labeled antigen and antibody-bound label separated from free antigen by PAS. Bound radioactivity was converted into in-house units using a high-titer standard with high ZnT8RA, ZnT8WA, or ZnT8QA reactivity. Intra-assay CV was for ZnT8RA 6%, ZnT8WA 5%, and ZnT8QA 4%. Inter-assay CV was for ZnT8RA 7%, ZnT8WA 8%, and ZnT8QA 10%. In the DASP inter-laboratory comparison (25), our laboratory had 66% workshop sensitivity for ZnT8RA, 50% for ZnT8WA, and 99% workshop specificity for ZnT8RA, 100% for ZnT8WA.
Islet cell cytoplasmic autoantibodies (ICA)
ICA were determined in a two-color indirect immunofluorescence assay performed on sections of frozen human pancreas, as described previously (26). Our laboratory participated in the 13th Immunology of Diabetes Workshop Standardization and showed 100% sensitivity and specificity. Levels of ICA are expressed in Juvenile Diabetes Foundation Units (JDF-U), using the world reference standard curve based on the international JDF reference sera (14).
HLA genotyping
HLA-DQB1 and DQA1 genotypes were typed by sequence-specific oligonucleotide probes as described (17) using a DELFIA Hybridization assay (Perkin Elmer, Boston, MA). The first set of probes defines the presence of HLA-DQB1*02, 0302, 0301, 0602, 0603, and 0604. The second set of probes defines the presence of additional DQB1 alleles. HLA-DQA1 probes defines the DQA1*0201, 03, and 05 alleles (13).
Data analysis
Statistical analyses were performed using SPSS statistical software (version 18.0; SPSS, Chicago, IL). Differences in proportions between groups were tested using the chi-square test. P values less than 0.05 were considered significant. LVAb distribution was evaluated using Quintile- Quintile normality plots.
Results
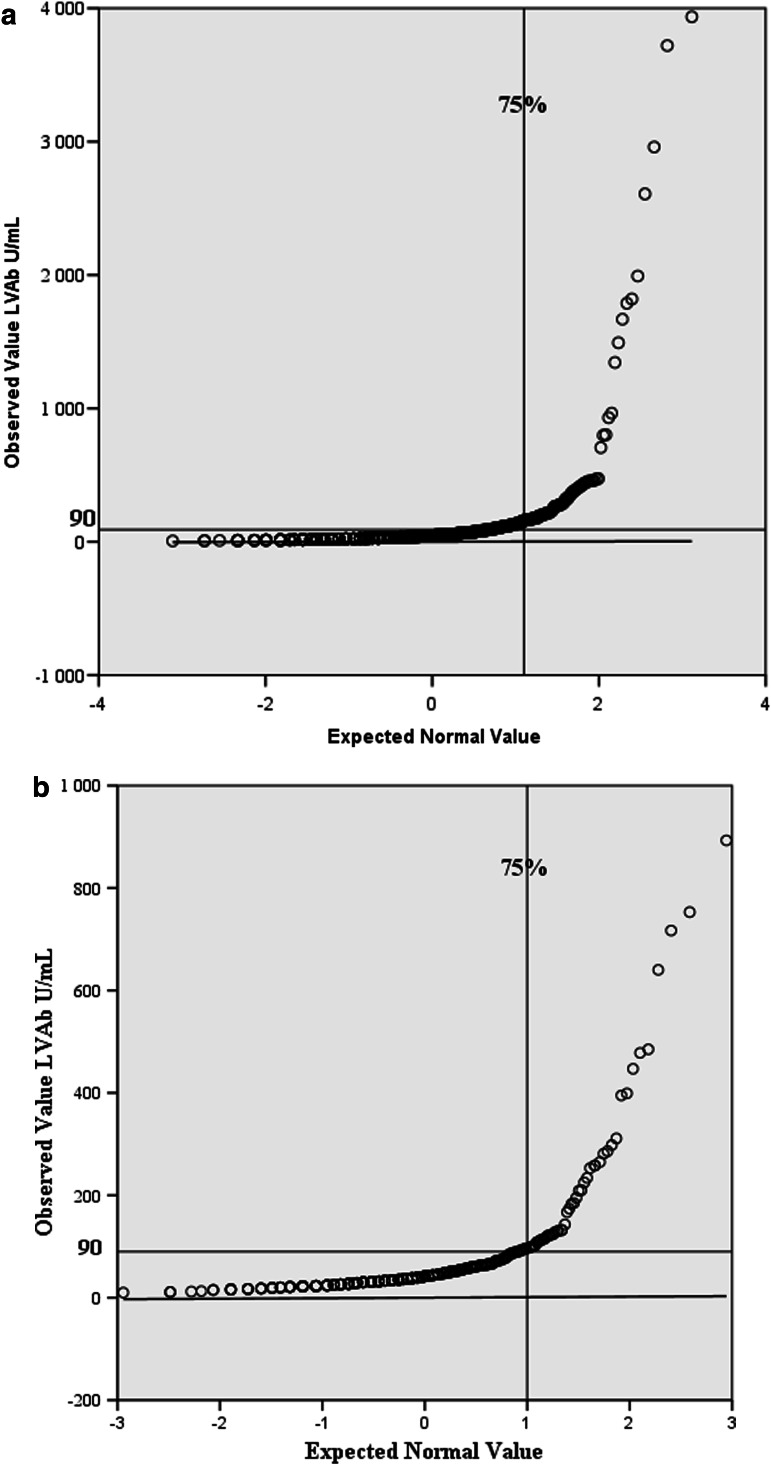
LVAb levels varied between 6 and 3936 U/mL (mean 90 U/mL) among the 676 newly diagnosed 0–18 year-old T1D patients (Fig. 1). The normality plot indicated higher LVAb levels in the 4th quartile of the serum samples from the T1D patients compared to the 309 healthy 11–13 years old controls. The controls had LVAb levels varying between 10 and 893 U/mL (mean 73 U/mL) (Fig. 1). A total of 170/676 (25%) T1D patients had binding levels in the 4th quartile (90–3936 U/mL) in contrast to 57/309 (18%), (90–893 U/mL), of the controls (p=0.02). Among the patients, LVAb in the 4th quartile was more often found in the lower age groups, 44% (n=45/102) in 0–4.99 years of age compared to 28% (63/228) in the 5–9.99 year olds, 20% (n=52/263) in the 10–14.99 year olds, and 12% (n=10/73) in the 15–18.8 year olds (p<0.0001). It was observed that 64 % of those with LVAb in the 4th quartile were <10 years old (n=108; p<0.0001) and 27% (n=45) belonged to the <5 year olds (p<0.0001).
FIG. 1.
Ljungan virus antibodies (LVAb) in U/mL in relation to normal distribution of all 676 type 1 diabetes patients newly diagnosed in 1996–2005 (a). The LVAb levels in 309 healthy controls obtained in 1989 are shown for comparison, demonstrating a similar distribution of possible LV exposure (b). The 4th quartile LVAb was>90 U/mL. Note: the y-axis with (a) max 4000, (b) max 1000 U/mL.
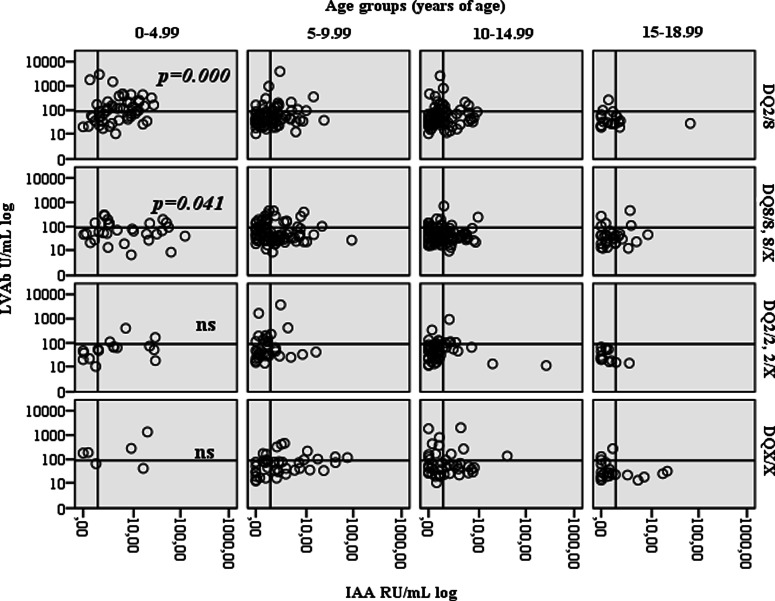
In the children <10 years of age, but not above, 4th quartile LVAb were related to HLA-DQ 2/8, 8/8, and 8/X genotypes ( p=0.006) (Table 1). In addition, there was an age-dependent association between 4th quartile LVAb and HLA-DQ2/8 in children being 0–4.99 years of age at diagnosis (p<0.0001, Fig. 2).
Table 1.
Relation Between 4th Quartile LVAb and HLA-DQ Genotypes
| |
HLA |
||||
|---|---|---|---|---|---|
| DQ2/8 | DQ8/8, 8/X | DQ2/2, 2/X | DQX/X | Total | |
| LVAb <4th quartile n | 152 | 182 | 92 | 78 | 504a |
| % within LVAb <4th quartile | 30 | 36 | 18 | 15 | 100 |
| LVAb in 4th quartile n | 64 | 51 | 23 | 32 | 170 |
| % within LVAb 4th quartile | 38 | 30 | 14 | 19 | 100 |
| Total n | 216 | 233 | 115 | 110 | 674 |
| % of total | 32 | 35 | 17 | 16 | 100 |
| P=0.105 | |||||
Two missing HLA values.
FIG. 2.
Ljungan virus antibodies (LVAb) in U/mL (log scale) in relation to age at diagnosis (years), HLA-DQ genotypes and levels IAA RU/mL (log scale). Young age at onset (0—4.99 years of age) among the HLA-DQ2/8 subjects correlated to high level LVAb and positive IAA (p=0.0001).
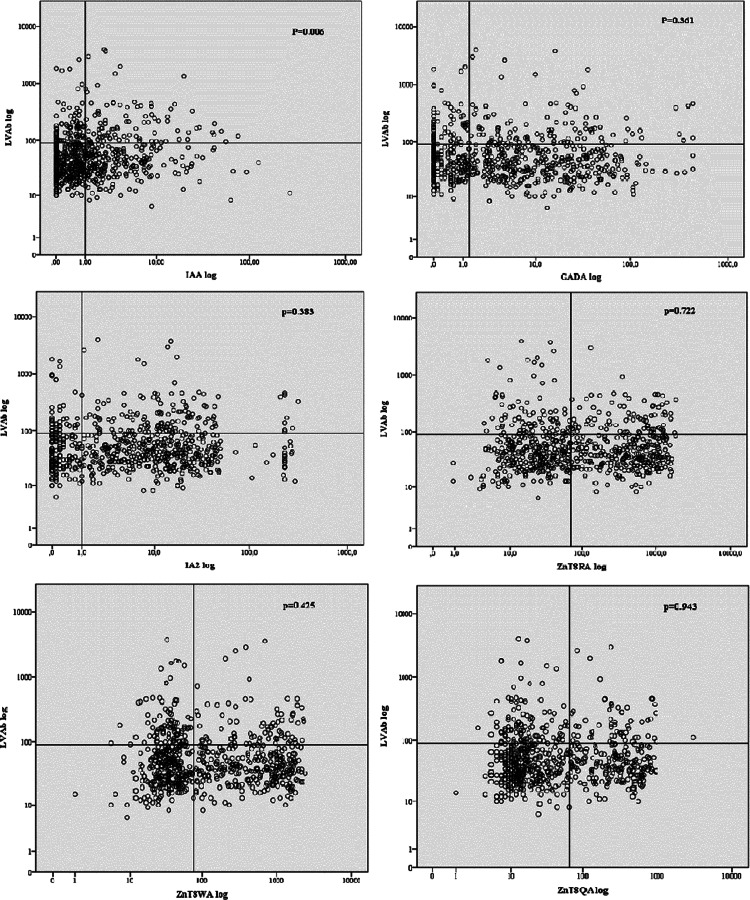
The 676 T1D children were tested for IAA (46%), IA-2A (75%), GADA (65%), ZnT8A (R-variant 49%, W-variant 50%, and Q-variant 36%) and ICA (77% positive). While neither GADA, IA-2A, ZnT8A, nor ICA were related to 4th quartile LVAb, it was found that 92/170 (54 %) with 4th quartile LVAb were also IAA positive (p=0.010) (Fig. 3). In relation to all IAA positive children, IAA and 4th quartile LVAb were also correlated in children both below 10 years of age 72/108 (67%; p=0.006) and below 5 years of age 40/45 (89%; p=0.006) (Table 2).
FIG. 3.
Ljungan virus antibodies (LVAb) in U/mL (log scale) in relation to DADA, IAA, IA-2A, ZnT8RA, ZnT8WA, and ZnT8QA (log scales). The vertical lines represent the cut-off for a positive autoantibody and the horizontal line the 4th quartile LVAb level. IAA (p=0.006) but not the other islet autoantibody levels correlated to high level LVAb.
Table 2.
4th Quartile LVAb in Relation to IAA, GADA, IA-2A, ZnT8A (All Three), and ICA in Different Age Groups
| |
|
Age groups (years of age) |
|||
|---|---|---|---|---|---|
| Antibodies | 0–4.99 | 5–9.99 | 10–14.99 | 15–18.99 | |
| % positive | LVAb in 4th quartile | 44 | 28 | 20 | 12 |
| IAA | 80 | 49 | 36 | 25 | |
| IA-2A | 78 | 78 | 74 | 69 | |
| GADA | 58 | 60 | 71 | 71 | |
| ICA | 78 | 80 | 78 | 69 | |
| ZnT8RA | 42 | 51 | 52 | 46 | |
| ZnT8WA | 40 | 48 | 56 | 47 | |
| ZnT8QA | 26 | 37 | 41 | 34 | |
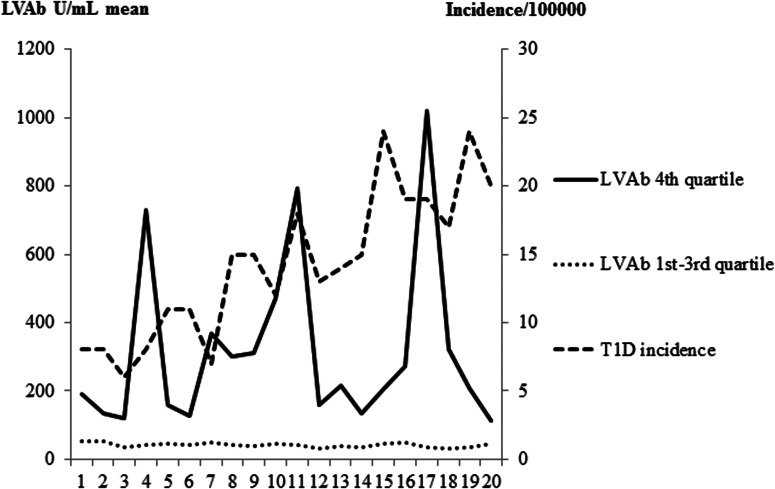
The incidence of T1D in Skåne during 1996–2005 increased from 14 to 46 patients per year per 100,000 children at 0–18 years of age (Fig. 4). It was noted that the 4th quartile LVAb varied markedly with peaks coinciding with some but not all winter seasons and did not exactly match the seasonality of T1D incidence in this Southern Sweden population,
FIG. 4.
Mean levels of Ljungan virus antibodies (LVAb) in U/mL within the 4th quartile (__ solid line) and in the 1st—3rd quartiles (….dotted line), respectively, in relation to season. Seasons from February 1996 until April 2005 are indicated as uneven numbers (1, 3, 5, etc) for the winter (October—March), and even numbers for the summer (April—September) seasons. The fluctuating levels of LVAb in the 4th quartile are compared to the increasing incidence (- - - -semi-dotted line) of T1D among children in Skåne.
Discussion
This study of 676 newly diagnosed T1D children in Skåne revealed that patients with 4th quartile LVAb were more common 1) when diagnosed below 10 years of age; 2) among the HLA DQ2/8, 8/8, and 8/X genotypes, and 3) in the IAA positive children below the age of 10. These results are consistent with our previous reports that LVAb levels tend to be high in the very young children (28,31). In addition, the present results support and extend our previous investigation in the Jämtland county that the proportion of children with 4th quartile LVAb were younger compared to the rest of the children (31). The two different studies conducted in these two different counties, one in the very South of Sweden (present study) and the other in the northern part of the country therefore showed comparable results. Furthermore, the new finding that high level LVAb were associated with the T1D high risk HLA DQ2/8, 8/8, and 8/X genotypes support the well-known association between HLA and the immune response to virus (33). The association is likely to be explained by HLA-DQ8 as there was no association between HLA-DQ 2/2 or 2/X (X is not 8) subjects. As IAA was associated with younger age at onset as well as with HLA DQ2/8, 8/8, and 8/X genotypes, it remains to be determined if high level LVAb was a trigger of IAA or if this covariance occurred by chance. Among these genotypes, only DQ2/8 was associated with young age at onset and it is therefore unlikely that the LVAb levels at young age at onset were solely explained by this genotype. We speculate that LVAb would be associated primarily with HLA DQ8 and that LVAb may develop in the young independent of IAA, despite the fact that this autoantibody is also associated with DQ8 (12).
The strength of the present study was the possibility to include essentially all 0–18-year-old children diagnosed with T1D during 1996–2005 in the county of Skåne (2). As all seven islet autoantibodies were analyzed along with HLA-DQ genotypes, our Skåne study offered a unique possibility to analyze antibodies against LV in an already defined population of T1D children. Importantly, our previous study only comprised 63 T1D patients from Jämtland, while the Skåne study offered an opportunity to analyze 676 patients ascertained over an almost 10-year period.
Our serological method for LVAb, which detects primarily IgG-antibodies to LV, was considered to adequately reveal a previous exposure to the virus. The large variation in LVAb levels in this and the previous Jämtland study (31) was comparable as both studies were cross-sectional and therefore would detect LVAb at different titers as the time of exposure to the virus could not be controlled for. The Q–Q plots (Fig. 1) suggest that many individuals have been exposed to LV, resulting in widely varying LVAb levels as the time between possible LV exposure and blood sampling may vary. The correlations between high level LVAb and young age is interesting with respect not only to a shorter prodrome of islet autoimmunity but also to increasing incidence of T1D in the young during the last decades (7,9).
A potential weakness is the cross-sectional character of the study design. Validation of our findings in relation to the development of islet autoimmunity and T1D among children who have one or several islet autoantibodies will require prospective population-based studies. Another weakness would be the use of PAS to separate free from antibody bound 35S-LV as IgG3 does not bind to Protein A. It may also be important to analyze LV-IgM for an immune response closer to the actual infection. Another potential weakness of the study was the lack of healthy controls ascertained during the same study period as the TID patients. The present controls were all 12 years of age and were ascertained prior to the TID study period. However, the controls still represent the same geographical area and were used mainly to test the distribution of LVAb levels in the population.
LV infection in relation to the risk for T1D was negative in the MIDIA study (38) as LV-RNA by PCR was not detected in stool samples from Norwegian children with high risk HLA genotype followed from birth for 28 months in 2001–2006. It is difficult to relate PCR and LVAb analyses to a possible LV infection, as it is unclear whether LV exposure is associated with symptoms. An important finding in our studies was still that LVAb were primarily found in the very young where symptoms of virus infections may not be obvious. Seroconversion of islet autoantibodies, especially IAA, takes place early, between 6 months and 2 years of age with peak incidence at 9 month to 2 years of age in children born to T1D parents (41). In the present study, the youngest T1D child was 1 year of age, and although maternal LVAb were unlikely, it cannot be excluded that the progression from IAA positivity to clinical onset of T1D accelerated after exposure to LV (37).
The fluctuating population pattern of bank voles, as reservoir for LV, and the similar fluctuation of T1D incidence raised the question that the two phenomena were related (28). However, climate changes during the last decade are thought to reduce the fluctuating population pattern (20). The time delay of about 2–3 years from vole population peak to T1D incidence peak may no longer be possible to document. Our data in Skåne where the bank vole population is not known to fluctuate to the same extent as in Northern Sweden still does not exclude the possibility that LV contribute to autoimmunity, T1D, or both. Regardless of time of virus exposure, the Skåne children also showed higher levels of LVAb in younger children and, in addition, an association with both HLA-DQ8 and IAA. Considering the low number of LV particles needed for infection and a possible long latency of virus replication (8), it may not be surprising that current laboratory methods have failed to detect virus in humans (38).
To conclude, this study showed significant association between 4th quartile LVAb and young age, as well as between 4th quartile LVAb and IAA in lower age groups of newly diagnosed T1D children in Skåne. This association was strengthen by the finding that both 4th quartile LVAb and IAA were significantly associated to high risk HLA in the lower age groups. The half year concordance between T1D incidence and LVAb levels in Jämtland (31) was not immediately apparent in our Skåne study. The presence of LVAb suggests that children with T1D may have been exposed to the LV. However, a possible contribution of LV exposure in the etiology and pathogenesis of T1D remains elusive. Taken together, TID children presented more elevated LVAb titers than controls, and among the T1D children, the youngest category of age represented a significant correlation between LVAb titers and HLA-DQ2/8, 8/8, and 8/X, as well as insulin autoantibodies.
Acknowledgments
We thank Ida Hansson, Anita Nilsson, and Ingrid Wigheden for their excellent technical assistance. Anna-Lena Nilsson was supported by a grant from Swedish Child Diabetes Foundation and the research by the Swedish Research Council (Grant 14064), Swedish Diabetes Association, National Institutes of Health (DK26190), UMAS Fund, the Knut and Alice Wallenberg Foundation, and the Skåne County Council for Research and Development.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.Andersson C. Larsson K. Vaziri-Sani F, et al. 2011 The three ZNT8 autoantibody variants together improve the diagnostic sensitivity of childhood and adolescent type 1 diabetes. Autoimmunity. 2011;44:394–405. doi: 10.3109/08916934.2010.540604. [DOI] [PubMed] [Google Scholar]
- 3.Bruining GJ. Association between infant growth before onset of juvenile type-1 diabetes and autoantibodies to IA-2. Netherlands Kolibrie study group of childhood diabetes. Lancet. 2000;356:655–656. doi: 10.1016/s0140-6736(00)02612-x. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Rode E. Sarmiento L. Tiberti C, et al. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia. 2003;46:1348–1353. doi: 10.1007/s00125-003-1179-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooke A. Review series on helminths, immune modulation and the hygiene hypothesis: How might infection modulate the onset of type 1 diabetes? Immunology. 2009;126:12–17. doi: 10.1111/j.1365-2567.2008.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlquist G. Frisk G. Ivarsson SA, et al. Indications that maternal coxsackie B virus infection during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia. 1995;38:1371–1373. doi: 10.1007/BF00401772. [DOI] [PubMed] [Google Scholar]
- 7.Dahlquist G. Mustonen L. Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Swedish Childhood Diabetes Study Group. Acta Paediatr. 2000;89:1231–1237. doi: 10.1080/080352500750027628. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrom JO. Tolf Fahlgren , et al. Replication of Ljungan virus in cell culture: The genomic 5'-end, infectious cDNA clones and host cell response to viral infections. Virus Res. 2007;130:129–139. doi: 10.1016/j.virusres.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.EURODIAB. Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 10.Frisk G. Nilsson E. Tuvemo T, et al. The possible role of Coxsackie A and echo viruses in the pathogenesis of type I diabetes mellitus studied by IgM analysis. J Infect. 1992;24:13–22. doi: 10.1016/0163-4453(92)90814-m. [DOI] [PubMed] [Google Scholar]
- 11.Gamble DR. Viral and epidemiological studies. Proc Royal Soc Med. 1975;68:256–258. doi: 10.1177/003591577506800432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham J. Hagopian WA. Kockum I, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 13.Graves PM. Norris JM. Pallansch MA, et al. The role of enteroviral infections in the development of IDDM: Limitations of current approaches. Diabetes. 1997;46:161–168. doi: 10.2337/diab.46.2.161. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum CJ. Palmer JP. Nagataki S, et al. Improved specificity of ICA assays in the Fourth International Immunology of Diabetes Serum Exchange Workshop. Diabetes. 1992;41:1570–1574. doi: 10.2337/diab.41.12.1570. [DOI] [PubMed] [Google Scholar]
- 15.Grubin CE. Daniels T. Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994;37:344–350. doi: 10.1007/BF00408469. [DOI] [PubMed] [Google Scholar]
- 16.Hagglof B. Blom L. Dahlquist G, et al. The Swedish childhood diabetes study: Indications of severe psychological stress as a risk factor for type 1 (insulin-dependent) diabetes mellitus in childhood. Diabetologia. 1991;34:579–583. doi: 10.1007/BF00400277. [DOI] [PubMed] [Google Scholar]
- 17.Hagopian WA. Erlich H. Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): Genetic criteria and international diabetes risk screening of 421,000 infants. Ped Diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermann R. M Knip M. R Veijola R, et al. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes—Indication of an increased environmental pressure? Diabetologia. 2003;46:420–425. doi: 10.1007/s00125-003-1045-4. [DOI] [PubMed] [Google Scholar]
- 19.Hober D. Sane F. Jaidane H, et al. Immunology in the clinic review series; Focus on type 1 diabetes and viruses: Role of antibodies enhancing the infection with Coxsackievirus-B in the pathogenesis of type 1 diabetes. Clin Exper Immunol. 2012;168:47–51. doi: 10.1111/j.1365-2249.2011.04559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornfeldt B. Hipkiss T. Eklund U. Fading out of vole and predator cycles? Proc Biol Sci. 2005;272:2045–2049. doi: 10.1098/rspb.2005.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hypponen E. Virtanen SM. Kenward MG, et al. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care. 2000;23:1755–1760. doi: 10.2337/diacare.23.12.1755. [DOI] [PubMed] [Google Scholar]
- 22.Johansson ES. Niklasson B. Tesh RB, et al. Molecular characterization of M1146, an American isolate of Ljungan virus (LV) reveals the presence of a new LV genotype. J Gen Virol. 2003;84:837–844. doi: 10.1099/vir.0.18792-0. [DOI] [PubMed] [Google Scholar]
- 23.Johansson S. Niklasson B. Maizel J, et al. Molecular analysis of three Ljungan virus isolates reveals a new, close-to-root lineage of the Picornaviridae with a cluster of two unrelated 2A proteins. J Virol. 2002;76:8920–8930. doi: 10.1128/JVI.76.17.8920-8930.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kockum I. Sanjeevi CB. Eastman S, et al. Complex interaction between HLA DR and DQ in conferring risk for childhood type 1 diabetes. Eur J Immunogenet. 1999;26:361–372. doi: 10.1046/j.1365-2370.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 25.Lampasona V. Schlosser M. Mueller PW, et al. Diabetes antibody standardization program: First proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem. 2011;57:1693–1702. doi: 10.1373/clinchem.2011.170662. [DOI] [PubMed] [Google Scholar]
- 26.Landin-Olsson M. Arnqvist HJ. Blohme G, et al. Appearance of islet cell autoantibodies after clinical diagnosis of diabetes mellitus. Autoimmunity. 1999;29:57–63. doi: 10.3109/08916939908995973. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg B. Ahlfors K. Carlsson A, et al. Previous exposure to measles, mumps, and rubella—But not vaccination during adolescence—Correlates to the prevalence of pancreatic and thyroid autoantibodies. Pediatrics. 1999;104:e12. doi: 10.1542/peds.104.1.e12. [DOI] [PubMed] [Google Scholar]
- 28.Niklasson B. Heller KE. Schonecker B, et al. Development of type 1 diabetes in wild bank voles associated with islet autoantibodies and the novel ljungan virus. Int J Exp Diabesity Res. 2003;4:35–44. doi: 10.1080/15438600303733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niklasson B. Hornfeldt B. Lundman B. Could myocarditis, insulin-dependent diabetes mellitus, and Guillain-Barre syndrome be caused by one or more infectious agents carried by rodents? Emerg Infect Dis. 1998;4:187–193. doi: 10.3201/eid0402.980206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niklasson B. Kinnunen L. Hornfeldt B, et al. A new picornavirus isolated from bank voles (Clethrionomys glareolus) Virology. 1999;255:86–93. doi: 10.1006/viro.1998.9557. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson AL. Lagerquist E. Lynch KF, et al. Temporal variation of Ljungan virus antibody levels in relation to islet autoantibodies and possible correlation to childhood Type 1 diabetes. Open Ped Med J. 2010;3:61–66. [Google Scholar]
- 32.Patterson CC. Dahlquist GG. Gyurus E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 33.Sadeharju K. Knip M. Hiltunen M, et al. 2003 The HLA-DR phenotype modulates the humoral immune response to enterovirus antigens. Diabetologia. 2003;46:1100–1105. doi: 10.1007/s00125-003-1157-x. [DOI] [PubMed] [Google Scholar]
- 34.Salminen K. Sadeharju K. Lonnrot M, et al. Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J Med Virol. 2003;69:91–98. doi: 10.1002/jmv.10260. [DOI] [PubMed] [Google Scholar]
- 35.Soltesz G. Patterson CC. Dahlquist G. Worldwide childhood type 1 diabetes incidence—What can we learn from epidemiology? Ped Diabetes. 2007;8(Suppl 6):6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 36.Stanway G. Kalkkinen N. Roivainen M, et al. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J Virol. 1994;68:8232–8238. doi: 10.1128/jvi.68.12.8232-8238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stene LC. Oikarinen S. Hyoty H, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: The Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tapia G. Cinek O. Rasmussen T, et al. No Ljungan virus RNA in stool samples from the Norwegian environmental triggers of type 1 diabetes (MIDIA) cohort study. Diabetes Care. 2010;33:1069–1071. doi: 10.2337/dc09-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaziri-Sani F. Delli AJ. Elding-Larsson H, et al. A novel triple mix radiobinding assay for the three ZnT8 (ZnT8-RWQ) autoantibody variants in children with newly diagnosed diabetes. J Immunol Methods. 2011;371:25–37. doi: 10.1016/j.jim.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon JW. The role of viruses and environmental factors in the induction of diabetes. Curr Topics Microbiol Immunol. 1990;164:95–123. doi: 10.1007/978-3-642-75741-9_6. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler AG. Bonifacio E. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55:1937–1943. doi: 10.1007/s00125-012-2472-x. [DOI] [PubMed] [Google Scholar]