Abstract
Significance
Wound healing is a complex physiological process involving a multitude of growth factors, among which transforming growth factor beta (TGF-β) has the broadest spectrum of effects. Animal studies have provided key information on the mechanisms of TGF-β action in wound healing and have guided the development of therapeutic strategies targeting the TGF-β pathway to improve wound healing and scarring outcome.
Recent Advances
Development of tissue-specific expression systems for overexpression or knockout of TGF-β signaling pathway components has led to novel insight into the role of TGF-β signaling in wound healing. This work has also identified molecules that might serve as molecular targets for the treatment of pathological skin conditions such as chronic wounds and excessive scarring (fibrosis).
Critical Issues
Many of the mouse models with genetic alterations in the TGF-β signaling pathway develop an underlying skin abnormality, which may pose some limitations on the interpretation of wound-healing results obtained in these animals. Also, TGF-β's pleiotropic effects on many cell types throughout all phases of wound healing present a challenge in designing specific strategies for targeting the TGF-β signaling pathway to promote wound healing or reduce scarring.
Future Directions
Further characterization of TGF-β signaling pathway components using inducible tissue-specific overexpression or knockout technology will be needed to corroborate results obtained in mouse models that display a skin phenotype, and to better understand the role of TGF-β signaling during distinct phases of the wound-healing process. Such studies will also provide a better understanding of how TGF-β mediates its autocrine, paracrine, and double paracrine effects on cellular responses in vivo during wound healing.

Kenneth W. Finnson, PhD
Scope and Significance
Wound healing is a complex physiological process characterized by the sequential overlapping phases of inflammation, proliferation, and maturation.1 Among the multitude of growth factors involved in wound healing, transforming growth factor beta (TGF-β) has the broadest spectrum of effects. TGF-β is a multifunctional growth factor that exerts pleiotropic effects on wound healing by regulating cell proliferation, differentiation, extracellular matrix (ECM) production, and immune modulation.1 Much of the current knowledge on TGF-β action in wound healing has been obtained from animal studies using incisional and/or excisional wounding models and manipulation of TGF-β signaling by addition of the exogenous TGF-β protein or anti–TGF-β neutralizing antibodies, or by genetic alteration in components of the TGF-β signaling pathway. The current review focuses on how the use of animal models have contributed to our understanding the role of TGF-β signaling in wound healing.
Translational Relevance
Animal models provide excellent experimental systems for elucidating molecular mechanisms by which TGF-β regulates wound-healing responses. Genetic manipulation in mice offers the unique opportunity to explore the function of endogenous TGF-β pathway components in wound healing. Other rodent (rat, rabbit) models are also useful since they display a scarring response that closely resembles that in humans. Animal models also play an important role in preclinical testing of agents that modulate TGF-β signaling in vivo, which are needed before proceeding to Phase 1 clinical testing for safety and tolerability in human subjects.
Clinical Relevance
Impaired wound healing and excessive scarring of the skin represent serious medical problems with limited options for treatment. Aberrant TGF-β signaling has been implicated in these conditions, suggesting that targeting the TGF-β pathway using therapeutic agents may improve wound healing and scarring outcome in these patients. Preclinical studies using animal models of wound healing and manipulation of TGF-β levels demonstrate the feasibility of this approach. Also, animal studies are needed for optimizing conditions before proceeding to a clinical setting.
Background
TGF-β was first identified in neoplastic tissues, which led to its name “transforming” growth factor.2,3 Later, TGF-βs were isolated and identified from normal tissues, and this soon led to a broad analysis of its physiological functions in various biological processes, including embryonic development and wound healing, and its pathophysiological role in cancer and fibrosis.4 The primary function of TGF-β as a stimulator of ECM production led to the exploration of its role as a wound-healing promoting factor.5 Further analyses have demonstrated the multifaceted role of TGF-βs on various cell populations, including epithelial, mesenchymal, and circulating hematopoetic cells involved in cutaneous wound healing. As elucidated in this review, exogenous and endogenous TGF-βs have dramatically different effects on cutaneous wound healing, emphasizing the spatial concentration and temporal dependence of its pleiotropic effects on distinct cell types and wound-healing events.
Overview of TGF-β Signaling
The TGF-β superfamily is a large family of structurally related molecules that include bone morphogenetic proteins, growth and differentiation factors, anti-müllerian hormone, activins, nodal, and TGF-βs.6 The three mammalian isoforms of TGF-β (TGF-β1, -β2, and -β3) share high (∼75%) sequence and structural similarity, but perform distinct functions in vivo.7 TGF-β isoforms are synthesized as homodimeric proproteins and undergo proteolytic cleavage in the trans-Golgi network by furin-like proprotein convertases, resulting in the formation of the mature TGF-β dimer. This dimer remains noncovalently associated with its propeptide, termed the latency-associated peptide (LAP), rendering the growth factor latent.8 TGF-βs are secreted as a multiprotein complex, in which the LAP portion of TGF-β is covalently linked by disulfide bonds with a protein known as the latent TGF-β binding protein. TGF-β must be released from this latent complex to bind to its receptors.8 Several activators of latent TGF-β have been identified, including thrombospondin 1 (TSP-1) and integrins, which activate TGF-β by dissociation from the latent complex.8
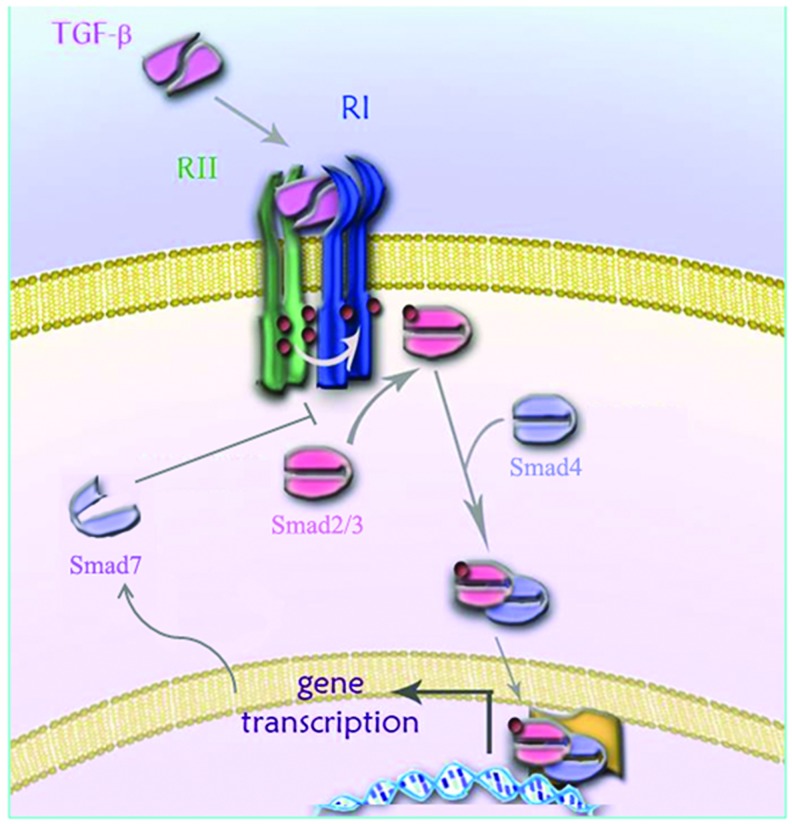
TGF-β signaling is transmitted by a pair of transmembrane serine/threonine kinase receptors, known as the type I and type II TGF-β receptors (TβRI, also known as activin receptor-like kinase 5 [ALK5], and TβRII, respectively).9 TGF-β first binds to TβRII, a constitutively active kinase, which then associates with and trans-phosphorylates ALK5, resulting in activation of ALK5 kinase activity.10 Activated ALK5 propagates the signal by phosphorylating intracellular Smad2 and Smad3 proteins. Phosphorylated Smad2/3 form a complex with Smad4, which shuttles into the nucleus and regulates target gene expression (Fig. 1).7 A subclass of Smads known as the inhibitory Smads (Smad6 and Smad7) play important roles in attenuating TGF-β signal transduction.11 Smad7 inhibits TGF-β signaling by binding to activated ALK5 and preventing Smad2/3 phosphorylation (Fig. 1).11 Smad7 also works as an adapter protein that recruits E3-ubiquitin ligases, Smad ubiquitination regulatory factors 1 and 2 (Smurf-1 and Smurf-2), to the activated TGF-β receptor complex, leading to ALK5 ubiquitination and proteasomal degradation.11 In addition to signaling via Smad proteins, TGF-β also activates other signaling pathways, including MAP kinases (ERK, p38, and JNK), PI3K/Akt, and Rho GTPase pathways.12
Figure 1.
Schematic diagram showing the main components of the TGF-β signaling pathway. TGF-β signals through a pair of transmembrane serine-threonine kinase receptors known as the type I (TβRI, also known as ALK5) and type II (TβRII) TGF-β receptors. TGF-β binds TβRII, a constitutively active kinase, which then associates with ALK5 resulting in the formation of a ternary signaling complex. TβRII phosphorylates ALK5 resulting in activation of the ALK5 kinase activity. Activated ALK5 then phosphorylates intracellular Smad2 and Smad3 proteins, which form a complex with Smad4 and enter the nucleus where they regulate gene transcription in co-operation with various coactivators or corepressors. Smad7 expression is induced by TGF-β and acts in a negative feedback loop to inhibit TGF-β signaling by binding to activated ALK5 and preventing Smad2/3 phosphorylation. ALK5, activin receptor-like kinase 5; TGF-β, transforming growth factor beta. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Animal Models
Manipulation of TGF-β signaling in wound healing using exogenously added molecules
Direct modulation of TGF-β levels
Initial observations on the potent effects of TGF-β in stimulating ECM production led to its assessment as a wound-healing agent.13 Injecting TGF-β into normal skin of newborn mice led to robust induction of angiogenesis and fibrosis. This included prominent new collagen synthesis and incorporation into the matrix. These observations prompted studies on administration of TGF-β to incisional wounds in rats, showing that TGF-β treatment resulted in better dermal healing, as indicated by prominent collagen deposition and significantly increased wound strength.14 Extending these observations to a perturbed wound-healing scenario, exogenous TGF-β was noted to promote healing in doxorubicin-treated rats, emphasizing its overarching role in wound healing.15 In some of the above studies, the use of neutralizing antibodies in vitro was able to block TGF-β–induced ECM production. Later, studies using exogenous neutralizing antibodies against TGF-βs in rat wounds were noted to reduce both the normal macrophage influx and the angiogenesis, collagen, and fibronectin content.16 Interestingly, the authors did not observe any detrimental changes in the wound-healing quality as assessed by the wound strength and tissue architecture. Further, studies using TGF-β isoform–specific neutralizing antibodies have demonstrated a specific role for TGF-β3 in promoting a scarless wound phenotype.17,18 A more recent study using a TGF-β3–specific neutralizing antibody has demonstrated an important role for endogenous TGF-β3 in excisional wound repair in vivo.19
Modulation of TGF-β bioavailability
Another strategy used to manipulate TGF-β action in vivo was to modify the levels of molecules that regulate TGF-β bioavailability. For example, decorin and fibromodulin, members of the small leucine-rich proteoglycan family, have been shown to inhibit TGF-β signaling in vitro by sequestering TGF-β in the ECM20 and to decrease TGF-β signaling in vivo during wound healing resulting in reduced scarring.21,22 Furthermore, the ectodomains of the TGF-β coreceptors betaglycan and CD109 have been shown to bind TGF-β and decrease TGF-β signaling in vitro,23–25 suggesting that they might decrease the bioavailability of TGF-β in vivo. In support of this notion, a peptide derived from the ectodomain of betaglycan (P144) was shown to ameliorate bleomycin-induced skin fibrosis.26 In addition, we have generated transgenic mice overexpressing CD109 in the epidermis (keratin [K] 14–driven) and showed that these mice display improved wound healing, reduced scarring,27 and resistance to bleomycin-induced skin fibrosis28 in manners consistent with reduced TGF-β signaling.
Modulation of TGF-β receptor activity using small molecules
Small molecule inhibitors that block ALK5 kinase activity have been tested for their efficacy to improve scarring in animal models. The ALK5 inhibitor, SB431542, has been shown to reduce scar formation in the eye after glaucoma filtration surgery in rabbits.29 SB431542 used in combination with recombinant human granulocyte colony-stimuating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) was shown to improve wound breaking strength in rat skin incisional wounds.30 A novel ALK5 inhibitor (CP-639180) was shown to reduce collagen deposition in a rat dermal incision wound-healing model.31 A recent study reported the discovery of a series of 2-(1H-pyrazol-1-yl)pyridines as ALK5 inhibitors with the potential to prevent dermal scarring.32 Topical application of one of these compounds (PF-03671148) to rat incisional wounds decreased fibrotic gene expression without altering the normal wound-healing process.32
Taken together, the above studies indicate that exogenously added TGF-β has the potential to promote wound healing by stimulating angiogenesis, immune cell infiltration, and ECM production, and that diminishing endogenous TGF-β action reduces scarring without adversely affecting wound-healing quality.
Genetic manipulation of endogenous TGF-βs in wound healing
A complementary approach to the above studies is to manipulate the expression of specific TGF-β pathway components by genetic approaches. Several mouse models with genetically modified components of the TGF-β signaling pathway have been generated and tested in wound-healing studies. This section highlights the main findings obtained from these studies.
Modulation of TGF-β activation from its latent complex
TSP-1
TSP-1 is a multidomain matrix glycoprotein that binds to the TGF-β latent complex and induces a conformational change in LAP that prevents LAP from conferring latency on mature TGF-β.33 Several studies using mice with genetic alterations in TSP-1 expression suggest that TSP-1 plays a critical role in wound healing. For example, TSP-1 knockout (KO) mice display delayed healing of excisional wounds with a decrease in active and total TGF-β1 levels in the wounds compared to wild-type (WT) mice.34 These findings were extended in a study demonstrating that TSP-1 KO mice exhibit impaired excisional wound healing with persistent granulation tissue, neovascularization, and inflammation.35 Importantly, topical application of a full-length recombinant TSP-1 protein or a TSP-1 peptide (amino acid sequence KRFK) that activates latent TGF-β, rescues the defective wound-healing phenotype and partially restores the local levels of TGF-β1 in TSP-1 KO excisional wounds.35 In addition, the rescuing effects of the TSP-1 protein and peptides in TSP-1 KO mouse wounds is partially reversed by addition of a TGF-β neutralizing antibody.35 Another study showed that transgenic mice overexpressing TSP-1 under the control of the K14 promoter to restrict transgene expression to the epidermis display impaired wound healing associated with reduced granulation tissue formation and diminished wound angiogenesis.36 Whether the effects of epidermal-specific TSP-1 overexpression on wound healing are mediated by TGF-β-dependent or TGF-β-independent mechanisms or both, remains to be determined.
Integrins
Integrins are cell-surface heterodimeric proteins consisting of an alpha and a beta subunit that mediate cell–ECM interactions and transmembrane signaling.8 In mammals, 18 alpha and 8 beta subunits have been characterized, and several integrins thought to participate in activation of latent TGF-β (integrins-α3, -β1, -β3, -β6) have been shown to be involved in the regulation of wound healing. For instance, skin from integrin-α3 KO mice grafted onto nude (immunodeficient) mice shows delayed re-epithelialization of excisional wounds as compared to WT littermate skin.37 Integrin-α3 KO mouse skin shows increased expression of Smad7, a major inhibitor of TGF-β signaling, and blockade of Smad7 expression in vivo using antisense oligonucleotides restored the re-epithelialization defect in the integrin-α3 KO skin.37 Although this study did not report any structural defects in integrin-α3 KO skin, earlier studies have shown that it displays a disorganized basement membrane with frequent blistering at the dermal–epidermal junction.38,39 A study using an epidermal-specific integrin-α3 KO mouse model (mediated by K14-Cre recombinase, K14-Cre/Itga3−/− mice) showed that they display a slightly enhanced wound re-epithelialization, suggesting that epidermal integrin-α3 delays, rather than facilitates, wound closure.40 Another study showed that K14-Cre/Itga3−/− mice exhibit impaired wound angiogenesis.41 Both studies using the K14-Cre/Itga3−/− mice reported a milder form of the skin phenotype compared to the global integrin-α3 KO mice. The discrepancies in the information obtained using global and tissue-specific integrin-β3 KO mice may reflect the more prominent skin defect in the global KO mice. Further studies using an inducible tissue-specific approach may resolve these discrepancies.
Mice with genetically modified integrin-β subunit expression have also been used to understand the role or integrins in wound healing. For example, integrin-β3 KO mice subjected to excisional wounds show improved wound healing with accelerated re-epithelialization and enhanced TGF-β1 signaling as compared to WT mice.42 Although not reported in this study, previous work has shown that integrin-β3 KO mice exhibit an underlying defect in angiogenesis43,44 which may interfere with the normal wound-healing process. This issue might be addressed in future wound-healing studies using an inducible skin (epidermal and/or dermal)-specific integrin-β3 KO mice. Another study has shown that mice containing an inducible (postnatal) fibroblast-specific deletion of integrin-β1 exhibit delayed excisional wound closure and diminished granulation tissue formation characterized by decreased ECM synthesis.45 Administration of exogenous TGF-β1 was shown to rescue the phenotype of these mice45 suggesting that dermal integrin-β1 promotes wound closure by increasing the TGF-β activity.
Research on integrin-β6 has revealed its potential role in the pathogenesis of chronic wounds. The observation that integrin-β6 expression is markedly increased in the epidermis in human chronic wounds prompted the question of whether transgenic mice overexpressing integrin-β6 in the epidermis display wound-healing abnormalities.46 Although the transgenic mice did not exhibit any differences in the rate of cutaneous wound closure as compared to WT mice, ∼25% of the transgenic mice developed chronic ulcers with elevated TGF-β1 expression.46 Other studies have shown that integrin-β6 plays an important role in the healing of compromised wounds. For example, integrin-β6 KO mice show delayed wound healing in older mice compared to age-matched WT mice,47 and during streptozotocin-induced diabetes as compared to diabetic WT littermates.48 The importance of TGF-β activation in mediating the wound-healing effects observed in integrin-β6 KO mice in the above studies was not determined and requires further investigation. Taken together, these studies suggest that endogenous integrin-β6 plays an important protective role during compromised wound healing and that its aberrant overproduction may lead to the development and/or progression of chronic nonhealing wounds.
Modulation of TGF-β1 expression
TGF-β1 KO (TGF-β1−/−) mice die at about 3–4 weeks of age from an autoimmune-like inflammatory response,49 which poses limitations on their application to wound-healing studies. One study showed that 10-day-old TGF-β1−/− mice (still nursing) subjected to excisional wounds display delayed re-epithelialization and reduced granulation tissue formation 10 days postwounding as compared to WT mice.50 Early wound healing in 10-day-old TGF-β1−/− mice proceeded relatively normally, presumably because of upregulation or functional redundancy of other growth factors50 or maternal rescue by means of TGF-β1 transmitted in milk before weaning,51 until complications arose from the inflammatory syndrome.50 To circumvent the problems encountered in the above studies, researchers opted to suppress the inflammatory response by treating TGF-β1−/− and WT mice with rapamycin, which prolongs the lifespan of TGF-β1−/− mice, and perform wound-healing studies in older (30-day-old, non-nursing) mice.52 Under these conditions, TGF-β1−/− mice display accelerated re-epithelialization and decreased granulation tissue formation as compared to WT mice.52 Another strategy used was to generate mice that were both TGF-β1−/− and immunodeficient (Scid−/− lacking B and T cells).53 Contrary to the hypothesis that wound repair would proceed normally in TGF-β1−/− Scid−/− mice, even at the later stages of healing, these mice showed a major delay in wound healing as compared to TGF-β1+/+ Scid−/− and TGF-β1+/− Scid−/− mice.53 Although the pathophysiological mechanism(s) underlying the delayed wound-healing response in TGF-β1−/− Scid−/− mice remains to be elucidated, these findings suggest an important synergistic interaction with TGF-β signaling and the immune system in mediating normal wound healing.
More recently, transgenic mice overexpressing active or latent forms of TGF-β1 in the epidermis have been developed. However, these models display skin phenotypes which limit their application to wound-healing studies. For example, transgenic mice overexpressing constitutively active TGF-β1 under the control of the K1 promoter, to restrict transgene expression to the spinous and granular layers of the epidermis, display shiny and tautly stretched skin with a thickened epidermis.54 Transgenic mice overexpressing constitutively active TGF-β1 driven by the K10 promoter, which restricts transgene expression suprabasally, showed hyperproliferation of epidermal cells in the absence of hyperplasia, suggesting an increase in epidermal cell turnover.55 The latter findings are in contrast to the expected growth inhibitory effect of TGF-β on epithelial cell proliferation and may be related to the use of the K10 promoter or other factors.55 In comparison, transgenic mice overexpressing latent TGF-β1 under the control of the K5 promoter, which targets transgene expression to the basal layer of the epidermis and hair follicles,56 developed inflammatory skin lesions, with gross appearance of psoriasis-like plaques.57 Interestingly, transgenic mice that overexpress latent TGF-β1 under the control of the K14 promoter, which also restricts transgene expression to the basal epidermal layer and hair follicles, display a scruffiness of the fur and thinner skin that is only observed in the homozygous transgenic mice (i.e., not in the heterozygote mice or WT littermates).58 Importantly, excisional wounds of both homozygous and heterozygous transgenic mice show a delay in re-epithelialization and increase in type I collagen mRNA expression as compared to wounds in WT littermates, suggesting a paracrine mechanism where epidermal transgene expression leads to stimulation of underlying dermal fibroblasts leading to fibrosis.58 A parallel study using these transgenic mice in a laser-induced thermal injury model showed that both homozygous and heterozygous transgenic mice have a delay in re-epithelialization associated with reduced keratinocyte proliferation and increased type I collagen mRNA production following burn injury as compared to WT mice.59 The similar results obtained using homozygous mice (which display an underlying skin abnormality) and heterozygote mice (which did not display the skin phenotype) support the notion that the effects of TGF-β1 transgene expression on wound healing are not a consequence of developmental defects or an underlying abnormality in the skin.
Modulation of TGF-β receptor expression
TβRII
TβRII KO mice show defects in the yolk sac hematopoiesis and vasculogenesis that results in embryonic lethality,60 precluding their application to wound-healing studies. Transgenic mice expressing a dominant-negative mutant form of TβRII driven by a mouse loricrin promoter, which expresses transgenes in both the basal and suprabasal layers of the epidermis, are viable, but display an overt skin phenotype characterized by thickened and wrinkled skin with epidermal hyperproliferation and hyperkeratosis.61 In addition, expression of a dominant-negative mutant form of TβRII driven by the fibroblast-specific mouse Col1A2 promoter leads to a paradoxical activation of TGF-β signaling and fibrosis.62 These studies suggest that endogenous TβRII plays a critical role in skin development and homeostasis and that manipulation of TβRII for wound-healing studies may require inducible tissue-specific manipulation of TβRII expression. Accordingly, two recent studies using inducible fibroblast-specific TβRII KO mice in an excisional wounding model demonstrated that postnatal abrogation of TβRII expression in the dermis led to defective wound contraction, reduced dermal scarring, and enhanced re-epithelialization.63,64 These findings highlight an important role for dermal TGF-β signaling in regulating cutaneous wound healing and scarring by autocrine, paracrine, and/or double paracrine mechanisms. Future studies using inducible epidermal-specific TβRII KO mice may advance our understanding of TGF-β signaling mechanisms that regulate epidermal–dermal interactions during wound healing and scarring.
TβRI (ALK5)
As with TβRII KO mice, ALK5 KO mice have defects in yolk sac angiogenesis resulting in embryonic lethality,65 necessitating the development of tissue-specific systems to manipulate ALK5 expression for in vivo studies. One group attempted to generate transgenic mice that express a (noninducible) fibroblast-specific, constitutively active form of ALK5 (caALK5), but all embryos died early during gestation.66 They then developed mice expressing an inducible fibroblast-specific caALK5 and found that postnatal induction of caALK5 expression led to a skin phenotype characterized by dermal fibrosis and a thinner epidermis.66 Another study generated mice with epithelium-specific (K14-Cre recombinase driven) deletion of ALK5 that do not have an overt skin phenotype, although they have craniofacial defects which interfere with normal feeding and reduce their lifespan.67 More recently, mice with a tamoxifen-inducible epithelium-specific (K14-CreER tam) deletion of ALK5 have been generated (to investigate the role of TGF-β signaling in head and neck squamous cell carcinoma)68 that would be amenable to wound-healing studies. Intriguingly, a recent study has discovered a regenerative phenotype in mice with a point mutation in ALK5.69 These mice display accelerated closure of ear punch wounds reminiscent of the perfect wound-healing response observed in ear punch wounds created in MRL/MpJ mice.70 A preliminary study showed a small but significant acceleration in cutaneous (dorsal skin) wound closure in the ALK5 mutant mice, but the healing architecture and histological appearance did not markedly differ between ALK5 mutant and WT mice.69 Further studies using these mice in incisional and excisional wound-healing models are needed to fully appreciate their potential for investigating TGF-β signaling dynamics in wound healing.
Modulation of Smad expression
Smad3
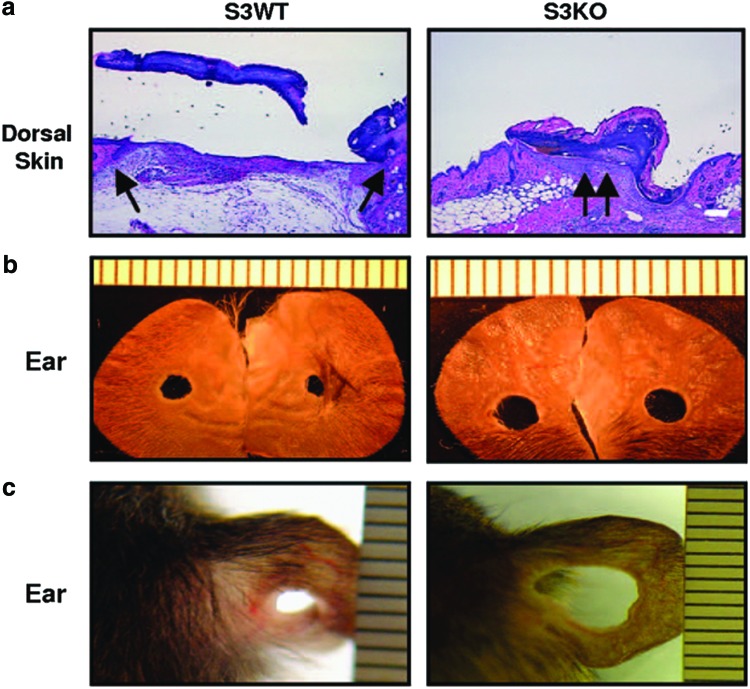
Because exogenous application of TGF-β has been shown to improve wound healing in mice, loss of Smad3 expression might be expected to have the opposite effect—delayed wound healing. On the contrary, Smad3 KO mice—which are viable and fertile, but display various defects, including impaired immune function71–73—show an accelerated cutaneous wound healing with a faster rate of re-epithelialization and an impaired local inflammatory response.74 The faster re-epithelialization involves altered keratinocyte growth and migration and the impaired inflammatory response was attributed to decreased chemotactic responses of monocytes to TGF-β.74 Smad3 KO mice also show accelerated wound closure in a tail-wounding model for delayed wound closure.75 Interestingly, excisional ear wounds made in Smad3 KO mice were shown to enlarge as compared to wounds in WT mice (Fig. 2).76 This result was attributed to changes in organization and expression of ECM molecules in the matrix leading to altered the mechanotransduction properties of these wounds.76 Other studies suggest that Smad3 mediates profibrotic/scarring responses, as evidenced by Smad3 KO mice being protected against cutaneous injury induced by ionizing radiation77 and having a resistance to bleomycin-induced skin fibrosis.78 Taken together, these results suggest that endogenous Smad3 delays re-epithelialization and promotes inflammation during early stages, but contributes to fibrosis (scar formation) at later stages of wound healing. Future studies using constitutive or inducible tissue-specific manipulation of Smad3 expression in the dermis, epidermis, and immune cells will be needed to more clearly define the role of Smad3 in cutaneous wound healing in the absence impaired immune function.
Figure 2.
Excisional wound healing in Smad 3 KO mice. Smad3 KO mice display opposite wound-healing responses in the dorsal skin versus the ear as described in section Modulation of Smad expression. (a) Dorsal skin 48 h after wounding stained with hematoxylin/eosin. Arrows point to epithelial wound margins. Scale bar: 100 μm. (b) Ear wounds 38 days after wounding. (c) Ear wounds in SVEV129 mice at 36 days after wounding. The differences in ear wounds are more apparent in the pure SVEV129 strain as compared with the mixed strain (C57BL/6×Black Swiss×SVEV129) shown in (b). KO, knockout. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Smad2
Studies on the function of Smad2 in wound healing have lagged behind studies on Smad3, possibly because Smad2 KO mice are embryonic lethal.79 However, transgenic mice overexpressing Smad2 under the control of the K14 promoter have been generated and display a skin phenotype with severe thickening and disorganization of the epidermis, irregular basement membrane, and dermal fibrosis.80 Another group showed that K14-Smad2 transgenic mice exhibit delayed healing of cutaneous incisional wounds as compared to WT mice, resulting from a defect in basal keratinocyte migration.81 However, the severity of the skin phenotype in K14-Smad2 transgenic mice was not reported in this study. Similar results showing a delay in wound healing due to inhibition of epithelial cell migration in K14-Smad2 mice were obtained using a gingival wound-healing model.82 These results are corroborated in a recent study showing that keratinocyte-specific Smad2 KO mice display the opposite effect—accelerated re-epithelialization with enhanced keratinocyte migration.83 Taken together, these studies suggest that epidermal Smad2 plays an important role in wound re-epithelialization.
Smad4
Smad4 is a common binding partner for Smad2 and Smad3, and Smad2/3-mediated responses require prior complex formation with Smad4 for their nuclear translocation and subsequent binding to specific TGF-β responsive promoter elements.84 Because Smad4 KO mice are embryonic lethal,85,86 researchers have developed tissue-specific Smad4 KO mice for in vivo studies. One study showed that deletion of Smad4 in epithelial tissues, including epidermis (mediated by MMTV-Cre recombinase), did not affect re-epithelialization, but led to several wound-healing defects in nonepidermal compartments, including delayed wound closure, increased angiogenesis, and increased inflammation.87 Although these conditional Smad4 KO mice have been shown to have hair follicle defects and squamous cell carcinoma formation,88 the wound-healing study reported using only nontumor-bearing mice.87 Another study using epidermal-specific Smad4 KO mice (mediated by K5-Cre recombinase), which also display impaired hair follicle cycling and form skin tumors,89 showed that these mice display accelerated re-epithelization leading to faster cutaneous wound repair.90 Although the reasons for the different results in the two studies are not known, they may be due to differences in tissue specificities of promoters, with MMTV-Cre activity directing a widespread pattern of expression88 and K5-Cre activity restricted to more basal layers of the epidermis.89 Future wound-healing studies using inducible epidermal-specific Smad4 KO mice may be needed to resolve the apparent discrepancies.
TAKE-HOME MESSAGES.
Basic science advances
Animal studies have advanced knowledge of the role of TGF-β signaling in the regulation of wound healing and scarring.
The occurrence of skin phenotypes in mice with global or conditional (tissue-specific) genetic modifications TGF-β signaling pathway signaling components underscores a critical need for the further development of inducible, tissue-specific expression systems for postnatal manipulation of TGF-β signaling in wound-healing studies.
Clinical science advances
Genetic manipulation of TGF-β pathway components in mice has led to the discovery of novel molecules (such as integrin β6) that are involved in the pathogenesis of chronic wounds and may represent novel targets for therapeutic treatment.
Relevance to clinical care
Characterization of the role of TGF-β signaling and components of this pathway in animal models of wound healing may lead to the development of a novel therapy to promote wound healing and/or reduce scarring.
Smad7
Smad7 is an inhibitory Smad that inhibits TGF-β–Smad2/3 signaling through different mechanisms (described in the section Overview of TGF-β signaling). Transgenic mice overexpressing Smad7 under the control of a K5 promoter (K5-Smad7 mice) display overt skin defects, which include epidermal hyperproliferation and aberrant hair follicle morphogenesis.56 However, K5-Smad7 mice expressing lower levels of the Smad7 transgene have no obvious skin abnormalities, and exhibit accelerated re-epithelialization and reduced inflammation in excisional wounds as compared to nontransgenic mice.91 More recently, transgenic mice expressing an inducible Smad7 transgene under the control of the K14 promoter have been generated.92 Postnatal induction of Smad7 expression was associated with accelerated re-epithelialization in excisional wounds with profound effects on the wound stroma, including reduced inflammation, angiogenesis, and type I collagen production.92 Taken together, these studies point to an important role for epidermal Smad7 expression in regulating epidermal–dermal interactions during wound healing by autocrine, paracrine, and possibly double paracrine mechanisms.
Summary and Concluding Remarks
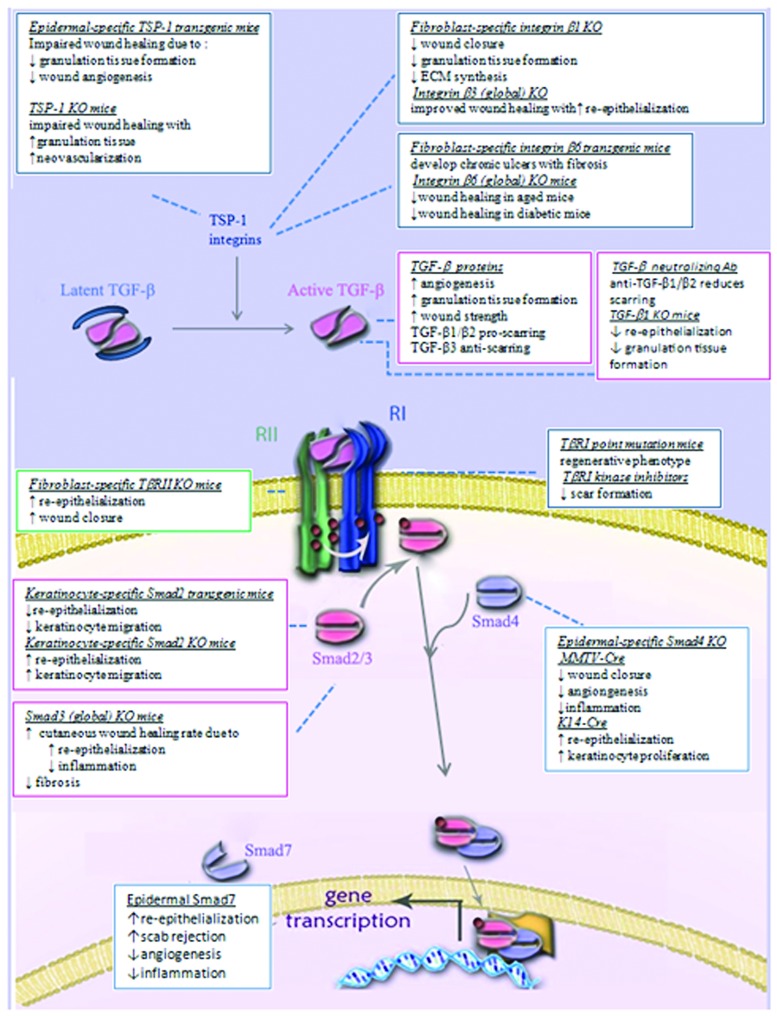
The complex role of TGF-βs in wound healing is evident from the reviewed literature. Besides the multifaceted role of TGF-β on specific cells of various lineages, the additional complexity due to its origin (exogenous versus endogenous), concentrations, and interactions (presence of synergistic or antagonistic factors, signaling cross talks) are all significant components determining its ultimate effect on the wound-healing outcome. Despite the shortcomings of using genetically modified mice, in some cases with underlying skin abnormalities, the information gathered from these models provide a foundation for future studies using more advanced inducible, tissue-specific expression systems to spatially and temporally control TGF-β signaling throughout the wound-healing process. The recent advances in our understanding of the mechanisms of TGF-β signaling—from ligand activation to intracellular signaling pathways, and their application to wound-healing studies—have not yielded results for immediate clinical application. However, these studies have immensely enriched our appreciation of the cellular mechanisms that control TGF-β responses during normal and pathological wound healing. Given the potent effects of TGF-β in these processes, future studies to identify components controlling the TGF-β-signaling pathways are likely to suggest new targets for therapeutic intervention to combat pathological wound-healing outcomes. Figure 3 depicts the main components of the TGF-β signaling pathway and a summarizes the results obtained from manipulation of this pathway in animal models.
Figure 3.
Schematic diagram summarizing available information obtained using animal models to investigate the role of TGF-β signaling in wound healing. The basic components of the TGF-β signaling pathway shown in Figure 1 are used here as a background to illustrate the phenotypic differences observed from manipulating the specific components in wound-healing studies. See text sections Manipulation of TGF-β signaling in wound healing using exogenously added molecules and Genetic manipulation of TGF-β signaling in wound healing for a detailed account of the signaling components manipulated and the specific effects on the wound-healing outcome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Abbreviations and Acronyms
- ALK5
activin receptor-like kinase 5
- ECM
extracellular matrix
- K1, K5, K10, K14
keratins-1, 5, 10, and 14
- KO
knockout
- LAP
latency-associated peptide
- Smurf
Smad ubiquitination regulatory factor
- TGF-β
transforming growth factor beta
- TSP-1
thrombospondin 1
- TβRII
type II TGF-β receptor
- WT
wild-type
Acknowledgments and Funding Sources
We would like to thank Albane Bizet and Anshuman Saksena (both members of the Philip Lab) for figure preparation. The work presented in this article was supported by a Canadian Institute of Health Research Grant to A.P. PRA is supported by the Intramural Research Program in NIDCR, NIH.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Dr. Kenneth Finnson is a Research Associate with Dr. Anie Philip in the Department of Surgery, McGill University, Montreal, Quebec, Canada. Dr. Praveen Arany is a clinical investigator at the National Institutes of Health in Bethesda, MD. His research focuses on the use of physical tools such as laser radiation to modulate TGF-β signaling during wound healing. Dr. Anie Philip is a Professor in the Department of Surgery, McGill University. Dr. Philip's research program focuses on understanding the molecular mechanisms of TGF-β signaling in wound healing, scarring, and fibrosis.
References
- 1.Penn JW. Grobbelaar AO. Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18. [PMC free article] [PubMed] [Google Scholar]
- 2.Moses HL. Branum EL. Proper JA. Robinson RA. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981;41:2842. [PubMed] [Google Scholar]
- 3.Roberts AB. Lamb LC. Newton DL. Sporn MB. De Larco JE. Todaro GJ. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci USA. 1980;77:3494. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts AB. Anzano MA. Lamb LC. Smith JM. Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci USA. 1981;78:5339. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn MB. Roberts AB. Shull JH. Smith JM. Ward JM. Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983;219:1329. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- 6.Wharton K. Derynck R. TGF-β family signaling: novel insights in development and disease. Development. 2009;136:3691. doi: 10.1242/dev.040584. [DOI] [PubMed] [Google Scholar]
- 7.Moustakas A. Heldin CH. The regulation of TGFβ signal transduction. Development. 2009;136:3699. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 8.Horiguchi M. Ota M. Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem. 2012;152:321. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santibanez JF. Quintanilla M. Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121:233. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- 10.Massague J. Gomis RR. The logic of TGF-β signaling. FEBS Lett. 2006;580:2811. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Yan X. Chen YG. Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J. 2011;434:1. doi: 10.1042/BJ20101827. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2008;19:128. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts AB. Sporn MB. Assoian RK. Smith JM. Roche NS. Wakefield LM. Heine UI. Liotta LA. Falanga V. Kehrl JH. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustoe TA. Pierce GF. Thomason A. Gramates P. Sporn MB. Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-β. Science. 1987;237:1333. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- 15.Cromack DT. Sporn MB. Roberts AB. Merino MJ. Dart LL. Norton JA. Transforming growth factor β levels in rat wound chambers. J Surg Res. 1987;42:622. doi: 10.1016/0022-4804(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 16.Shah M. Foreman DM. Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor β. Lancet. 1992;339:213. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 17.McCollum PT. Bush JA. James G. Mason T. O'Kane S. McCollum C. Krievins D. Shiralkar S. Ferguson MW. Randomized phase II clinical trial of avotermin versus placebo for scar improvement. Br J Surg. 2011;98:925. doi: 10.1002/bjs.7438. [DOI] [PubMed] [Google Scholar]
- 18.Shah M. Foreman DM. Ferguson MW. Neutralising antibody to TGF-β 1, 2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107(Pt 5):1137. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- 19.Le M. Naridze R. Morrison J. Biggs LC. Rhea L. Schutte BC. Kaartinen V. Dunnwald M. Transforming growth factor β3 is required for excisional wound repair in vivo. PLoS One. 2012;201(7):e48040. doi: 10.1371/journal.pone.0048040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildebrand A. Romarís M. Rasmussen LM. Heinegård D. Twardzik DR. Border WA. Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302(Pt 2):527. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z. Nguyen C. Zhang X. Khorasani H. Wang JZ. Zara JN. Chu F. Yin W. Pang S. Le A. Ting K. Soo C. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-β3 signaling. J Invest Dermatol. 2012;131:769. doi: 10.1038/jid.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvinen TA. Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing, suppresses scar formation in mice. Proc Natl Acad Sci USA. 2010;107:21671. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man XY. Finnson KW. Baron M. Philip A. CD109, a TGF-β co-receptor, attenuates extracellular matrix production in scleroderma skin fibroblasts. Arthritis Res Ther. 2012;14:R144. doi: 10.1186/ar3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilchis-Landeros MM. Montiel JL. Mendoza V. Mendoza-Hernandez G. Lopez-Casillas F. Recombinant soluble betaglycan is a potent and isoform-selective transforming growth factor-β neutralizing agent. Biochem J. 2001;355:215. doi: 10.1042/0264-6021:3550215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam B. Larouche D. Germain L. Hooper N. Philip A. Characterization of a 150 kDa accessory receptor for TGF-β1 on keratinocytes: direct evidence for a GPI anchor and ligand binding of the released form. J Cell Biochem. 2001;83:494. doi: 10.1002/jcb.1074. [DOI] [PubMed] [Google Scholar]
- 26.Santiago B. Gutierrez-Cañas I. Dotor J. Palao G. Lasarte JJ. Ruiz J. Prieto J. Borrás-Cuesta F. Pablos JL. Topical application of a peptide inhibitor of transforming growth factor-β1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol. 2005;125:450. doi: 10.1111/j.0022-202X.2005.23859.x. [DOI] [PubMed] [Google Scholar]
- 27.Vorstenbosch J. Gallant-Behm C. Trzeciak A. Roy S. Mustoe T. Philip A. Transgenic mice overexpressing CD109 in the epidermis display decreased inflammation and granulation tissue and improved collagen architecture during wound healing. Wound Repair Regen. 2013;21:235. doi: 10.1111/wrr.12023. [DOI] [PubMed] [Google Scholar]
- 28.Vorstenbosch J. Al-Ajmi H. Winocour S. Trzeciak A. Philip A. CD109 overexpression ameliorates skin fibrosis in a bleomycin-induced mouse model of scleroderma. Arthritis Rheum. 2013;65:1378. doi: 10.1002/art.37907. [DOI] [PubMed] [Google Scholar]
- 29.Xiao YQ. Liu K. Shen JF. Xu GT. Ye W. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Invest Ophthalmol Vis Sci. 2009;50:1698. doi: 10.1167/iovs.08-1675. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama K. Ishii G. Ochiai A. Esumi H. Improvement of the breaking strength of wound by combined treatment with recombinant human G-CSF, recombinant human M-CSF, and a TGF-β1 receptor kinase inhibitor in rat skin. Cancer Sci. 2008;99:1021. doi: 10.1111/j.1349-7006.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian F. Render J. Ren XD. Chio C. Chan K. Boys M. Lala DS. Pocalyko D. An activin receptor-like kinase 5 inhibitor reduces collagen deposition in a rat dermal incision wound healing model. Plastic Reconstr Surg. 2011;128:451e. doi: 10.1097/PRS.0b013e31822b65c7. [DOI] [PubMed] [Google Scholar]
- 32.Boys ML. Bian F. Kramer JB. Chio CL. Ren XD. Chen H. Barrett SD. Sexton KE. Iula DM. Filzen GF. Nguyen MN. Angell P. Downs VL. Wang Z. Raheja N. Ellsworth EL. Fakhoury S. Bratton LD. Keller PR. Gowan R. Drummond EM. Maiti SN. Hena MA. Lu L. McConnell P. Knafels JD. Thanabal V. Sun F. Alessi D. McCarthy A. Zhang E. Finzel BC. Patel S. Ciotti SM. Eisma R. Payne NA. Gilbertsen RB. Kostlan CR. Pocalyko DJ. Lala DS. Discovery of a series of 2-(1H-pyrazol-1-yl)pyridines as ALK5 inhibitors with potential utility in the prevention of dermal scarring. Bioorg Med Chem Lett. 2012;22:3392. doi: 10.1016/j.bmcl.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Murphy-Ullrich JE. Poczatek M. Activation of latent TGF-β by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 34.Agah A. Kyriakides TR. Lawler J. Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nör JE. Dipietro L. Murphy-Ullrich JE. Hynes RO. Lawler J. Polverini PJ. Activation of latent TGF-β1 by thrombospondin-1 is a major component of wound repair. Oral Biosci Med OBM. 2005;2:153. [PMC free article] [PubMed] [Google Scholar]
- 36.Streit M. Velasco P. Riccardi L. Spencer L. Brown LF. Janes L. Lange-Asschenfeldt B. Yano K. Hawighorst T. Iruela-Arispe L. Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds LE. Conti FJ. Silva R. Robinson SD. Iyer V. Rudling R. Cross B. Nye E. Hart IR. Dipersio CM. Hodivala-Dilke KM. α3β1 integrin-controlled Smad7 regulates re-epithelialization during wound healing in mice. J Clin Invest. 2008;118:965. doi: 10.1172/JCI33538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiPersio CM. Hodivala-Dilke KM. Jaenisch R. Kreidberg JA. Hynes RO. Alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Arcangelis A. Mark M. Kreidberg J. Sorokin L. Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- 40.Margadant C. Raymond K. Kreft M. Sachs N. Janssen H. Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell K. Szekeres C. Milano V. Svenson KB. Nilsen-Hamilton M. Kreidberg JA. DiPersio CM. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122:1778. doi: 10.1242/jcs.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds LE. Conti FJ. Lucas M. Grose R. Robinson S. Stone M. Saunders G. Dickson C. Hynes RO. Lacy-Hulbert A. Hodivala-Dilke K. Accelerated re-epithelialization in β3-integrin-deficient- mice is associated with enhanced TGF-β1 signaling. Nat Med. 2005;11:167. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 43.Hodivala-Dilke KM. McHugh KP. Tsakiris DA. Rayburn H. Crowley D. Ullman-Culleré M. Ross FP. Coller BS. Teitelbaum S. Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds LE. Wyder L. Lively JC. Taverna D. Robinson SD. Huang X. Sheppard D. Hynes RO. Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 45.Liu S. Xu SW. Blumbach K. Eastwood M. Denton CP. Eckes B. Krieg T. Abraham DJ. Leask A. Expression of integrin β1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 46.Hakkinen L. Koivisto L. Gardner H. Saarialho-Kere U. Carroll JM. Lakso M. Rauvala H. Laato M. Heino J. Larjava H. Increased expression of β6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AlDahlawi S. Eslami A. Hakkinen L. Larjava HS. The αvβ6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen. 2006;14:289. doi: 10.1111/j.1743-6109.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen JN. Steffensen B. Hakkinen L. Krogfelt KA. Larjava HS. Skin wound healing in diabetic beta6 integrin-deficient mice. Apmis. 2010;118:753. doi: 10.1111/j.1600-0463.2010.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shull MM. Ormsby I. Kier AB. Pawlowski S. Diebold RJ. Yin M. Allen R. Sidman C. Proetzel G. Calvin D. Annunziata N. Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown RL. Ormsby I. Doetschman TC. Greenhalgh DG. Wound healing in the transforming growth factor-β-deficient mouse. Wound Repair Regen. 1995;3:25. doi: 10.1046/j.1524-475X.1995.30108.x. [DOI] [PubMed] [Google Scholar]
- 51.Letterio JJ. Geiser AG. Kulkarni AB. Roche NS. Sporn MB. Roberts AB. Maternal rescue of transforming growth factor-β1 null mice. Science. 1994;264:1936. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- 52.Koch RM. Roche NS. Parks WT. Ashcroft GS. Letterio JJ. Roberts AB. Incisional wound healing in transforming growth factor-β1 null mice. Wound Repair Regen. 2000;8:179. doi: 10.1046/j.1524-475x.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- 53.Crowe MJ. Doetschman T. Greenhalgh DG. Delayed wound healing in immunodeficient TGF-β1 knockout mice. J Invest Dermatol. 2000;115:3. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 54.Sellheyer K. Bickenbach JR. Rothnagel JA. Bundman D. Longley MA. Krieg T. Roche NS. Roberts AB. Roop DR. Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc Natl Acad Sci USA. 1993;90:5237. doi: 10.1073/pnas.90.11.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui W. Fowlis DJ. Cousins FM. Duffie E. Bryson S. Balmain A. Akhurst RJ. Concerted action of TGF-β1 and its type II receptor in control of epidermal homeostasis in transgenic mice. Genes Dev. 1995;9:945. doi: 10.1101/gad.9.8.945. [DOI] [PubMed] [Google Scholar]
- 56.He W. Li AG. Wang D. Han S. Zheng B. Goumans MJ. Ten Dijke P. Wang XJ. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J. 2002;21:2580. doi: 10.1093/emboj/21.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li A. Wang D. Feng X. Wang X. Latent TGFβ1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23:1770. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan T. Ghahary A. Demare J. Yang L. Iwashina T. Scott PG. Tredget EE. Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-β1 transgenic mouse. Wound Repair Regen. 2002;10:177. doi: 10.1046/j.1524-475x.2002.11101.x. [DOI] [PubMed] [Google Scholar]
- 59.Yang L. Chan T. Demare J. Iwashina T. Ghahary A. Scott PG. Tredget EE. Healing of burn wounds in transgenic mice overexpressing transforming growth factor-β1 in the epidermis. Am J Pathol. 2001;159:2147. doi: 10.1016/s0002-9440(10)63066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oshima M. Oshima H. Taketo MM. TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 61.Wang XJ. Greenhalgh DA. Bickenbach JR. Jiang A. Bundman DS. Krieg T. Derynck R. Roop DR. Expression of a dominant-negative type II transforming growth factor β (TGF-β) receptor in the epidermis of transgenic mice blocks TGF-β-mediated growth inhibition. Proc Natl Acad Sci USA. 1997;94:2386. doi: 10.1073/pnas.94.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denton CP. Zheng B. Evans LA. Shi-wen X. Ong VH. Fisher I. Lazaridis K. Abraham DJ. Black CM. de Crombrugghe B. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor β (TGFβ) receptor leads to paradoxical activation of TGFβ signaling pathways with fibrosis in transgenic mice. J Biol Chem. 2003;278:25109. doi: 10.1074/jbc.M300636200. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Ferrer M. Afshar-Sherif AR. Uwamariya C. de Crombrugghe B. Davidson JM. Bhowmick NA. Dermal transforming growth factor-β responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 2010;176:98. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denton CP. Khan K. Hoyles RK. Shiwen X. Leoni P. Chen Y. Eastwood M. Abraham DJ. Inducible lineage-specific deletion of TβRII in fibroblasts defines a pivotal regulatory role during adult skin wound healing. J Invest Dermatol. 2009;129:194. doi: 10.1038/jid.2008.171. [DOI] [PubMed] [Google Scholar]
- 65.Itoh F. Itoh S. Carvalho RL. Adachi T. Ema M. Goumans MJ. Larsson J. Karlsson S. Takahashi S. Mummery CL. Dijke PT. Kato M. Poor vessel formation in embryos from knock-in mice expressing ALK5 with L45 loop mutation defective in Smad activation. Lab Invest. 2009;89:800. doi: 10.1038/labinvest.2009.37. [DOI] [PubMed] [Google Scholar]
- 66.Sonnylal S. Denton CP. Zheng B. Keene DR. He R. Adams HP. Vanpelt CS. Geng YJ. Deng JM. Behringer RR. de Crombrugghe B. Postnatal induction of transforming growth factor-β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007;56:334. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- 67.Dudas M. Kim J. Li WY. Nagy A. Larsson J. Karlsson S. Chai Y. Kaartinen V. Epithelial and ectomesenchymal role of the type I TGF-β receptor ALK5 during facial morphogenesis and palatal fusion. Dev Biol. 2006;296:298. doi: 10.1016/j.ydbio.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bian Y. Terse A. Du J. Hall B. Molinolo A. Zhang P. Chen W. Flanders KC. Gutkind JS. Wakefield LM. Kulkarni AB. Progressive tumor formation in mice with conditional deletion of TGF-β signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 2009;69:5918. doi: 10.1158/0008-5472.CAN-08-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J. Johnson K. Li J. Piamonte V. Steffy BM. Hsieh MH. Ng N. Zhang J. Walker JR. Ding S. Muneoka K. Wu X. Glynne R. Schultz PG. Regenerative phenotype in mice with a point mutation in transforming growth factor β type I receptor (TGFBR1) Proc Natl Acad Sci USA. 2011;108:14560. doi: 10.1073/pnas.1111056108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark LD. Clark RK. Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 71.Yang X. Letterio JJ. Lechleider RJ. Chen L. Hayman R. Gu H. Roberts AB. Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 1999;18:1280. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Datto MB. Frederick JP. Pan L. Borton AJ. Zhuang Y. Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol Cell Biol. 1999;19:2495. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu Y. Richardson JA. Parada LF. Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 74.Ashcroft GS. Yang X. Glick AB. Weinstein M. Letterio JL. Mizel DE. Anzano M. Greenwell-Wild T. Wahl SM. Deng C. Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 75.Falanga V. Schrayer D. Cha J. Butmarc J. Carson P. Roberts AB. Kim SJ. Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen. 2004;12:320. doi: 10.1111/j.1067-1927.2004.012316.x. [DOI] [PubMed] [Google Scholar]
- 76.Arany PR. Flanders KC. Kobayashi T. Kuo CK. Stuelten C. Desai KV. Tuan R. Rennard SI. Roberts AB. Smad3 deficiency alters key structural elements of the extracellular matrix and mechanotransduction of wound closure. Proc Natl Acad Sci USA. 2006;103:9250. doi: 10.1073/pnas.0602473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flanders KC. Sullivan CD. Fujii M. Sowers A. Anzano MA. Arabshahi A. Major C. Deng C. Russo A. Mitchell JB. Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lakos G. Takagawa S. Chen SJ. Ferreira AM. Han G. Masuda K. Wang XJ. DiPietro LA. Varga J. Targeted disruption of TGF-β/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol. 2004;165:203. doi: 10.1016/s0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nomura M. Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 80.Ito Y. Sarkar P. Mi Q. Wu N. Bringas P., Jr Liu Y. Reddy S. Maxson R. Deng C. Chai Y. Overexpression of Smad2 reveals its concerted action with Smad4 in regulating TGF-β-mediated epidermal homeostasis. Dev Biol. 2001;236:181. doi: 10.1006/dbio.2001.0332. [DOI] [PubMed] [Google Scholar]
- 81.Hosokawa R. Urata MM. Ito Y. Bringas P., Jr. Chai Y. Functional significance of Smad2 in regulating basal keratinocyte migration during wound healing. J Invest Dermatol. 2005;125:1302. doi: 10.1111/j.0022-202X.2005.23963.x. [DOI] [PubMed] [Google Scholar]
- 82.Tomikawa K. Yamamoto T. Shiomi N. Shimoe M. Hongo S. Yamashiro K. Yamaguchi T. Maeda H. Takashiba S. Smad2 decelerates re-epithelialization during gingival wound healing. J Dent Res. 2012;91:764. doi: 10.1177/0022034512451449. [DOI] [PubMed] [Google Scholar]
- 83.Yang X. Teng Y. Hou N. Fan X. Cheng X. Li J. Wang L. Wang Y. Wu X. Yang X. Delayed re-epithelialization in Ppm1a gene-deficient mice is mediated by enhanced activation of Smad2. J Biol Chem. 2011;286:42267. doi: 10.1074/jbc.M111.292284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross S. Hill CS. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40:383. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Yang X. Li C. Xu X. Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci USA. 1998;95:3667. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sirard C. de la Pompa JL. Elia A. Itie A. Mirtsos C. Cheung A. Hahn S. Wakeham A. Schwartz L. Kern SE. Rossant J. Mak TW. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Owens P. Engelking E. Han G. Haeger SM. Wang XJ. Epidermal Smad4 deletion results in aberrant wound healing. Am J Pathol. 2010;176:122. doi: 10.2353/ajpath.2010.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiao W. Li AG. Owens P. Xu X. Wang XJ. Deng CX. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- 89.Yang L. Mao C. Teng Y. Li W. Zhang J. Cheng X. Li X. Han X. Xia Z. Deng H. Yang X. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- 90.Yang L. Li W. Wang S. Wang L. Li Y. Yang X. Peng R. Smad4 disruption accelerates keratinocyte reepithelialization in murine cutaneous wound repair. Histochem Cell Biol. 2012;138:573. doi: 10.1007/s00418-012-0974-8. [DOI] [PubMed] [Google Scholar]
- 91.Han G. Li AG. Owens P. Wang XJ. Overexpression of Smad7 in keratinocytes accelerates cutaneous wound healing. Wound Repair Regen. 2004;12:A11. [Google Scholar]
- 92.Han G. Li F. Ten Dijke P. Wang XJ. Temporal Smad7 transgene induction in mouse epidermis accelerates skin wound healing. Am J Pathol. 2011;179:1768. doi: 10.1016/j.ajpath.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]