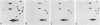Abstract
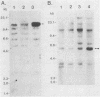
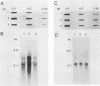
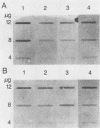
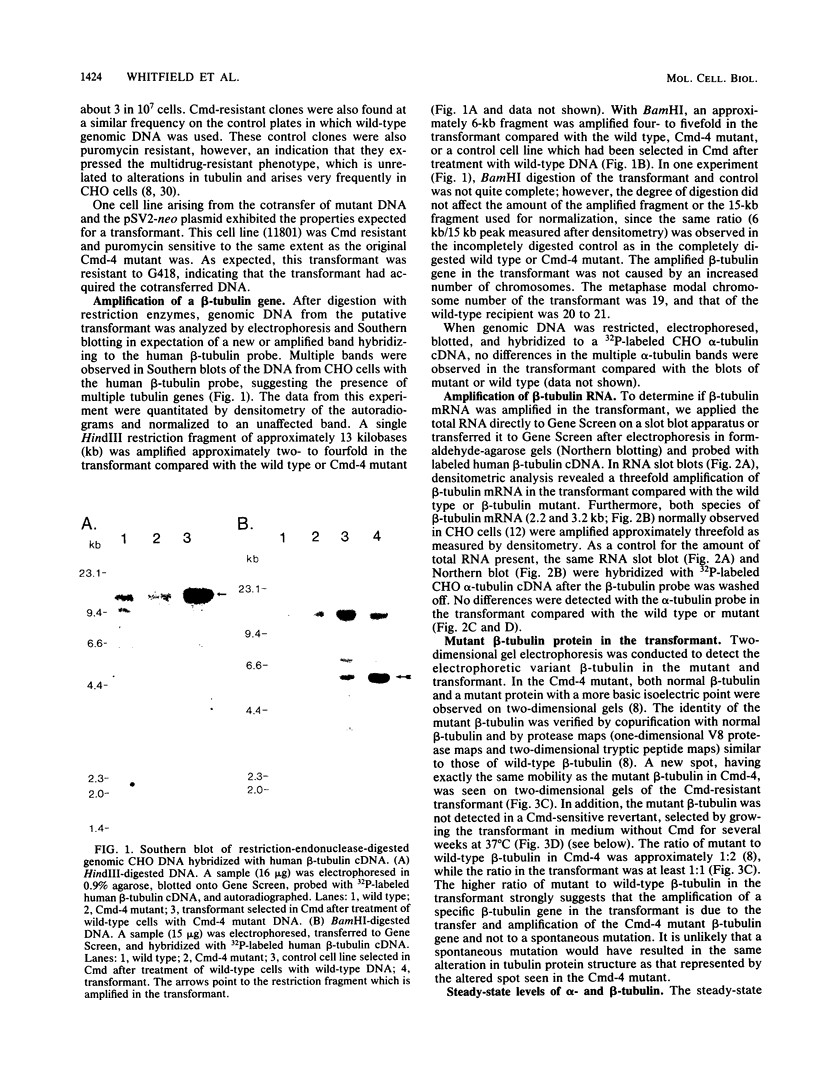
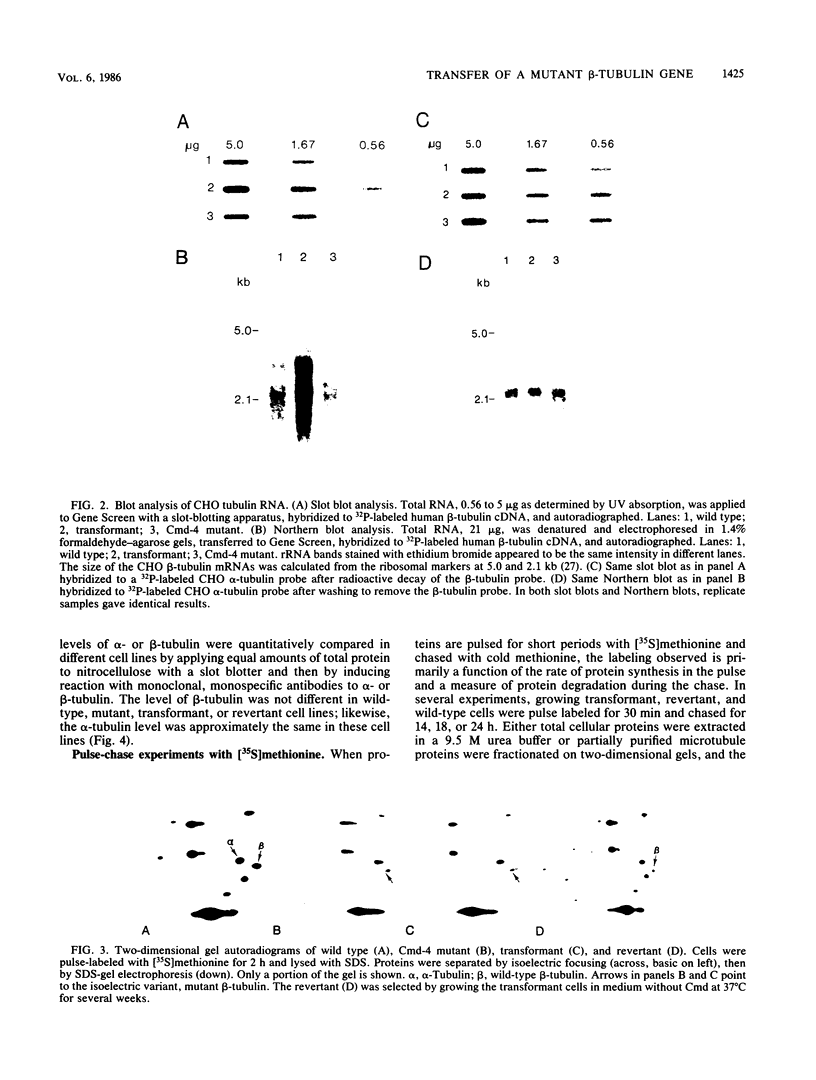
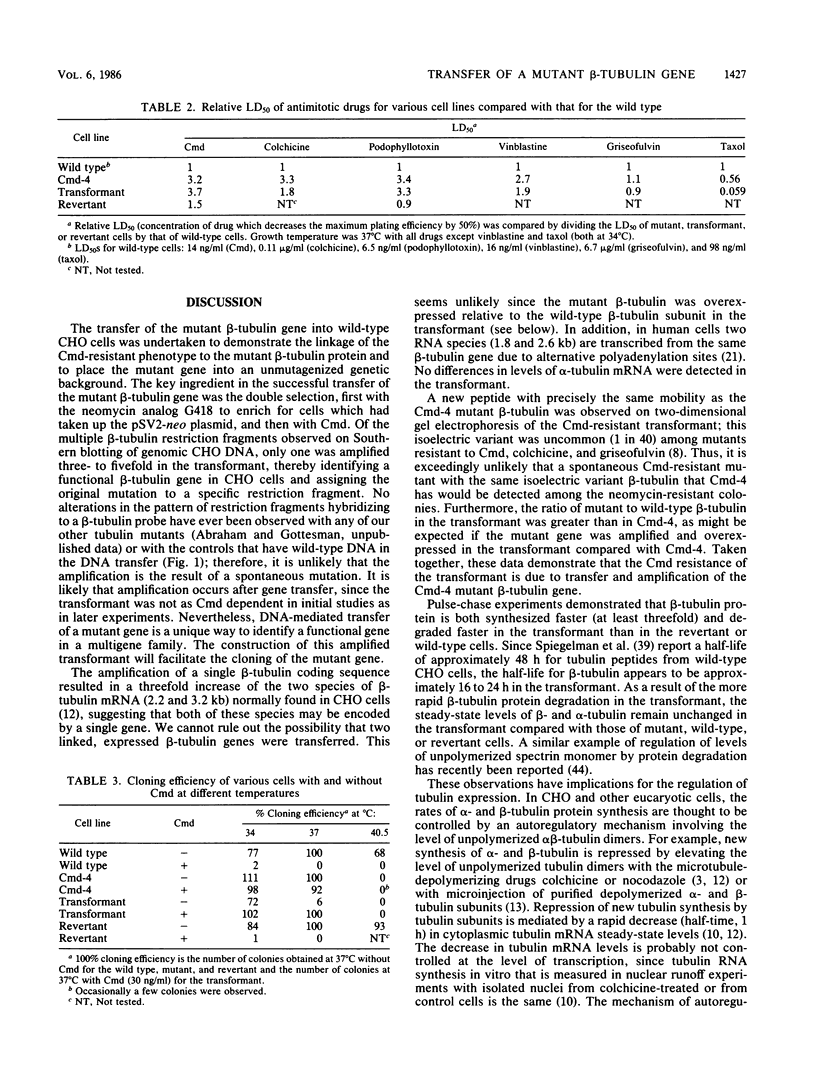
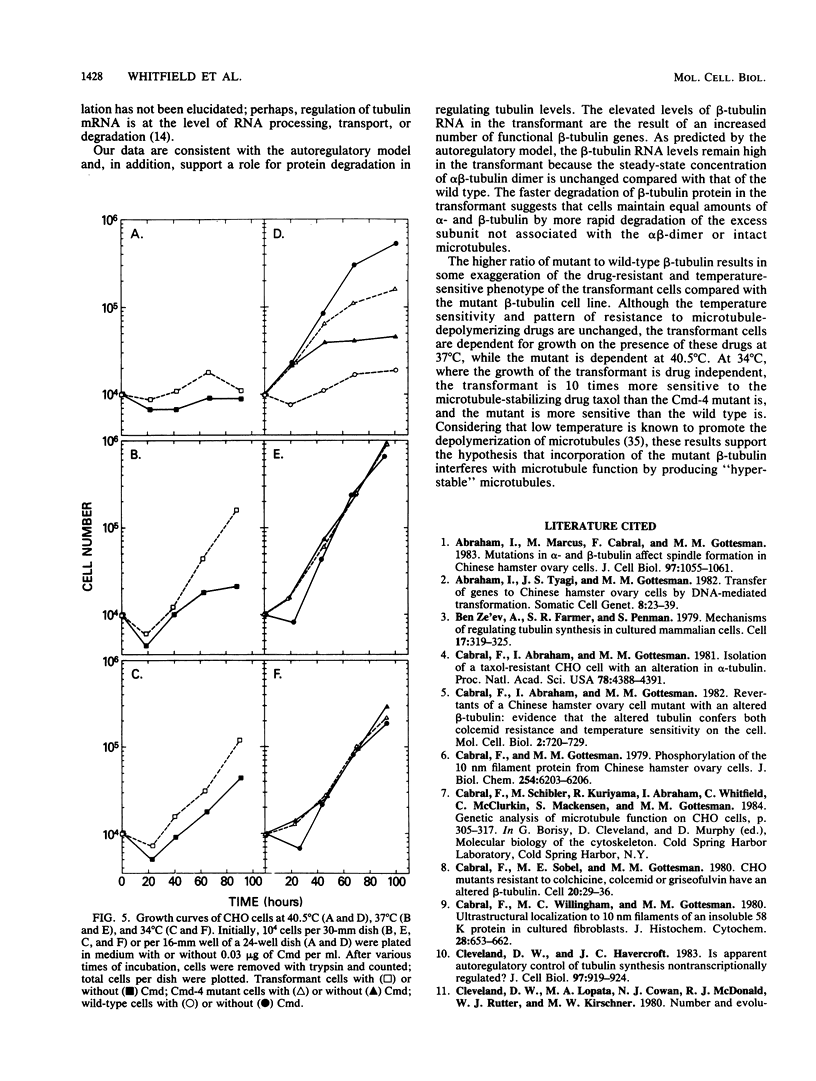
Total genomic DNA from a temperature-sensitive, colcemid-resistant Chinese hamster ovary (CHO) cell mutant expressing an electrophoretic variant beta-tubulin was used to transform wild-type CHO cells to colcemid-resistant cells at 37 degrees C. Southern blot analysis of the transformant demonstrated the three- to fivefold amplification of one of many beta-tubulin sequences compared with that of the wild type or mutant, thereby identifying a functional tubulin gene in CHO cells. This amplification of one tubulin-coding sequence resulted in a threefold increase in two beta-tubulin mRNA species, suggesting that both species may be encoded by a single gene. Pulse-chase experiments showed that in the transformant, total beta-tubulin was synthesized and degraded faster than in the revertant or wild-type cells, so that the steady-state levels of beta-tubulin and alpha-tubulin were unchanged in the transformant compared with those of wild-type, mutant, or revertant cells. Increased ratios of mutant to wild-type beta-tubulin made the transformant dependent on microtubule-depolymerizing drugs for growth at 37 but not 34 degrees C and supersensitive to the microtubule-stabilizing drug taxol at 34 degrees C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham I., Marcus M., Cabral F., Gottesman M. M. Mutations in alpha- and beta-tubulin affect spindle formation in Chinese hamster ovary cells. J Cell Biol. 1983 Oct;97(4):1055–1061. doi: 10.1083/jcb.97.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham I., Tyagi J. S., Gottesman M. M. Transfer of genes to Chinese hamster ovary cells by DNA-mediated transformation. Somatic Cell Genet. 1982 Jan;8(1):23–39. doi: 10.1007/BF01538648. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Isolation of a taxol-resistant Chinese hamster ovary cell mutant that has an alteration in alpha-tubulin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4388–4391. doi: 10.1073/pnas.78.7.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Revertants of a Chinese hamster ovary cell mutant with an altered beta-tubulin: evidence that the altered tubulin confers both colcemid resistance and temperature sensitivity on the cell. Mol Cell Biol. 1982 Jun;2(6):720–729. doi: 10.1128/mcb.2.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M. Phosphorylation of the 10-nm filament protein from Chinese hamster ovary cells. J Biol Chem. 1979 Jul 25;254(14):6203–6206. [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Cabral F., Willingham M. C., Gottesman M. M. Ultrastructural localization to 10 nm filaments of an insoluble 58K protein in cultured fibroblasts. J Histochem Cytochem. 1980 Jul;28(7):653–662. doi: 10.1177/28.7.7391554. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Havercroft J. C. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983 Sep;97(3):919–924. doi: 10.1083/jcb.97.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Pittenger M. F., Feramisco J. R. Elevation of tubulin levels by microinjection suppresses new tubulin synthesis. Nature. 1983 Oct 20;305(5936):738–740. doi: 10.1038/305738a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Pittenger M. F., Lopata M. A. Autoregulatory control of expression of alpha and beta tubulin. J Submicrosc Cytol. 1983 Jan;15(1):353–358. [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. J., Hua L., Liau G., Gal S., Graham D. E., Sobel M., Gottesman M. M. Malignant transformation and tumor promoter treatment increase levels of a transcript for a secreted glycoprotein. Mol Cell Biol. 1985 Mar;5(3):466–473. doi: 10.1128/mcb.5.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. E., Medina D., Smith G. H. Increased concentration of an indigenous proviral mouse mammary tumor virus long terminal repeat-containing transcript is associated with neoplastic transformation of mammary epithelium in C3H/Sm mice. J Virol. 1984 Mar;49(3):819–827. doi: 10.1128/jvi.49.3.819-827.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havercroft J. C., Cleveland D. W. Programmed expression of beta-tubulin genes during development and differentiation of the chicken. J Cell Biol. 1984 Dec;99(6):1927–1935. doi: 10.1083/jcb.99.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Raff E. C., Raff R. A., Kaufman T. C. Mutation in a testis-specific beta-tubulin in Drosophila: analysis of its effects on meiosis and map location of the gene. Cell. 1980 Sep;21(2):445–451. doi: 10.1016/0092-8674(80)90481-x. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Raff R. A., Kaufman T. C., Raff E. C. Mutation in a structural gene for a beta-tubulin specific to testis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3991–3995. doi: 10.1073/pnas.76.8.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G., Binder L. I., Gottesman M. M. Tubulin composition and microtubule nucleation of a griseofulvin-resistant Chinese hamster ovary cell mutant with abnormal spindles. Exp Cell Res. 1985 Oct;160(2):527–539. doi: 10.1016/0014-4827(85)90199-5. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Gilmartin M. E., Hall J. L., Cowan N. J. Three expressed sequences within the human beta-tubulin multigene family each define a distinct isotype. J Mol Biol. 1985 Mar 5;182(1):11–20. doi: 10.1016/0022-2836(85)90023-3. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spiegelman B. M., Penningroth S. M., Kirschner M. W. Turnover of tubulin and the N site GTP in Chinese hamster ovary cells. Cell. 1977 Nov;12(3):587–600. doi: 10.1016/0092-8674(77)90259-8. [DOI] [PubMed] [Google Scholar]
- Thompson W. C., Asai D. J., Carney D. H. Heterogeneity among microtubules of the cytoplasmic microtubule complex detected by a monoclonal antibody to alpha tubulin. J Cell Biol. 1984 Mar;98(3):1017–1025. doi: 10.1083/jcb.98.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Chow L. T., Wefald F. C., Cowan N. J. Structure of two human alpha-tubulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(1):96–100. doi: 10.1073/pnas.79.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]
- Woods C. M., Lazarides E. Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: regulation of spectrin topogenesis by beta-spectrin degradation. Cell. 1985 Apr;40(4):959–969. doi: 10.1016/0092-8674(85)90356-3. [DOI] [PubMed] [Google Scholar]