Abstract
It has been previously shown that anti-dengue virus (DENV) nonstructural protein NS1 antibodies could act as autoantibodies that direct against one or more of the host's own proteins, which has potential implications for dengue hemorrhagic fever pathogenesis. In the present study, we have employed suppression subtractive hybridization (SSH) to identify the differentially expressed genes from human microvascular endothelial cells (HMEC-1) in response to anti-dengue virus type 2 NS1 antibodies (anti-DENV2 NS1 Abs). A total of 35 clones from the SSH cDNA library were randomly selected for further analysis using bioinformatics tools after vector screening. After searching for sequence homology in NCBI GenBank database with BLASTN and BLASTX programs, 23 obtained sequences with significant matches (E-values <1×10−4) in the SSH library. The predicted genes in the subtracted library include immune response molecules (CD59 antigen preproprotein preproprotein, MURR1), signal transduction molecules (Nuclear casein kinase and cyclin-dependent kinase substrate 1), calcium-binding proteins (S100A6, Annexin A2 isoform 1/2), and cell-membrane component (Yip1 domain family). From these clones, 5 upregulated genes were selected for differential expression profiling by real-time RT-PCR to confirm their upregulated status. The results confirmed their differential upregulation, and thus verified the success of SSHs and the likely involvement of these genes in dengue pathogenesis.
Introduction
Dengue virus (DENV), which is a member of the genus Flavivirus, family Flaviviridae, is transmitted to humans by the Aedes mosquitoes, causing dengue fever (DF), and in some severe cases it may develop into dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (19,20). Since the identification and naming of the disease in 1779, there have been cyclic outbreaks in Asia, Africa, and South America. However, the pathogenic mechanisms are not fully understood yet and there is currently no available vaccine for protection against all four serotypes of dengue virus or effective therapeutic medication for patients presenting with these severe forms.
DF is an acute, self-limited febrile illness, whereas DHF and DSS are life-threatening and characterized by plasma leakage, low platelet counts, liver damage, and, in the most severe cases, death due to shock (21). Plasma leakage, also called the hemorrhagic manifestations, occurs once vessel endothelium is disrupted and is followed by the loss of its barrier function. Although dengue virus-infected endothelial cells in vitro had been reported (6,24), there is no direct evidence indicating DENV infection of endothelial cells in vivo (21). It is likely that different mechanisms are involved in the disease pathogenesis, including complement activation (4,32), lymphocytes and cytokines Tsunami (3,8), or cross-reactive antibodies to endothelial cells (2,12).
Antibodies generated by mice to the dengue virus nonstructural protein 1 (NS1) have been shown to cross-react with human fibrinogen, platelets, and endothelial cells (12,13). Serum samples from DHF/DSS patients show higher endothelium binding activity than do those of people with dengue fever (30). Anti-DENV NS1 antibodies act as autoantibodies that cross-react with host proteins and noninfected endothelial cells and trigger intracellular signaling leading endothelial dysfunction (2,29,31). However, the mechanism of vascular permeability and hemostatic disorders as a result of endothelium dysfunction caused by the autoimmune pathogenesis is still not fully understood.
In order to gain insight into the intracellular signaling triggered by the anti-endothelial cell autoantibodies, we employed suppression subtractive hybridization (SSH) to construct cDNA libraries for identifying differentially expressed genes from human microvascular endothelial cells (HMEC-1) in response to anti-dengue virus type 2 NS1 antibodies immunoglobulin G (IgG) (anti-DENV2 NS1 Abs). From genes in this SSH libraries, five upregulated genes were selected for differential expression profiling by real-time RT-PCR to confirm their upregulated status. The information provided from this study will enhance the understanding of anti-NS1 antibodies' role in dengue hemorrhagic fever pathogenesis.
Materials and Methods
Preparation of recombinant NS1 and generation of anti-NS1 Abs
The full-length cDNA of NS1 from DENV-2 virus New Guinea C strain (NGC) was cloned to expression vector pPICZαB (Invitrogen) to establish a pPICZαB-NS1 plasmid; the recombinant NS1 protein expression was induced by adding methanol as described before (49). Recombinant NS1 protein immunogen was prepared and antibodies against NS1 protein were generated by the immunization of New Zealand rabbits. Anti-NS1 polyclonal antibody was purified by caprylic acid-ammonium sulfate precipitation and protein A resin (GenScript, USA). Then the specificity, sensitivity, and valence of the antibody were detected by Western blot and ELISA. The antibodies were quantified by UV280, as we previously described (27).
Cell culture
Human microvascular endothelial cell line-1 (HMEC-1) was obtained from the Centers for Disease Control, Atlanta, GA (1) and passed in culture plates containing endothelial cell growth medium (Gibco, MCDB131, Cat. 10372-019) composed of 10% FBS (Gibco), 1 μg/mL hydrocortisone (Sigma-Aldrich), 10 ng/mL epidermal growth factor (Becton-Dickinson), 10 mM L-Glutamine (Gibco), and antibiotics. Cells were detached using 0.25% trypsin-EDTA (Gibco).
Anti-NS1 Abs cell binding assay
HMEC-1 cells were grown in culture flasks and incubated with 5 mL anti-DENV2 NS1 Abs (8 μg/mL) or control rabbit IgG at 37°C for 1 h. After washing three times with PBS, the cells were incubated with FITC-conjugated anti-rabbit IgG at 4°C for 40 min and analyzed by indirect immunofluresence. In addition, more than 1×106 HMEC-1 cells were collected for binding assay using flow cytometry (Coulter EPICS Elite Flow Cytometer Sorter).
RNA extraction and cDNA synthesis
RNA was extracted from around 2×106 HMEC-1 cells at eight time points (2, 4, 6, 8, 10, 12, 24, and 48 h) post incubation with anti-NS1 Abs using RNeasy Mini Kit (Qiagen). Normal rabbit IgG incubated cells were used as control. After the extraction, total RNA was visualized on a 1.0% agarose Tris–acetate–ethylenediaminetetraacetic acid (TAE) gel. Total RNA in each sample was quantified using a BioSpec-mini spectrophotometer (Shimadzu Biotech, Nakagyo-ku, Kyoto, Japan). For construction of the cDNA libraries for SSH, each sample was created by combining 2 μg RNA from each time point. The cDNA synthesis was performed using 3 μg of RNA from each sample using the SMART™ PCR cDNA Synthesis Kit (Clontech, CA), in accordance with the manufacturer's protocols.
Construction of suppression subtractive hybridization library and differential screening
A suppression subtractive hybridization (SSH) library was prepared with the PCR-Select™ cDNA subtraction kit (Clontech), according to the manufacturer's instructions. Briefly, the forward-subtracted library was constructed where the cDNAs generated from HMEC-1 cells cultured with anti-NS1 Abs served as the “tester”, while cDNAs generated from HMEC-1 cells cultured with control rabbit antibodies served as the “driver”, as well as the reverse-subtracted library where the samples exchanged their positions of tester and driver. The forward-subtracted and the reverse-subtracted cDNA library were cloned using the pGEM-T Easy Kit (Promega, WI, USA). Then, 5 μL of the ligation reaction was used to transform Trans10 competent cells (TransGen, Guangzhou, China) for subsequent ampicillin-resistant colony selection and blue-white screening. To construct probes for differential screening, the forward-subtraction and reverse-subtraction cDNAs as well as the unsubtracted cDNA libraries were labeled with digoxigenin, and Southern blot was performed for colony PCR positive clones to exclude the false positive ones using DIG High Prime DNA Labeling and Detection Starter Kit I, according to the manufacturer's instructions. Results analysis was done according to the manual of the PCR-Select™ differential screening kit (Clontech).
Sequencing and homology search
The differentially expressed clones were sequenced using an automated DNA sequencer (Invitrogen, Shanghai, China) and analyzed using the online BLAST program from NCBI's website (http://blast.ncbi.nlm.nih.gov/). The minimum length of cDNA matching the mRNA sequence was set to 200 bp, when selecting SSH-derived clones for further investigation (11,34).
Quantitative RT-PCR
For quantitative RT-PCR, the specific oligonucleotide primers to five selected genes including ADP-ribosylation factor-like 6 interacting protein “ARL6IP” (forward: 5′-ACCACAAGGTACAACATCAGTGCAATAA-3′ and reverse: 5′-TCTATGACTTTCACTCTAAGACTGGCAA-3′), aryl hydrocarbon receptor nuclear translocator isoform 1/3 “ARNT1/3” (forward: 5′-AATGTGCCTTATGTTTGTATGTCTGGAG-3′ and reverse: 5′-GTTACTACCCTCACCCTTTACCTTCCTC-3′), CD59 (forward:5′-GGCTCCAAAGCACTATGTCACCTACCTC-3′ and reverse: 5′-AATCAGTCCTGTCCTTGGCAAGCTCTTC-3′), nuclear ubiquitous casein kinase and cyclin-dependent kinase substrate 1 “NUCKS1” (forward: 5′-AACTTTGCTTTCCACAACACTTCATAAC-3′ and reverse: 5′-TTTATTGAAGGGTTTCCAGGACTGCGTG-3′), S100A6 (forward: 5′-TACAATTACCCACCACTGGATTTGACTC-3′ and reverse: 5′-ACCAGGAGGTGAACTTCCAGGAGTATGT-3′) and β-actin (forward: 5′-GAACCTCTCGTTGCCGATGGTG-3′ and reverse: 5′-GAAGCTGTGCTACGTGGCTCTG-3′) were used in the experiment. Two samples of RNA were prepared as mentioned before. Quantitative RT-PCR was performed to detect differences in transcript levels of the selected ESTs at different time points. Amplification was determined using SYBR Premix Ex Taq (Takara, Guangzhou, China) in the CFX96™ Real-Time PCR Detection System (Bio-Rad, CA, USA). Each real-time PCR reaction was done in triplicate. The results were presented as the relative expression ratios of the target genes, which were standardized to the expression level of the constitutive β-actin gene. The data were analyzed using SPSS statistics 17.0 (Chicago, USA). Statistical significance was calculated by paired-samples t-test and one-way ANOVA. Significance was accepted at p<0.05.
Results
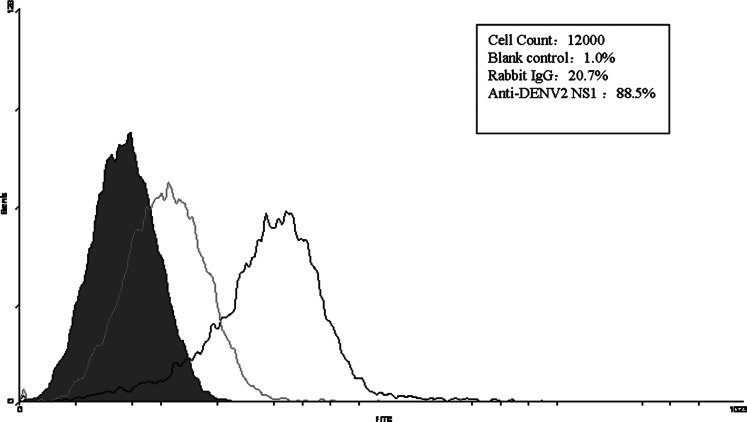
The antibodies against DENV2 NGC strain NS1 protein were obtained by the immunization of New Zealand rabbits with the recombinant expressed NS1 protein. The antibody could specifically recognize native DENV2 NS1 protein analyzed by Western blot and the titer of the antiserum was up to 1:6000 analyzed by ELISA (27). The binding assay showed that the anti-DENV2 NS1 antibodies could cross-react with HMEC-1 cells by using immunofluorescence assay (data not shown) and flow cytometry (Fig. 1).
FIG. 1.
Binding of anti-DENV2 NS1 antibodies and HMEC-1 cells analyzed using flow cytometry. HMEC-1 cells were incubated with anti-DENV2 NS1 antibodies (black), rabbit IgG (gray), or no antibodies (solid). The results is shown as a representative of triplicate experiments.
A subtracted cDNA library enriched in differentially expressed genes in HMEC-1 response of anti-DENV2 NS1 Abs was generated using SSH. The subtraction efficiency was evaluated by comparing the transcript abundance of G3PDH before and after subtraction. The result showed that G3PDH was not detectable in the subtracted cDNA samples, whilst the presence of G3PDH was discovered in the nonsubtracted cDNA samples whenever the subtraction was forward or reverse (data not shown), implying that the SSHs were successful.
Sequence analysis of subtracted cDNA library
A total of 35 clones from the SSH cDNA library were randomly selected and sequenced using the M13 reverse primer. After searching for sequence homology in NCBI GenBank database with BLASTN and BLASTX programs, 23 obtained sequences with significant matches (E-values <1×10−4) in the SSH library. Known genes were categorized according to their putative functions (Table 1). The predicted genes in the subtracted library include immune response molecules (CD59 antigen preproprotein preproprotein, MURR1), signal transduction molecules (Nuclear casein kinase and cyclin-dependent kinase substrate 1), calcium-binding proteins (S100A6, Annexin A2 isoform 1/2), and cell-membrane component (Yip1 domain family).
Table 1.
Upregulated and Downregulated Genes in the HMEC-1 Response of Anti-DENV2 NS1 Abs Identified by Suppression Subtractive Hybridization (SSH)
| Functional categories | Homologous gene | Upregulate/downregulate | GenBank Accession No | No of clones | E-value (% Identity) |
|---|---|---|---|---|---|
| Immune response regulator | CD59 antigen preproprotein preproprotein | upregulate | NT_009237.18 | 1 | 0.0 (99%) |
| MURR1 (COMM domain-containing protein 1) | downregulate | NT_022184.15 | 2 | 4e-145 (99%) | |
| Apoptosis regulator | ADP-ribosylation factor-like 6 interacting protein | upregulate | NT_010393.16 | 1 | 9e-36 (100%) |
| Calcium-binding protein | S100 calcium-binding protein A6 | upregulate | NT_004487.19 | 1 | 3e-103 (100%) |
| Annexin A2 isoform 1 | downregulate | NM_001002858.2 | 2 | 0.0 (98%) | |
| Annexin A2 isoform 2 | downregulate | NM_004039.2 | 1 | 0.0 (99%) | |
| Cell growth | Spindle and KT associated 2 isoform 1/2 | upregulate | NT_010783.15 | 3 | 4e-159 (100%) |
| MAD2-like 1 | upregulate | NT_016354.19 | 1 | 5e-151 (99%) | |
| Centromere protein Q | upregulate | NT_007592.15 | 2 | 5e-57 (98%) | |
| Proteasome | Proteasome (prosome, macropain) subunit, alpha type 8 | upregulate | NT_010966.14 | 1 | 0.0 (98%) |
| Protein synthesis and processing | Ribosomal protein L10 | upregulate | NT_167198.1 | 2 | 1e-49 (100%) |
| Eukaryotic translation initiation factor 2A | downregulate | NT_005612.16 | 1 | 0.0 (100%) | |
| Transcriptional regulation | Aryl hydrocarbon receptor nuclear translocator isoform 1/3 | downregulate | NT_004487.19 | 1 | 8e-108 (99%) |
| Cell-membrane component | Yip1 domain family, membrane 6 | upregulate | NT_011669.17 | 1 | 1e-60 (100%) |
| Signal transduction | Nuclear casein kinase and cyclin-dependent kinase substrate 1 | upregulate | NT_004487.19 | 1 | 5e-74 (99%) |
| Hypothetical protein | Hypothetical protein LOC746 | upregulate | NT_167190.1 | 1 | 1e-50 (100%) |
Determination of differentially expressed cDNAs by real-time qPCR
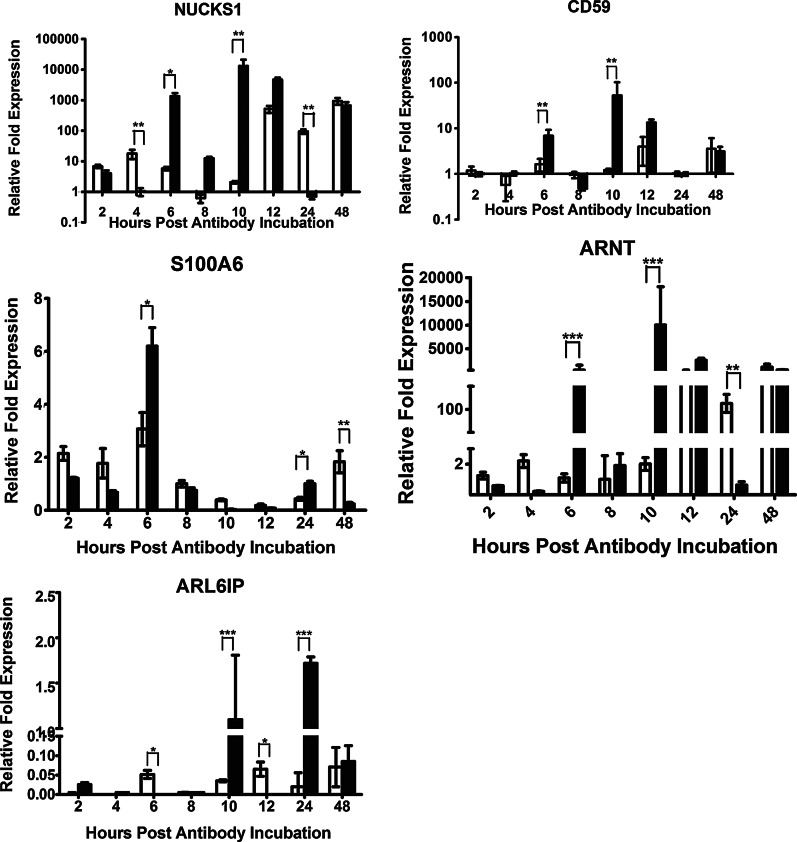
To confirm the differential expression of genes identified from the SSH library and to understand gene expression variations coupled with HMEC-1 response of anti-DENV2 NS1 Abs, nuclear ubiquitous casein kinase and cyclin-dependent kinase substrate 1 (NUCKS1), CD59 preproprotein, S100A6, aryl hydrocarbon receptor nuclear translocator isoform 1/3 (ARNT1/3), and ADP-ribosylation factor-like 6 interacting protein (ARL6IP) were selected for real-time PCR based evaluation of their relative expression levels (Fig. 2). The expression of NUCKS1 was upregulated from 6 h up to 48 h (except for at 24 h) post anti-DENV2 NS1 Abs incubation. The transcripts of NUCKS1 gene showed much higher in the anti-DENV2 NS1 Abs-treated group than control Abs-treated group at 6 h, 8 h, and 10 h, but their transcripts levels were almost the same at 12 h and 48 h. Strangely, the expression levels of NUCKS1 were much lower in the anti-DENV2 NS1 Abs-treated group than control Abs-treated group at 24 h. The expression of CD59 was also upregulated after 6 h post anti-DENV2 NS1 Abs incubation, except for a relatively lower expression level at 8 h and 24 h, respectively. The transcript levels were considerably higher in the anti-DENV2 NS1 Abs-treated group than control Abs-treated group at 6 h and 10 h. However, the expression of S100A6 increased only at 6 h and 24 h when compared with control Abs treated group. The expression pattern of ARNT was almost the same as NUCKS1 and CD59: the transcript levels increased after 6 h of anti-DENV2 NS1 Abs incubation except for a relatively lower expression level at 8 h and 24 h, respectively. The transcript levels were consistently higher in the anti-DENV2 NS1 Abs-treated group than control Abs-treated group at 6 h and 10 h, while they were almost the same at 12 h and 48 h. The expression of ARL6IP was upregulated after 10 h up to 48 h post anti-DENV2 NS1 Abs incubation except 12 h. The transcript levels were consistently higher in the anti-DENV2 NS1 Abs-treated group than control Abs-treated group at 10 h and 24 h.
FIG. 2.
Relative fold expression of five different target genes, nuclear ubiquitous casein kinase and cyclin-dependent kinase substrate 1 (NUCKS1), CD59, S100A6, aryl hydrocarbon receptor nuclear translocator isoform 1/3 (ARNT1/3), and ADP-ribosylation factor-like 6 interacting protein (ARL6IP), expressed in the HMEC-1 cells incubated with anti-NS1 Abs (solid bar) compared to normal rabbit IgG incubated cells (open bar) and standardized against β-actin as the internal reference, were analyzed by real-time RT-PCR at 2, 4, 6, 8, 10, 12, 24, and 48 hours post incubation. Samples from each time point were tested in triplicate and the data represent the mean (±1 standard deviation) relative expression level was used for analysis that the target gene is downregulated, the same, or upregulated, respectively, compared with the control (*p<0.05; **p<0.01).
Discussion
Plasma leakage and hemorrhagic are the hallmarks of dengue hemorrhagic fever. Although studies showed Abs directed against DENV NS1 would cross-react with endothelial cells and cause damage, the pathogenic mechanism, however, has not been well understood (12,30). To develop a better understanding of the intracellular signaling triggered by the anti-endothelial cell autoantibodies, SSH is a powerful approach adopted herein to identify endothelial cell-responsive genes upon anti-DENV NS1 Abs incubation. Suppression subtractive hybridization (SSH), which is a technology that combines normalization and subtraction in a single procedure based primarily on suppression polymerase chain reaction (PCR), allows the isolation of differentially expressed cDNAs with specific genomic sequences (11). SSH could be employed to construct differentially expressed gene libraries between anti-DENV NS1 Abs treated and normal endothelial cells. From our SSH library, we found certain genes involved in various cellular functions that related to immune response regulation, cell growth, and transcriptional regulation.
Nuclear ubiquitous casein and cyclin-dependent kinases substrate (NUCKS) is a nuclear DNA binding protein occurring in almost all types of human cells, adult and fetal tissues (18,41). It has all the features of being a housekeeping gene (18). The abundance of NUCKS was found in rapidly growing cells, as well as in certain cancers, suggests that it may be involved in facilitating and maintaining activity of transcription of some genes during rapid proliferation and in cancer (39). The importance of NUCKS remains still unknown and disputable. Overexpression of NUCKS is probably related to high levels of transcription. Future studies should also pay additional attention in elucidating potential functions of specific post-translational modifications of NUCKS in etiology and progression of this disease.
Complement plays a well-known important role as a multi-functional complex system comprising more than 30 proteins, in host defense against infectious agents, in the removal of apoptotic cells and immune complexes, and in the modulation of the adaptive immune system (36). CD59 is the key regulator of the terminal pathway which inhibits the formation of membrane attack complex (MAC), that limits host cell damage by binding to C5b-8 or C5b-9 and preventing leakage or lysis of the host cell membrane (23,33). Cells with normal levels of the complement regulatory proteins DAF, MCP, and CD59 defend themselves against immediate lysis, but the attack persists as long as more antigen is expressed, more antibody is available, and complement levels remain sufficient to maintain the attack. Further study is needed to reveal the potential role of CD59 in response against leakage of endothelial cells triggered by anti-DENV NS1 Abs.
The family of S100 proteins is one of the largest subfamilies of EF-hand proteins. The S100A6 (calcyclin) protein, belongs to the S100 protein family, is a 10.5 kDa Ca2+-binding protein (38,47). The function of S100A6 is not clear at present, but it has been suggested that it may be involved in cell proliferation, cytoskeletal dynamics, and tumorigenesis. S100A6 expression at the protein and/or mRNA level can be upregulated by multiple factors, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), serum, TNFα, retinoic acid, estrogen, palmitate, vasopressin, and gastrin (5,10,16,25,28,42,43). The interaction of S100A6 and annexins, tropomyosin, caldesmon, and calponin suggest that S100A6 may play a regulatory role in the actin cytoskeleton (14,16,17,46,48). In this research, we found that S100A6 was upregulated following anti-DENV NS1 Abs treating; as a result, we suggest that S100A6 might be implicated in the increasing of endothelial cells permeability response to the antibody, thus it is attractive to examine the function of S100A6 for being a signal molecules and its interaction with the corresponding proteins.
The AhR nuclear translocator (ARNT; also known as HIF-1β) is the dimerization partner of the aryl hydrocarbon receptor (AhR) in the nucleus. The AhR-ARNT heterodimer has been shown to regulate the expression of several batteries of genes involved in diverse signaling pathways, including phase I/II metabolism, inflammation, and cell cycling (7,15). The AhR is a member of the PAS (Per-ARNT-Sim) superfamily of proteins. Physiologically, many of these proteins act by sensing molecules and stimuli from the cellular environment, and initiating signaling cascades to elicit the appropriate cellular responses. Some experimental results suggest that AhR plays an important role during development and maintenance of the hematopoietic and immune system (22,35,40,44). Our SSH library also revealed an upregulated state of this gene, so it merits further functional investigation of ARNT in endothelial cells response.
ADP-ribosylation factor-like 6 interacting protein 1 (ARL6IP1) is an apoptotic regulator, as well as involved in protein transport, membrane trafficking, or cell signaling during hematopoietic maturation (37,45). Mouse ARL6IP1 was confirmed to physically interact with mouse ARL6, which belongs to ARF/ARL family in the Ras superfamily (9,26). However, the biological functions of ARL6IP1 have not been fully elucidated. It raises the interesting question for further study of whether ARL6IP1 might be also implicated in the endothelial cells response to anti-DENV NS1 Abs.
In conclusion, subtractive hybridization libraries of HMEC-1 response of anti-DENV2 NS1 Abs were successfully constructed and some differentially expressed genes were identified. The information indicated there were many genes involved in the pathogenic mechanism directly or indirectly through signaling molecules. This baseline study can serve as a starting point for further work on the cell-autoantibody cross-reaction mechanism and increase our understanding of dengue pathogenesis.
Acknowledgments
This study was supported by Fund of Technology Program of Guangdong Province of P.R. China (2011B031800360).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ades EW. Candal FJ. Swerlick RA. George VG. Summers S. Bosse DC. Lawley TJ. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 2.Avirutnan P. Punyadee N. Noisakran S, et al. Vascular leakage in severe infections: A potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193:1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 3.Basu A. Chaturvedi UC. Vascular endothelium: The battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–299. doi: 10.1111/j.1574-695X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S. Kazatchkine MD. Pathogenesis of dengue: An alternative hypothesis. Southeast Asian J Trop Med Public Heath. 1990;21:652–657. [PubMed] [Google Scholar]
- 5.Busch AK. Cordery D. Denyer GS. Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic beta-cell function. Diabetes. 2002;51:977–987. doi: 10.2337/diabetes.51.4.977. [DOI] [PubMed] [Google Scholar]
- 6.Buttthep P. Bunyaratvej A. Bhamarapravati N. Dengue virus and endothelial cell: A related phenomenon to thrombocytopenia and granulocytopenia in dengue haemorrhagic fever. Southeast Asian J Trop Med Public Heath. 1993;24:246–249. [PubMed] [Google Scholar]
- 7.Casado FL. Singh KP. Gasiewicz TA. The aryl hydrocarbon receptor: Regulation of hematopoiesis and involvement in the progression of blood diseases. Blood Cells Mol Dis. 2010;44:199–206. doi: 10.1016/j.bcmd.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi UC. Shrivatava R. Tripathi RK. Nagar R. Dengue virus-specific suppressor T cells: Current perspectives. FEMS Immunol Med Microbiol. 2007;50:285–299. doi: 10.1111/j.1574-695X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 9.Clark J. Moore L. Krasinskas A. Way J. Battey J. Tamkun J. Kahn RA. Selective amplification of additional members of the ADP-ribosylation factor (ARF) family: Cloning of additional human and Drosophila ARF-like genes. Proc Natl Acad Sci USA. 1993;90:8952–8956. doi: 10.1073/pnas.90.19.8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtois-Coutry N. Le Moellic C. Boulkroun S, et al. Calcyclin is an early vasopressin-induced gene in the renal collecting duct. Role in the long-term regulation of ion transport. J Biol Chem. 2002;277:25728–25734. doi: 10.1074/jbc.M112435200. [DOI] [PubMed] [Google Scholar]
- 11.Diatchenko L. Lau YF. Campbell AP, et al. Suppression subtractive hybridization: A method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falconar AKI. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesion proteins and binds to human endothelial cells: Potential implications in haemorrhagic fever pathogenesis. Arch Virol. 1997;142:897–916. doi: 10.1007/s007050050127. [DOI] [PubMed] [Google Scholar]
- 13.Falconar AKI. Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design. Clin Vaccine Immunol. 2007;14:493–504. doi: 10.1128/CVI.00371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipek A. Gerke V. Weber K. Kuźnicki J. Characterization of the cell-cycle-regulated protein calcyclin from Ehrlich ascites tumor cells. Identification of two binding proteins obtained by Ca2(+)-dependent affinity chromatography. Eur J Biochem. 1991;195:795–800. doi: 10.1111/j.1432-1033.1991.tb15768.x. [DOI] [PubMed] [Google Scholar]
- 15.Furness SG. Whelan F. The pleiotropy of dioxin toxicity-xenobiotic misappropriation of the aryl hydrocarbon receptor's alternative physiological roles. Pharmacol Ther. 2009;124:336–353. doi: 10.1016/j.pharmthera.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzo F. Lauret E. Ferrari S. Baserga R. Growth factor regulation of the promoter for calcyclin, a growth-regulated gene. J Biol Chem. 1988;263:4758–4763. [PubMed] [Google Scholar]
- 17.Golitsina NL. Kordowska J. Wang CL. Lehrer SS. Ca2+-dependent binding of calcyclin to muscle tropomyosin. Biochem Biophys Res Commun. 1996;220:360–365. doi: 10.1006/bbrc.1996.0410. [DOI] [PubMed] [Google Scholar]
- 18.Grundt K. Haga IV. Aleporou-Marinou V. Drosos Y. Wanvik B. Østvold AC. Characterisation of the NUCKS gene on human chromosome 1q32.1 and the presence of a homologous gene in different species. Biochem Biophys Res Commun. 2004;323:796–801. doi: 10.1016/j.bbrc.2004.08.153. [DOI] [PubMed] [Google Scholar]
- 19.Guzmán MG. Kourí G. Díaz M, et al. Dengue, one of the great emerging health challenge of the 21st century. Expert Rev Vaccines. 2004;3:511–520. doi: 10.1586/14760584.3.5.511. [DOI] [PubMed] [Google Scholar]
- 20.Halstead SB. The XXth century dengue pandemic: Need for surveillance and research. World Health Stat Q. 1992;45:292–298. [PubMed] [Google Scholar]
- 21.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 22.Hahn ME. Aryl hydrocarbon receptors: Diversity and evolution. Chem Biol Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Harada R. Okada N. Fujita T. Okada H. Purification of 1F5 antigen that prevents complement attack on homologous cell membranes. J Immunol. 1990;144:1823–1828. [PubMed] [Google Scholar]
- 24.Hathriat P. Isarangkura P. Srichaikul T. Suvatte V. Mitrakul C. Abnormal hemostasis in dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 1993;24:80–85. [PubMed] [Google Scholar]
- 25.Hong EJ. Park SH. Choi KC. Leung PC. Jeung EB. Identification of estrogen regulated genes by microarray analysis of the uterus of immature rats exposed to endocrine disrupting chemicals. Reprod Biol Endocrinol. 2006;4:49–61. doi: 10.1186/1477-7827-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingley E. Williams JH. Walker CE, et al. A novel ADP-ribosylation like factor (ARL6) interacts with the protein-conducting channel SEC61β subunit. FEBS Lett. 1999;495:69–74. doi: 10.1016/s0014-5793(99)01188-6. [DOI] [PubMed] [Google Scholar]
- 27.Jiang L. Tang YX. Yin Y. Fang DY. Zhou JM. Jiang LF. Expression of the secreted NS1 of DENV2 virus and the preparation of the rabbit polyclonal anti-NS1 antibodies. Re Dai Yi Xue Za Zhi. 2009;9:601–604. (Chinese) [Google Scholar]
- 28.Kucharczak J. Pannequin J. Camby I. Decaestecker C. Kiss R. Martinez J. Gastrin induces overexpression of genes involved in human U373 glioblastoma cell migration. Oncogene. 2001;20:7021–7028. doi: 10.1038/sj.onc.1204882. [DOI] [PubMed] [Google Scholar]
- 29.Lin CF. Chiu SC. Hsiao YL, et al. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J Immunol. 2005;174:395–403. doi: 10.4049/jimmunol.174.1.395. [DOI] [PubMed] [Google Scholar]
- 30.Lin CF. Lei HY. Shiau AL, et al. Antibodies from dengue patients sera cross-react with endothelial cells and induce damage. J Med Virol. 2003;69:82–90. doi: 10.1002/jmv.10261. [DOI] [PubMed] [Google Scholar]
- 31.Lin CF. Lei HY. Shiau AL, et al. Endothelial cell apopotosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol. 2002;169:657–664. doi: 10.4049/jimmunol.169.2.657. [DOI] [PubMed] [Google Scholar]
- 32.Malasit P. Complement and dengue haemorrhagic fever/shock syndrome. Southeast Asian J Trop Med Public Heath. 1987;18:316–320. [PubMed] [Google Scholar]
- 33.Meri S. Morgan BP. Davies A, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71:1–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Munir S. Singh S. Kaur K. Kapur V. Suppression subtractive hybridization coupled with microarray analysis to examine differential expression of genes in virus infected cells. Biol Proced Online. 2004;6:94–104. doi: 10.1251/bpo77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negishi T. Kato Y. Ooneda O, et al. Effects of aryl hydrocarbon receptor signaling on the modulation of Th1/Th2 balance. J Immunol. 2005;175:7348–7356. doi: 10.4049/jimmunol.175.11.7348. [DOI] [PubMed] [Google Scholar]
- 36.Pangburn MK. Ferreira1 VP. Cortes1 C. Discrimination between host and pathogens by the complement system. Vaccine. 2008;26:115–121. doi: 10.1016/j.vaccine.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettersson M. Bessonova M. Gu HF. Groop LC. Jonsson JI. Characterization, chromosomal localization, and expression during hematopoietic differentiation of the gene encoding Arl6ip, ADP ribosylation-like factor-6 interacting protein (ARL6) Genomics. 2000;68:351–354. doi: 10.1006/geno.2000.6278. [DOI] [PubMed] [Google Scholar]
- 38.Potts BC. Smith J. Akke M, et al. The structure of calcyclin reveals a novel homodimeric fold for S100 Ca(2+)-binding proteins. Nat Struct Biol. 1995;2:790–796. doi: 10.1038/nsb0995-790. [DOI] [PubMed] [Google Scholar]
- 39.Schaner ME. Ross DT. Ciaravino G, et al. Gene expression patterns in ovarian carcinomas. Mol Biol Cell. 2003;14:4376–4386. doi: 10.1091/mbc.E03-05-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt JV. Su GH. Reddy JK. Simon MC. Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Nat Acad Sci USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stvold AC. Norum JH. Mathiesen S. Wanvik B. Sefland I. Grundt K. Molecular cloning of a mammalian nuclear phosphoprotein NUCKS, which serves as a substrate for Cdk1in vivo. Eur J Biochem. 2001;268:2430–2440. doi: 10.1046/j.1432-1327.2001.02120.x. [DOI] [PubMed] [Google Scholar]
- 42.Tonini GP. Casalaro A. Cara A. Di Martino D. Inducible expression of calcyclin, a gene with strong homology to S-100 protein, during neuroblastoma cell differentiation and its prevalent expression in Schwannlike cell lines. Cancer Res. 1991;51:1733–1737. [PubMed] [Google Scholar]
- 43.Tsoporis JN. Izhar S. Parker TG. Expression of S100A6 in cardiac myocytes limits apoptosis induced by tumor necrosis factor-alpha. J Biol Chem. 2008;283:30174–30183. doi: 10.1074/jbc.M805318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veldhoen M. Hirota K. Westendorf AM. Buer J. Dumoutier L. Renauld JC. Stockinger B. The aryl hydrocarbon receptor links T(H)17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 45.Wang GL. Shi X. Salisbury E. Timchenko NA. Regulation of apoptotic and growth inhibitory activities of C/EBPalpha in different cell lines. Exp Cell Res. 2008;314:1626–1639. doi: 10.1016/j.yexcr.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wills FL. McCubbin WD. Kay CM. Smooth muscle calponin-caltropin interaction: Effect on biological activity and stability of calponin. Biochemistry. 1994;33:5562–5569. doi: 10.1021/bi00184a027. [DOI] [PubMed] [Google Scholar]
- 47.Wojda U. Kuźnicki J. Calcyclin from mouse Ehrlich ascites tumor cells and rabbit lung form non-covalent dimmers. Biochim Biophys Acta. 1994;1209:248–252. doi: 10.1016/0167-4838(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 48.Zeng FY. Gerke V. Gabius HJ. Identification of annexin II, annexin VI, and glyceraldehyde-3-phosphate dehydrogenase as calcyclin-binding proteins in bovine heart. Int J Biochem. 1993;25:1019–1027. doi: 10.1016/0020-711x(93)90116-v. [DOI] [PubMed] [Google Scholar]
- 49.Zhou JM. Tang YX. Fang DY, et al. Secreted expression and purification of dengue 2 virus full-length nonstructural glycoprotein NS1 in Pichia pastoris. Virus Genes. 2006;33:27–32. doi: 10.1007/s11262-005-0036-6. [DOI] [PubMed] [Google Scholar]