Abstract
Cardiometabolic disorders have been shown to impair coronary microvascular functions leading to diminished cardiac performance and increased mortality. In this review, we focus on the molecular pathomechanisms of impaired endothelium-dependent and flow-induced dysregulation of coronary vasomotor tone in cardiometabolic disorders such as obesity, diabetes mellitus or hyperhomocysteinemia based on animal experiments and human studies. We also briefly summarize the relationship among key signaling mechanisms that contribute to the development of coronary dysfunctions in these disorders, which may help develop new targets for efficient cardiometabolic prevention and treatments.
Keywords: Coronary circulation, Obesity, Type 2 diabetes mellitus, Hyperhomocysteinemia, Wall shear stress, Endothelial function
Introduction
The prevalence of metabolic syndrome (central obesity, high fasting glucose, dyslipidemia and hypertension) exceeds 30 % in the western European Region as well as in the United States. In the background of cardiovascular complications, disorders of microcirculation and endothelial dysfunctions precede atherosclerosis. The healthy endothelium responds to various stimuli by production of factors that regulate vascular tone, smooth muscle cell proliferation and vessel wall inflammation.
In this review, we focus on the endothelial regulation of coronary vasomotor functions. This role is achieved by production and release of vasoactive molecules that dilate or constrict the coronary vessels, thereby playing an important role in the maintenance of tissue oxygen supply (and is also involved in the optimal remodeling of vessels). The proportional contribution of various endothelium derived mediators shows gender characteristics, likely responsible for the different prevalence and nature of cardiovascular diseases in women and men [1, 2, 3].
Dysregulation of coronary circulation in cardiometabolic diseases
Cardiometabolic disorders [e.g., visceral obesity, type 2 DM (T2DM), hypertension, etc.] have been shown to be associated with coronary microvascular dysfunction, shown by reduced coronary venous pO2, diminished vasodilation to endothelium-dependent and independent agonists. Such coronary microvascular disorders are mainly based on endothelial dysfunction, and precede atherosclerosis. They have been associated with left ventricular systolic and diastolic contractile dysfunction in humans and animal models [for reviews see [1, 2, 3]].
All the components of the metabolic syndrome can individually impair endothelial functions. Decreased NO availability appears to play a major role in this coronary endothelial dysfunction. Additionally, reduced availability of other vasodilator agents (including prostacyclin and EDHF) and simultaneously increased activity of vasoconstrictor substances (including endothelin-1 and Ang II) may also play role.
Obesity: Endothelial dysfunction and potential pathomechanisms
We have demonstrated that high-fat diet (HFD, 60 % fat calories) impairs NO mediation of endothelium-dependent dilations of carotid and skeletal muscle arterioles in rats, primarily because of an increased xanthine oxidase-derived superoxide anion production. Our findings suggest that HFD promotes the development of oxidative stress and endothelial dysfunction in arterioles [4]. However, in coronary arterioles of obese rats, endothelium-dependent acetylcholine (ACh)-induced arteriolar dilations were essentially preserved. This response was not significantly affected by eNOS inhibition, whereas the dilator responses to NO donors were significantly enhanced. Upon administration of soluble guanylyl-cyclase (sGC) inhibitor, the dilator response was reduced to the control level. These findings indicate that in HFD obesity, NO sensitivity of coronary arterioles is enhanced, due to the increased sGC activity [5]. Thus, despite the impaired NO bioavailability, this adaptive mechanism may contribute to the maintenance of NO-mediated vascular signaling [5]. Similar results have been obtained from isolated human coronary arterioles from obese hypertensive patients [6].
The exact origin and nature of factor(s) that may contribute to the activation of sGC are still unknown. (1) It seems plausible that the lack of NO may lead to enhanced sGC sensitivity. (2) Moreover, TNF-mediated vascular inflammation has important consequences with respect to the downstream signaling of NO, although, in our respective study, the level of systemic inflammation was not increased (a possible involvement of localized vascular wall inflammation cannot be excluded). (3) Enhanced xanthine oxidase-derived superoxide anion production was demonstrated by us in HFD rats [4]. Thus, it is likely that reactive oxygen species, in addition to their well-known effect on NO bioavailability, may play a role in the upregulation of the downstream sGC enzyme activity in the coronary microvessels. It remains to be clarified to what extent and how long the upregulation of sGC may contribute to the maintenance of NO-mediated coronary vasomotor responses to provide adequate perfusion of the myocardium (Fig. 1).
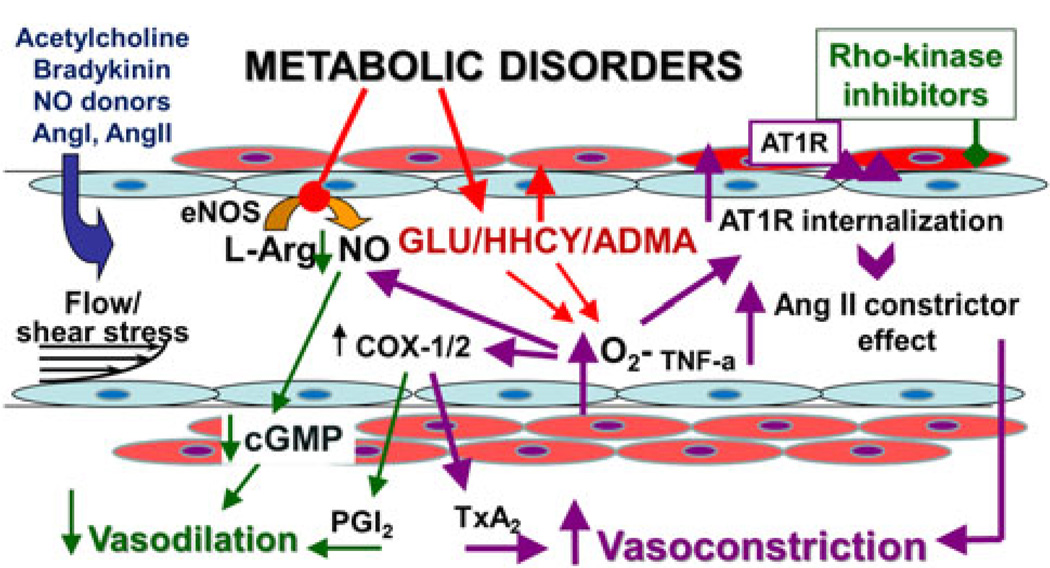
Fig. 1.
This schematic figure illustrates the endothelial pathomechanisms, which contribute to the dysregulation of coronary vascular tone in metabolic diseases. eNOS endothelial nitric oxide synthase, L-Arg L-arginine, NO nitric oxide, GLU glucose, HHCY hyperhomocysteinaemia, ADMA asymmetric-dimethylarginine, O2− superoxide anion, COX cyclooxygenase, PGI2 prostaglandin I2, TxA2 thromboxane A2, Ang angiotensin, AT1R angiotensin II type 1 receptor, TNF-a tumor necrosis factors-alpha
Type 2 diabetes mellitus/hyperglycemia: Endothelial dysfunction and potential pathomechanisms
Type 2 diabetes mellitus is associated with a markedly increased incidence of cardiovascular diseases, accounting for ~70 % of diabetic mortality [1, 2, 3]. Vasomotor dysfunction of microvessels is an early manifestation of the vascular complications in T2DM. Alterations in local vasomotor mechanisms, such as reduced endothelium-dependent dilation have been reported. Changes in the local vasomotor mechanisms of peripheral microvessels may significantly influence vascular resistance in T2DM (Fig. 1) [7].
In diabetic patients, despite the presence of normal coronary arteries, the coronary flow reserve is reduced. In animal models of diabetes mellitus, reduced agonist- and flow-induced endothelium-dependent dilations of coronary arterioles were shown by us [7].
On the other hand, in human observations Bagi’s group found that bradykinin elicited greater coronary vasodilation in diabetic patients than in controls [8]. Non-selective inhibition of COXs by indomethacin failed to affect bradykinin-induced arteriolar dilation in the non-diabetic group, but it significantly reduced bradykinin-induced responses to the control level in coronary arterioles of diabetic patients. Additionally, selective inhibition of COX-2 also reduced bradykinin-induced coronary dilations in the diabetic group, but had no effect in controls. Because the reduction in bradykinin-induced dilation by the selective COX-2 inhibitor was the same as indomethacin-induced inhibition, it suggests that in coronary arterioles of diabetic patients bradykinin elicits dilator prostaglandin release primarily via stimulating COX-2. Compared to controls we have found a marked COX-2 immunostaining in coronary arterioles of diabetic patients, which was localized both to the endothelial and smooth muscle layers of arteriolar wall [8]. These results suggest that in diabetic coronary arterioles, increased COX-2 expression may contribute to an enhanced release of dilator prostaglandins on stimulation [8] (Fig. 1).
The importance and the underlying mechanisms responsible for the enhanced COX-2 expression in diabetic coronary arterioles are not fully understood. Recently, the hypothesis has been raised that during the development of DM, adaptive mechanisms may compensate for the impaired vascular function, e.g., loss of NO mediation of dilation has been shown to be associated with an enhanced EDHF activity. In addition, at the site of atherosclerotic plaques, an upregulation of COX-2 enzyme and consequent increased prostacyclin production has been proposed. Also, a specific role for COX-2-derived prostaglandins has been suggested in flow-induced adaptive vascular remodeling in an animal model of atherosclerosis. Our findings show that in human coronary arterioles upregulation of COX-2 and bradykinin-induced release of dilator prostaglandins may serve as an adaptive mechanism aiming to reduce the detrimental effects of diabetes on coronary blood flow [8]. Experimental evidence suggests that underlying mechanism(s) responsible for the upregulation of COX-2 in diabetes are oxidative stress and low-grade inflammation [8], as illustrated in Fig. 1.
Recently, a potential role of the vascular renin–angiotensin systems (RAS) in diabetic coronary disorders has also been raised. Clinical studies demonstrated the effectiveness of Ang II AT1 receptor antagonists (originally developed for hypertension therapy) in the treatment of diabetic vascular complications. Enhanced AT1 receptor signaling elicits increased vasomotor tone and peripheral vascular resistance; therefore, it may contribute to diabetes-related vascular remodeling. Thus, it is possible that diabetes alters the function of AT1 receptors in the vessel wall. In healthy individuals, upon stimulation by Ang II, AT1 receptors are rapidly desensitized and then internalized, becoming unavailable for further stimulation. This negative-feedback regulation of AT1 receptor availability is an important physiological mechanism to prevent prolonged effects of Ang II.
In pre-diabetic metabolic syndrome, Ang II-induced AT1 receptor-mediated coronary constriction has been demonstrated to be augmented in dogs. It may contribute to impaired control of coronary blood flow via increases in circulating Ang II and/or coronary arteriolar AT1 receptor density. Our studies in muscle arterioles also indicate that hyperglycemia leads not only to an enhanced but also a sustained arterial constriction to Ang II [9]. These vasomotor changes in muscles are likely to be due to increased activation of vascular RhoA/Rho-kinase elicited by high glucose, which results in a sustained vascular availability of AT1 Ang II receptors, as illustrated in Fig. 1 [9]. Further studies are needed to test similar mechanisms in the diabetic coronary microcirculatory disorders.
Hyperhomocysteinemia: Endothelial dysfunction and potential pathomechanisms
A high plasma level of homocysteine (HHcy) is a reliable indicator of cardiovascular disease. Homocysteine (Hcy) is a thiol(sulfhydryl) group—containing amino acid that is formed during metabolism of the essential amino acid methionine. Plasma homocysteine concentration can be increased by alterations in genetic and nutritional factors, such as various enzyme (cystathionebeta-synthase, methyltetrahydrofolate-reductase) abnormalities and deficiency of vitamins (folic acid, cyanocobalamin, pyridoxal phosphate).
Chronic hyperhomocysteinemia (HHcy) elicits its adverse vascular effects by altering endothelial function, i.e., flow-induced vasodilation in microvessels, including small coronary arteries [10]. Elevation of plasma homocysteine concentration impairs flow-induced dilation of coronary arterioles by decreasing bioavailability of NO, an effect that can be reversed by superoxide scavengers. Our results in isolated coronary vessels of hyperhomocysteinemic rats suggest that high plasma homocysteine level elicits activation of tumor necrosis factor-alpha (TNF-a), that in turn activates NAD(P)H-oxydase producing superoxide anions. In these events, elevated levels of asymmetric-dimethylarginine (ADMA) may also play a role. Elevated levels of free radicals reduce the bioavailability of vasodilator NO (via scavenging NO and forming peroxynitrite) leading to impaired coronary vasomotor regulation [10], as illustrated in Fig. 1.
Conclusions and perspectives (for translational research)
The prevalence of cardiometabolic disorders shows a dynamic increase with the progressive aging of the population in the western European Region. Better understanding of the underlying endothelial and microvascular pathomechanisms contributing to cardiovascular diseases may help to develop new targets for efficient prevention and conservative, acute and emergency treatments to improve the cardiometabolic health of humans.
Acknowledgments
The authors apologize, because many excellent reviews and studies could not be cited due to space limitation. Support: American Heart Association, Founders Affiliate, 0855910D, NIH PO-1 HL-43023, the Hungarian National Science Research Fund (OTKA) K71591 and K67984, SROP-4.2.1/B-10/2/KONV-2010-0002, SROP-4.2.2.A-11/1/KONV-2012-0024.
Footnotes
Submitted to Internal and Emergency Medicine (Official Journal of the Italian Society of Internal Medicine) as Proceedings of the 2013 Tuscany Endocrinology And Metabolism Conference (4–6 April, 2013, Pisa, Italy).
Conflict of interest None.
Contributor Information
Akos Koller, Email: akos.koller@aok.pte.hu, Department of Pathophysiology and Gerontology, Medical School, J. Szentagothai Res. Centre, University of Pecs, 12. Szigeti Str, 7624 Pecs, Hungary; Department of Physiology, New York Medical College, Valhalla, NY, USA.
Marta Balasko, Department of Pathophysiology and Gerontology, Medical School, J. Szentagothai Res. Centre, University of Pecs, 12. Szigeti Str, 7624 Pecs, Hungary.
Zsolt Bagi, Vascular Biology Center, Medical College of Georgia, GRU, Augusta, GA 30912, USA.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20(2):140–146. doi: 10.1016/j.numecd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52(4):848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdei N, Tóth A, Pásztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006;291(5):H2107–H2115. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- 5.Jebelovszki E, Kiraly C, Erdei N, Feher A, Pasztor ET, Rutkai I, Forster T, Edes I, Koller A, Bagi Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: role of soluble guanylate cyclase activation. Am J Physiol Heart Circ Physiol. 2008;294(6):H2558–H2564. doi: 10.1152/ajpheart.01198.2007. [DOI] [PubMed] [Google Scholar]
- 6.Fulop T, Jebelovszki E, Erdei N, Szerafin T, Forster T, Edes I, Koller A, Bagi Z. Adaptation of vasomotor function of human coronary arterioles to the simultaneous presence of obesity and hypertension. Arterioscler Thromb Vasc Biol. 2007;27(11):2348–2354. doi: 10.1161/ATVBAHA.107.147991. [DOI] [PubMed] [Google Scholar]
- 7.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilation of coronary arterioles in type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H1404–H1410. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- 8.Szerafin T, Erdei N, Fülöp T, Pasztor ET, Edes I, Koller A, Bagi Z. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006;99(5):e12–e17. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 9.Bagi Z, Feher A, Cassuto J, Akula K, Labinskyy N, Kaley G, Koller A. Increased availability of angiotensin AT 1 receptors leads to sustained arterial constriction to angiotensin II in diabetes role for Rho-kinase activation. Br J Pharmacol. 2011;163(5):1059–1068. doi: 10.1111/j.1476-5381.2011.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]