Abstract
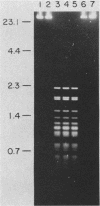
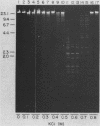
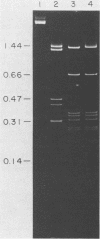
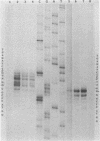
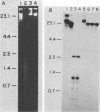
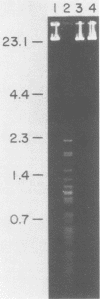
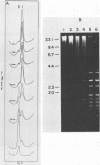
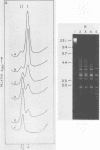
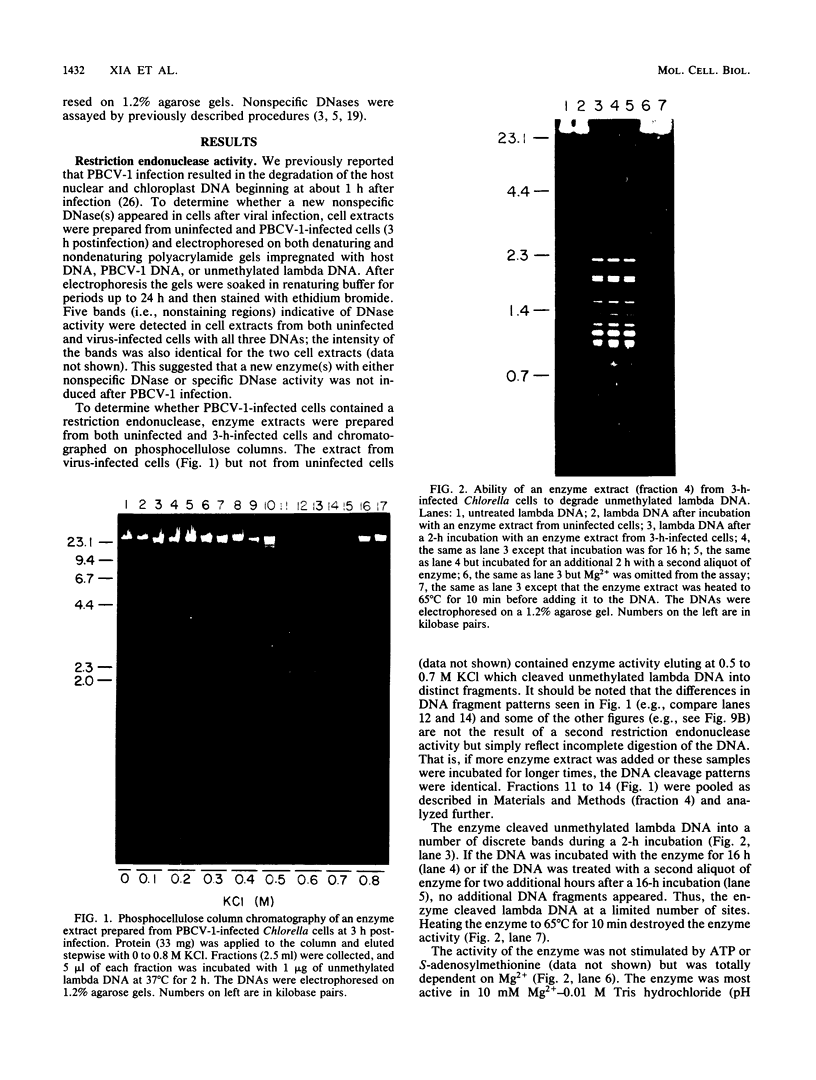
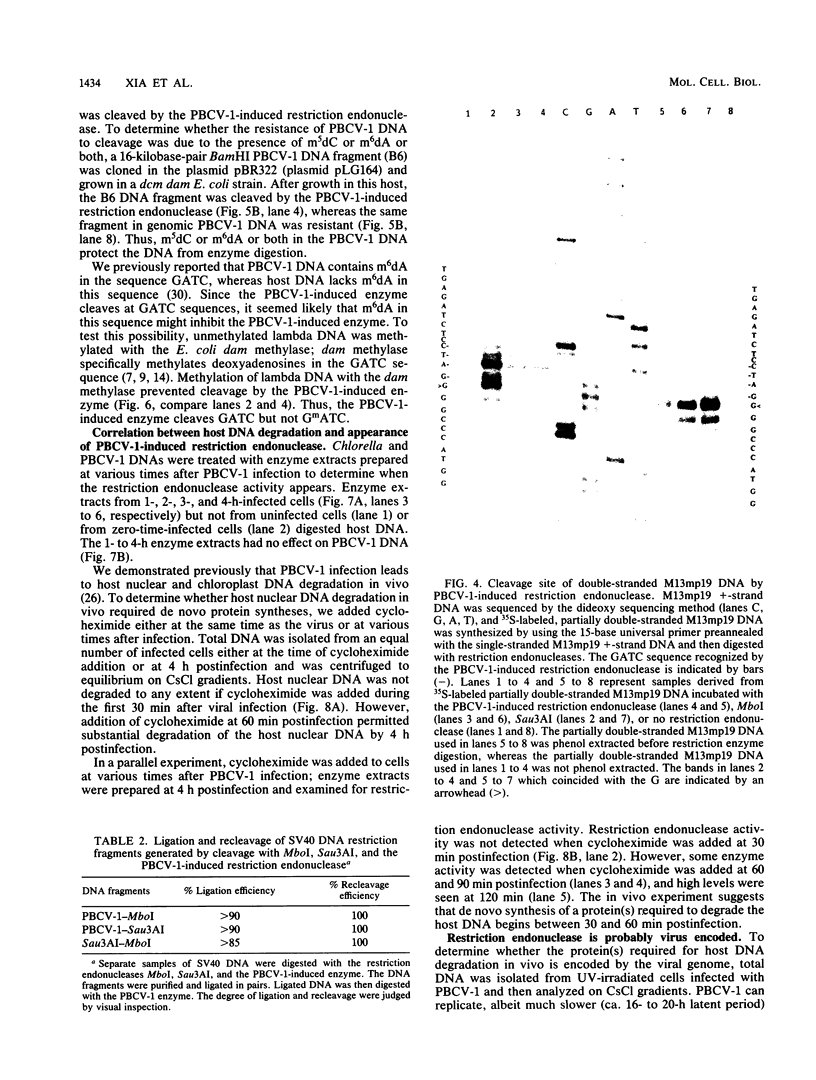
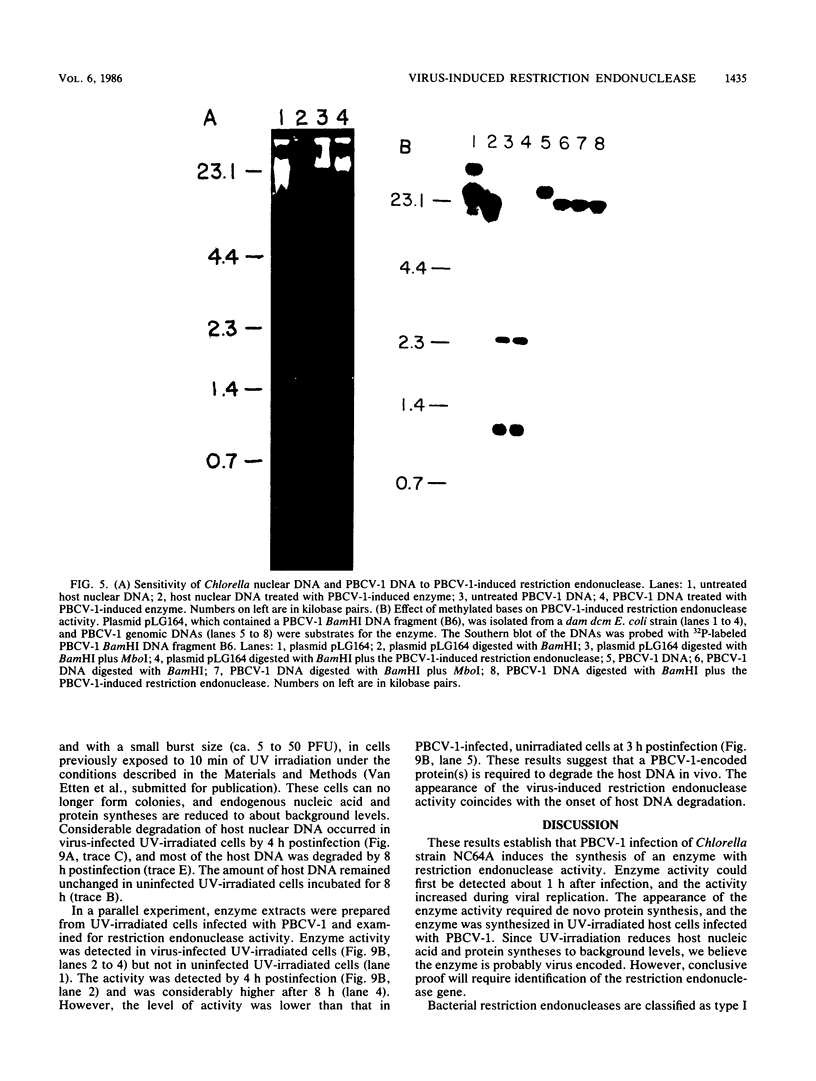
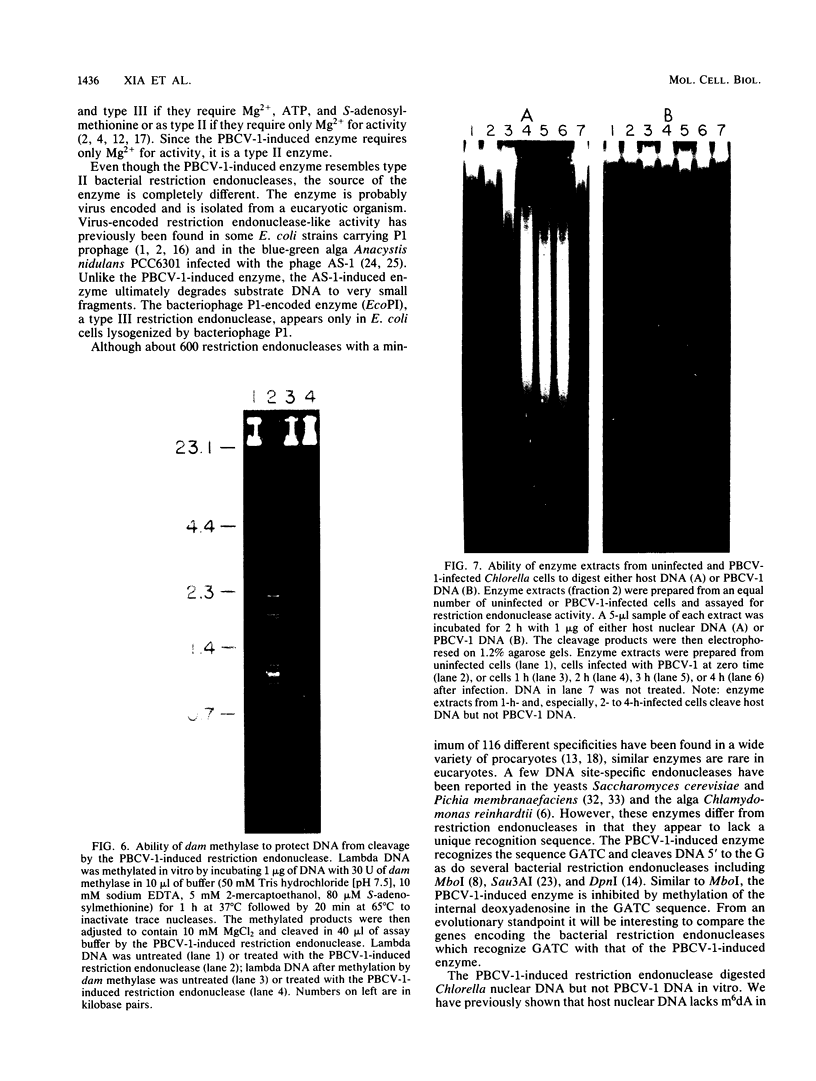
An enzyme was isolated from a eucaryotic, Chlorella-like green alga infected with the virus PBCV-1 which exhibits type II restriction endonuclease activity. The enzyme recognized the sequence GATC and cleaved DNA 5' to the G. Methylation of deoxyadenosine in the GATC sequence inhibited enzyme activity. In vitro the enzyme cleaved host Chlorella nuclear DNA but not viral DNA because host DNA contains GATC and PBCV-1 DNA contains GmATC sequences. PBCV-1 DNA is probably methylated in vivo by the PBCV-1-induced methyltransferase described elsewhere (Y. Xia and J. L. Van Etten, Mol. Cell. Biol. 6:1440-1445). Restriction endonuclease activity was first detected 30 to 60 min after viral infection; the appearance of enzyme activity required de novo protein synthesis, and the enzyme is probably virus encoded. Appearance of enzyme activity coincided with the onset of host DNA degradation after PBCV-1 infection. We propose that the PBCV-1-induced restriction endonuclease participates in host DNA degradation and is part of a virus-induced restriction and modification system in PBCV-1-infected Chlorella cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H., Scibienski E., Slocum H., Roulland-Dussoix D. The in vitro restriction of the replicative form of W.T. and mutant fd phage DNA. Virology. 1971 Dec;46(3):703–710. doi: 10.1016/0042-6822(71)90072-9. [DOI] [PubMed] [Google Scholar]
- Brown T. L., Yet M. G., Wold F. Substrate-containing gel electrophoresis: sensitive detection of amylolytic, nucleolytic, and proteolytic enzymes. Anal Biochem. 1982 May 1;122(1):164–172. doi: 10.1016/0003-2697(82)90266-4. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Roberts R. J., Myers P. A., Sager R. A site-specific single-strand endonuclease from the eukaryote Chlamydomonas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2687–2691. doi: 10.1073/pnas.74.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi B., Reiser J., Pirrotta V. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J Mol Biol. 1979 Feb 25;128(2):143–163. doi: 10.1016/0022-2836(79)90123-2. [DOI] [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Gelinas R. E., Myers P. A., Roberts R. J. Two sequence-specific endonucleases from Moraxella bovis. J Mol Biol. 1977 Jul;114(1):169–179. doi: 10.1016/0022-2836(77)90290-x. [DOI] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. G. Separation and isolation of DNA fragments using linear polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1980;65(1):305–319. doi: 10.1016/s0076-6879(80)65041-1. [DOI] [PubMed] [Google Scholar]
- Kauc L., Piekarowicz A. Purification and properties of a new restriction endonuclease from Haemophilus influenzae Rf. Eur J Biochem. 1978 Dec;92(2):417–426. doi: 10.1111/j.1432-1033.1978.tb12762.x. [DOI] [PubMed] [Google Scholar]
- Kessler C., Neumaier P. S., Wolf W. Recognition sequences of restriction endonucleases and methylases--a review. Gene. 1985;33(1):1–102. doi: 10.1016/0378-1119(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Carraway M., Frey A. Z., Brown L., Arraj J. A. Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet. 1983;192(1-2):288–289. doi: 10.1007/BF00327681. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1985;13 (Suppl):r165–r200. doi: 10.1093/nar/13.suppl.r165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schleif R. Assaying of organisms for the presence of restriction endonucleases. Methods Enzymol. 1980;65(1):19–23. doi: 10.1016/s0076-6879(80)65004-6. [DOI] [PubMed] [Google Scholar]
- Skrdla M. P., Burbank D. E., Xia Y., Meints R. H., Van Etten J. L. Structural proteins and lipids in a virus, PBCV-1, which replicates in a Chlorella-like alga. Virology. 1984 Jun;135(2):308–315. doi: 10.1016/0042-6822(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Monfoort C. H., Schiphof R., Stobberingh E. E. A restriction endonuclease from Staphylococcus aureus. Nucleic Acids Res. 1976 Nov;3(11):3193–3202. doi: 10.1093/nar/3.11.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M., Szmidt A. E., Török I. Evidence for a restriction/modification-like system in Anacystis nidulans infected by cyanophage AS-1. Eur J Biochem. 1983 Mar 1;131(1):137–141. doi: 10.1111/j.1432-1033.1983.tb07240.x. [DOI] [PubMed] [Google Scholar]
- VAN Etten J. L., Burbank D. E., Kuczmarski D., Meints R. H. Virus infection of culturable chlorella-like algae and dlevelopment of a plaque assay. Science. 1983 Feb 25;219(4587):994–996. doi: 10.1126/science.219.4587.994. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Meints R. H., Kuczmarski D., Burbank D. E., Lee K. Viruses of symbiotic Chlorella-like algae isolated from Paramecium bursaria and Hydra viridis. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3867–3871. doi: 10.1073/pnas.79.12.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Schuster A. M., Girton L., Burbank D. E., Swinton D., Hattman S. DNA methylation of viruses infecting a eukaryotic Chlorella-like green alga. Nucleic Acids Res. 1985 May 24;13(10):3471–3478. doi: 10.1093/nar/13.10.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe H., Shibata T., Ando T. Site-specific endo-deoxyribonucleases in eukaryotes: endonucleases of yeasts, Saccharomyces and Pichia. J Biochem. 1981 Dec;90(6):1623–1632. doi: 10.1093/oxfordjournals.jbchem.a133637. [DOI] [PubMed] [Google Scholar]
- Watabe H., Shibata T., Iino T., Ando T. Purification of a eukaryotic site-specific endonuclease, Endo.Sce I, from Saccharomyces cerevisiae and effectors on its specificity and activity. J Biochem. 1984 Jun;95(6):1677–1690. doi: 10.1093/oxfordjournals.jbchem.a134781. [DOI] [PubMed] [Google Scholar]
- Xia Y. N., Van Etten J. L. DNA methyltransferase induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1440–1445. doi: 10.1128/mcb.6.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]