Abstract
This study evaluates the robustness of a magnetic resonance (MR) fat quantification method to changes in R2* caused by an intravenous infusion of superparamagnetic iron oxide (SPIO) contrast agent. The R2* and proton density fat fraction (PDFF) were measured in liver and spine in 14 subjects using an investigational sequence (IDEAL IQ) provided by the MR scanner vendor. Measurements were made before and after SPIO infusion. Results showed SPIO significantly increased R2* in both liver (p = 8.8 × 10−8) and spine (p = 1.3 × 10−2) but PDFFs were not significantly different in either the liver (p = 5.5 × 10−1) or the spine (p = 5.6 × 10−1). These results confirm that the IDEAL IQ method of fat quantification is robust to changes in R2*.
INTRODUCTION
The amount of fat in a tissue is an important biomarker of disease states and numerous magnetic resonance (MR) methods have been developed for estimating fat content (1,2). In clinical MR imaging, the quantity of interest is the proton density fat fraction (PDFF). The PDFF is so-named to differentiate it from the fat composition measured by other modalities, which may use a different physical unit or scale to quantify fat content (e.g. Hounsfield unit or percentage of cells containing a fat droplet).
The PDFF in a given sample is defined as the number of protons in fat (triglyceride) divided by the total number of protons (water plus triglyceride). However, some MR methods for measuring PDFF are confounded by signal relaxation, in the sense that the measured quantity depends on relaxation parameters as well as the fat content. The R2* is clinically relevant as a confounding factor since iron frequently accumulates in the liver and spine and causes rapid signal decay. R2* can also vary with MR scanner settings including field strength, voxel size and bandwidth, which can introduce variability into the PDFF measurement. For these reasons, an important consideration in evaluating methods of measuring PDFF is the robustness to R2*.
A conventional method for PDFF estimation that clearly exhibits the confounding effect of R2* is the 2-pt Dixon method, which can be shown (3) to measure the quantity
where IP and OP are the in-phase and opposed-phase signal intensities and TE is the difference in echo time between the IP and OP acquisitions. Clearly PDFF2–pt is not a simple measure of PDFF, and so its use as a biomarker of the fat content in tissues is problematic.
Current state-of-the-art methods for measuring PDFF take into account the R2*, as well as other confounds including field inhomogeneity, R1 relaxation, fat spectrum, noise bias and eddy currents. The literature on the topic is large and the reader is referred to a recent review article and conference proceedings for a survey of contemporary methods (2). The method considered in the present study is based on Refs (4–6) and its implementation was provided as an investigational research sequence called IDEAL IQ by the vendor.
The goal of the present study is to assess whether a current state-of-the-art method for fat quantification (IDEAL IQ) is robust to changes in R2* induced by an intravenous infusion of superparamagnetic iron oxide (SPIO) contrast agent. The methodology serves as a model of iron overload in organs and is similar to that of previous studies (7,8).
METHODS
Study Design and Research Subjects
This was a cross-sectional observational research study, approved by our Institutional Review Board (IRB) and compliant with Health Insurance Portability and Accountability Act (HIPAA). We enrolled 14 adult subjects with underlying liver disease (eight with chronic hepatitis C viral infection, six with non-alcoholic fatty liver disease) to undergo a research MR examination of the liver and lumbar spine before and after SPIO infusion. The study sample included 12 men and two women, ranging in age from 19 to 63 years (mean age 39 years). All subjects gave written informed consent prior to participating in the study.
MR Imaging
Subjects were scanned at 3.0T (Signa HDx, GE Healthcare, Milwaukee, WI) in axial or sagittal planes with an 8-element torso phased-array coil centered over the liver after at least two hours fasting. Respiratory bellows were applied to monitor breathing and subjects were instructed to breath-hold during the image acquisition. A dielectric pad was placed between coil and the abdominal wall.
A 3D multiple echo spoiled gradient echo sequence was used with a flyback echo train of 3, interleaved twice, to give a total of 6 echos. The TR/first TE/ΔTE were approximately 8/1/1 ms (precise timings varied depending on field of view since minimum values were automatically selected). Other parameters were: field of view 35–45 cm, matrix 256×160×16, slice thickness 8 mm, bandwidth 1500 Hz/pixel and Array Spatial Sensitivity Encoding Technique (ASSET) parallel imaging factor 2. Scan times were approximately 25 seconds. A low flip angle of 5° was used to reduce the effects of R1 relaxation (including those induced by SPIO contrast agent).
A SPIO contrast agent (Feridex, Bayer Healthcare, Wayne, NJ) was prepared as a suspension diluted in 100 ml of 5% dextrose and infused intravenously through a 5 micron filter over 30 min (iron dose 10 μmol/kg of body weight) at a rate of 2–4 ml/min. Imaging was performed before and after the SPIO infusion (typical time interval 120 min).
Image Analysis
After acquisition, maps of the R2* and fat fraction were calculated using an investigational method supplied by the MR vendor (IDEAL IQ). The maps were transferred offline and measurements taken using custom-written Matlab software (The Mathworks, Natick, MA). A radiology resident (2 months experience in clinical MR and 4 years experience in research MR) selected representative axial or sagittal slices of the liver and of the spine in each subject.
Two regions of interest (ROIs) were selected for each subject, one in the liver, and another in the spine. Each ROI contained approximately 170 to 180 voxels. The liver ROIs were selected to include liver parenchyma and avoid focal lesions, biliary or vascular structures, and imaging artifact. The spine ROIs were selected in the center of a lower thoracic or upper lumbar (T12 to L3) vertebral body for each subject, and was positioned to include vertebral body bone marrow and avoid areas of partial voluming and image artifact.
Each ROI was inspected and manually adjusted, when necessary, to account for subject motion between scans. The mean signal intensity within the ROIs was recorded giving eight numbers per subject: liver R2* pre, liver R2* post, liver PDFF pre, liver PDFF post, spine R2* pre, spine R2* post, spine PDFF pre, and spine PDFF post.
Visual inspection of data showed extensive breathing motion artifact in Subject 13’s data, and the liver data for this subject was not included in the analysis. Subject 13’s spine data was not affected by the motion artifact and was included.
Statistical Analyses
The difference of the estimated PDFF before and after the administration of SPIO was calculated in the liver (liver PDFF post – liver PDFF pre) for each subject. A one sample t-test was performed on the difference values to determine if SPIO significantly changed PDFF estimation. This was repeated for the liver R2* measurements. The entire process was then repeated for the spine values.
RESULTS
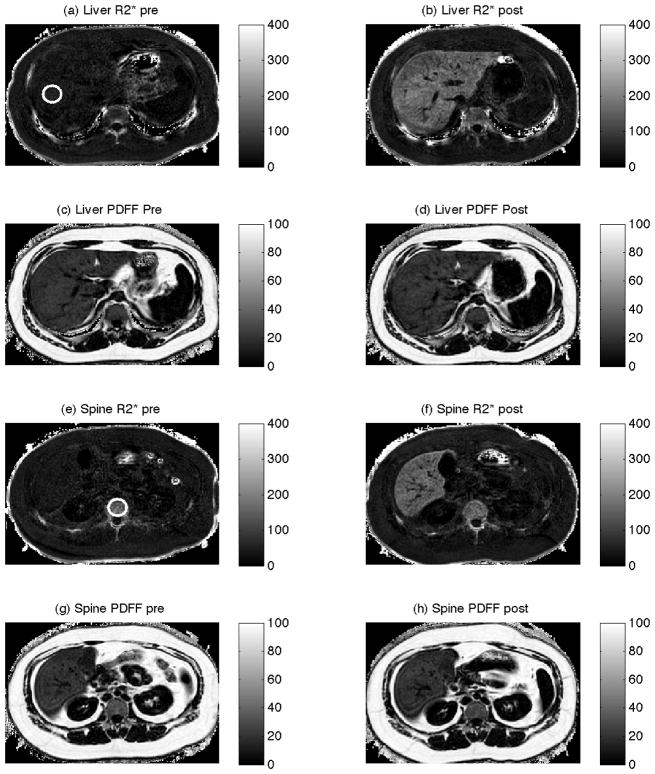
Figure 1 shows data from a representative subject (Subject 3), with the white circles in panels (a) and (e) indicating the liver and spine ROIs respectively. The top row shows representative axial slices of liver R2* measurements before (a) and after (b) the administration of SPIO. The liver parenchyma shows dramatically increased R2* after administration of SPIO, consistent with the known effect of SPIO. The second row shows representative axial slices of liver PDFF measurements before (c) and after (d) the administration of SPIO. Qualitatively, the liver PDFF maps are very similar, suggesting that PDFF estimates are unaffected by R2*. The third row shows representative axial slices of spine R2* measurements before (e) and after (f) the administration of SPIO. The vertebral body R2* maps appear similar before and after administration of SPIO, suggesting SPIO has little effect on the spine’s R2*. Axial slices showing the spine PDFF before (g) and after (h) the administration of SPIO are also visually similar (bottom row).
Figure 1.
Axial data from Subject 3 before (left column) and after (right column) the administration of SPIO. The first and third rows are measurements of R2* (s−1), and the second and fourth rows are measurements of proton density fat fraction (PDFF) (%). The axial slices from the top two rows were selected to evaluate the liver, and the bottom two rows were selected to evaluate the spine. Circles in panels (a) and (e) indicate regions of interest in the liver and spine.
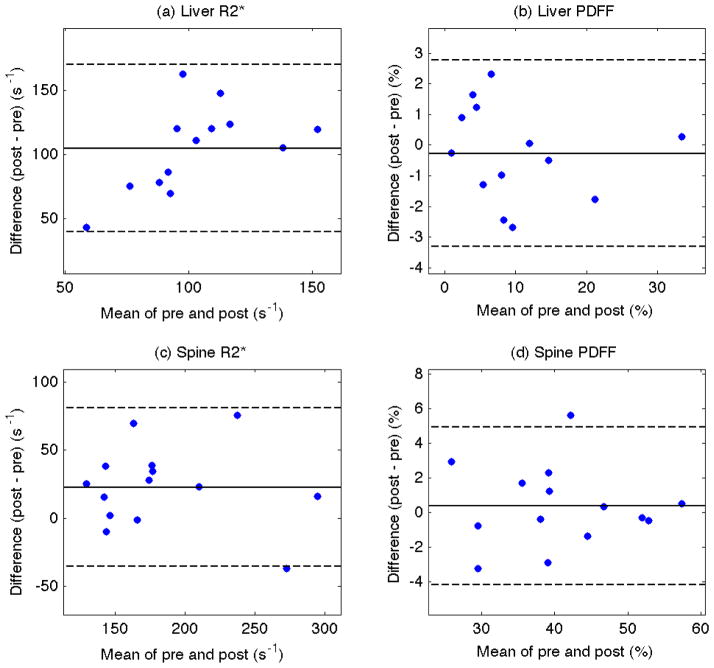
Table 1 gives the mean R2* and PDFF in liver and spine for each subject before and after the administration of SPIO. The second to last row shows the mean difference computed across subjects and the last row shows the results of the one sample t-test on the difference (post – pre) across subjects. Consistent with Figure 1, SPIO significantly increases the liver R2* (p = 8.8 × 10−8; mean difference 104.7 s−1). The Bland-Altman plot in Figure 2a shows the liver R2* difference for every subject is positive. Despite the dramatic increase in liver R2*, the liver PDFF measurements are not significantly different (p = 5.5 × 10−1; mean difference −0.27%). This is depicted in the Bland-Altman plot in Figure 2b, which shows relatively symmetric distribution of liver PDFF difference values about zero.
Table 1.
R2* and fat fraction (PDFF) in the liver and spine before and after SPIO administration. The liver data for subject 13 was not included due to breathing motion artifact.
| Subject | Liver R2* (s−1) | Liver PDFF (%) | Spine R2* (s−1) | Spine PDFF (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 85.5 | 190.6 | 5.4 | 7.7 | 156.6 | 195.3 | 39.4 | 45.0 |
| 2 | 16.2 | 178.8 | 8.5 | 7.5 | 128.1 | 198.1 | 46.5 | 46.9 |
| 3 | 55.0 | 178.6 | 22.0 | 20.2 | 144.9 | 146.9 | 31.1 | 27.9 |
| 4 | 57.9 | 127.3 | 1.0 | 0.8 | 199.4 | 275.1 | 57.1 | 57.6 |
| 5 | 49.1 | 127.1 | 33.3 | 33.5 | 123.8 | 162.0 | 24.4 | 27.3 |
| 6 | 47.3 | 158.4 | 3.2 | 4.8 | 160.3 | 188.1 | 34.7 | 36.4 |
| 7 | 39.0 | 186.7 | 6.1 | 4.8 | 159.4 | 194.1 | 38.0 | 40.3 |
| 8 | 49.2 | 169.6 | 11.9 | 12.0 | 166.3 | 164.9 | 29.9 | 29.1 |
| 9 | 48.5 | 134.5 | 14.8 | 14.3 | 198.6 | 221.7 | 38.2 | 37.9 |
| 10 | 37.0 | 80.1 | 9.5 | 7.1 | 116.5 | 141.8 | 40.5 | 37.7 |
| 11 | 35.4 | 155.2 | 1.9 | 2.8 | 133.9 | 149.3 | 52.1 | 51.8 |
| 12 | 38.6 | 113.9 | 3.8 | 5.1 | 286.8 | 303.1 | 38.6 | 39.8 |
| 13 | - | - | - | - | 148.6 | 138.4 | 53.0 | 52.5 |
| 14 | 92.4 | 212.0 | 10.9 | 8.2 | 291.5 | 254.3 | 45.2 | 43.9 |
| Mean Difference | 104.7 | −0.27 | 22.7 | 0.37 | ||||
| p-value | 8.8 × 10−8 | 5.5 × 10−1 | 1.3 × 10−2 | 5.6 × 10−1 | ||||
Figure 2.
Bland-Altman plots of R2* (left column) and proton density fat fraction (right column) measurements for each subject from the liver (top row) and spine (bottom row). The difference (y-axis) versus mean (x-axis) of the signal intensities in the regions of interest of each subject are shown. The mean difference (solid horizontal line) and ± 1.96 standard deviation (dash lines) are also shown.
Although a change in R2* with SPIO administration was not visually appreciable in the spine in Figure 1, SPIO was found to significantly increase R2* in the spine (p = 1.3 × 10−2; mean difference 22.7 s−1). As depicted in the Bland-Altman plot in Figure 2c, most but not all subjects showed an increase in spine R2* after SPIO (the majority of spine R2* differences are positive). The spine PDFF, however, was not significantly different pre- and post- SPIO (p = 5.6 × 10−1; mean difference = 0.37%), which is indicated in the Bland-Altman plot in Figure 2d.
DISCUSSION
Fat fraction measurements typically rely on multiecho methods that attribute the signal change during an echo time interval to chemical shift interference between water and fat. However R2* decay also causes a signal change over this same interval and R2* is traditionally a confound for estimating fat using clinical IP/OP imaging.
In this study, we experimentally increased both the liver R2* and spine R2* using SPIO contrast agent and measured the proton density fat fraction (PDFF) measurements pre- and post- infusion using a recent MR fat quantification method (IDEAL IQ). Correction for R2* employed by IDEAL IQ assumes a monoexponential signal decay, which has been shown in previous studies to provide robustness to R2* variability (7–10). The PDFF measured by IDEAL IQ was not significantly affected by SPIO in either liver or spine, indicating that this method is robust to differences in R2*.
One interesting observation was the R2* in the spleen did not change as much as the liver. The spleen signal is sometimes used to correct liver signal to normalize for non-fat related differences between IP and OP images (11,12). While not specifically noted in the above studies, spleen correction will also partly compensate for R2* decay. A relatively minor point to make is that, since the R2* can differ between spleen and liver (at least in the case of SPIO particles), a spleen correction may not fully correct for R2* decay in the liver and should not substitute for R2* correction.
A limitation of this study is the absence of a fat fraction measurement gold standard, given we did not perform biopsies to obtain histologic quantification of fat fraction. This should not affect interpretation of our study since each subject serves as his independent control and comparison of the pre and post SPIO values would mitigate the effect of any systemic bias. In addition, several prior studies have evaluated the accuracy of MR fat fraction estimation in the absence of SPIO (13,14), and found the approach (i.e. with corrections for known confounding factors) to be accurate with respect to independent references. Taking the pre SPIO PDFF as a reference, the present study can be considered to validate the IDEAL IQ method in the presence of R2* decay.
A second limitation of the present study was a variation in protocol across subjects; specifically, eight subjects were scanned in axial sections and six subjects were scanned in sagittal sections. The plane of image acquisition is not thought to significantly affect the interpretation of results because careful visual evaluation of the raw data showed comparable data quality in both axial and sagittal data. Analysis performed using only the eight subjects acquired in axial section (not shown) gave very similar findings, except that the increase in spine R2* with SPIO was no longer statistically significant (p = 9.2 × 10−2, mean difference = 26.1 s−1).
In conclusion, we have tested whether the proton density fat fraction (PDFF) measured in the liver and spine using an investigational MR technique (IDEAL IQ) is significantly affected by changes in R2*. Our findings were that the PDFF is not significantly affected by R2* and hence the technique is robust to R2* variability.
Acknowledgments
The authors thank GE Healthcare for research support and Ann Shimikawa, Kang Wang and Huanzhou Yu for technical help. Research grants were provided by Berlex (Bayer) and from NIH grants R01 (#DK075128) and R21 (#CA162718). The study was ancillary (#AS009) to the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). The NASH CRN is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (UCSD #1U01DK061734, Data Coordinating Center #5U01DK061730) and the National Institute of Child Health and Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28(3):543–558. doi: 10.1002/jmri.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hu HH, Bornert P, Hernando D, et al. ISMRM workshop on fat-water separation: insights, applications and progress in MRI. Magn Reson Med. 2012;68(2):378–388. doi: 10.1002/mrm.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26(3):347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54(3):636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60(5):1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Shimakawa A, Hines CD, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med. 2011;66(1):199–206. doi: 10.1002/mrm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bydder M, Shiehmorteza M, Yokoo T, et al. Assessment of liver fat quantification in the presence of iron. Magn Reson Imaging. 2010;28(6):767–776. doi: 10.1016/j.mri.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuaki T, Yoshimitsu K, Zimine I, Saiki S, Cauteren MV, Miyati T. Estimation of fat fraction considering T2* decay in liver after SPIO injection. Proc Intl Soc Mag Reson Med. 2009:4092. [Google Scholar]
- 9.Horng D, Hernando D, Hines C, Reeder S. Comparison of R2* correction methods for accurate fat quantification in fatty liver. Journal of Magnetic Resonance Imaging. doi: 10.1002/jmri.23835. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen KH, Schroeder ME, Hamilton G, Sirlin CB, Bydder M. Robustness of fat quantification using chemical shift imaging. Magn Reson Imaging. 2012;30(2):151–157. doi: 10.1016/j.mri.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelblinger C, Krssak M, Maresch J, et al. Hepatic steatosis assessment with (1)H-spectroscopy and chemical shift imaging at 3.0T before hepatic surgery: Reliable enough for making clinical decisions? Eur J Radiol. 2012;81(11):2990–2995. doi: 10.1016/j.ejrad.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques--initial experience. Radiology. 2005;237(2):507–511. doi: 10.1148/radiol.2372040539. [DOI] [PubMed] [Google Scholar]
- 13.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36(1):22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joe E, Lee JM, Kim KW, et al. Quantification of hepatic macrosteatosis in living, related liver donors using T1-independent, T2*-corrected chemical shift MRI. J Magn Reson Imaging. 2012 doi: 10.1002/jmri.23738. [DOI] [PubMed] [Google Scholar]